Abstract
A small library of bis- and tetraamides was synthesized by the Ugi reaction with α-ketoglutaric acid, tert-butyl isocyanide, aromatic aldehydes, and aromatic amines. When o-azidoanilines were used, azidated peptidomimetics were obtained, the post-cyclization of which by the aza-Wittig reaction yielded a series of substituted 3-(3-oxo-3,4-dihydroquinoxalin-2-yl)propanoic acids containing a pharmacophore quinoxalinone moiety. The tandem Ugi/aza-Wittig combination was also carried out in a one-pot procedure without isolation of the intermediate.
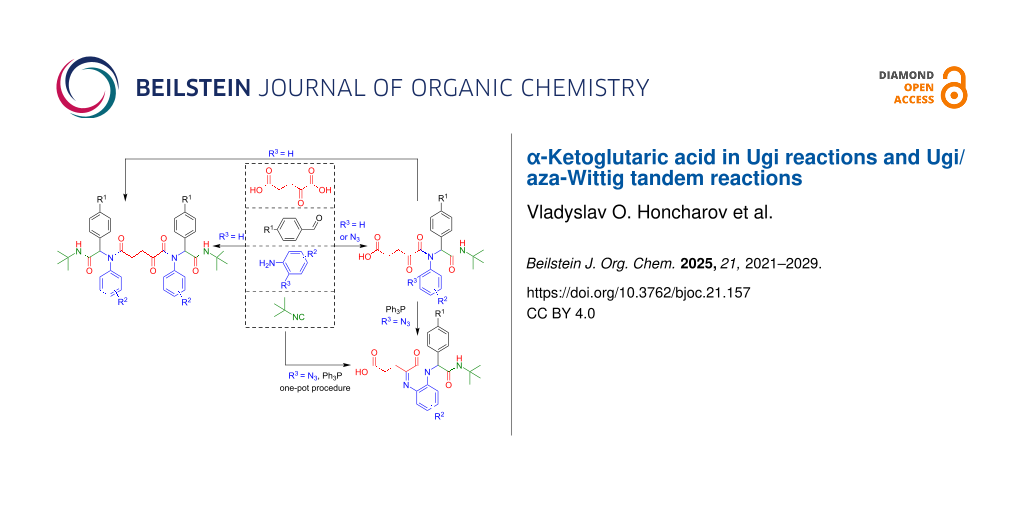
Graphical Abstract
Introduction
Multicomponent reactions are powerful tools in organic chemistry that enable the synthesis of structurally complex and multifunctional compounds from three or more starting materials in a single synthetic step. They are widely used in drug discovery because, unlike conventional linear synthesis strategies, they enable the preparation of libraries of organic compounds in higher yields and with significantly less time, resources, and chemical waste [1-5].
The Ugi reaction, discovered in 1959 by Ivar Karl Ugi [6], is one of the classic multicomponent reactions, widely used as a green alternative for the synthesis of active pharmaceutical ingredients [7] and opens up the possibility of creating new compounds from the class of peptidomimetics [8-10], which often exhibit diverse biological effects [11] including antidiabetic [12], antiviral [13], antibacterial [8,14,15], and anticancer [16,17] activities.
The use of the Ugi reaction followed by post-cyclization is an effective strategy that yields diverse heterocycle-containing peptidomimetics and requires a minimal number of steps [18]. For example, Mazur et al. [19] developed an efficient method for the preparation of benzodiazepinone derivatives, which showed promising psychotropic effects [20-24], using a tandem combination of Ugi/azide–alkyne cycloaddition reactions. From this point of view, azido amines are promising reagents for use in the Ugi reaction, opening up the possibility of synthesizing various heterocyclic systems such as quinazolines [25], diazepinones [25,26], quinoxalinones [27], diazocinones [28], and imidazolines [29] due to the exceptional reactivity of the azido group. However, the use of azido amines in the Ugi reaction together with oxoacids, which have an additional reaction center for post-cyclizations, is scarcely reported in the literature [25,30,31].
Among the numerous tandem synthetic approaches, the combination of Ugi/aza-Wittig reactions is of particular interest. In recent years, several works have been dedicated to the synthesis of nitrogen-containing heterocyclic compounds using various modifications of this sequence [32-36]. Among others, Yan et al. [31] described a facile way to quinoxalinone derivatives using an Ugi/Staudinger/aza-Wittig tandem combination with pyruvic acid or phenylglyoxylic acid.
Quinoxalinones are regarded as privileged heterocyclic moieties in the development of new pharmacologically active organic compounds (Figure 1) [37]. These structures are of great interest to chemists because of their broad spectrum of potential biological effects, including anticancer [38], antibacterial [39] and anti-HIV [40] activities. Furthermore, a quinoxaline-containing commercial drug, caroverine, has been proven effective in the treatment of tinnitus [41]. In addition, in vivo studies in mice and rats with various quinoxalinone derivatives have shown favorable analgesic and anti-inflammatory properties as well as low toxicity of these substances [42]. Biological activity screening also suggests that quinoxalinone-based compounds can inhibit aldose reductase [43], α-amylase and α-glucosidase [44], as well as key enzymes involved in the processes of saturated fatty acid conversion [45] and glycogenolysis [46]. Therefore, these molecules can be considered as potential antidiabetic agents.
Figure 1: Some biologically active quinoxalinone derivatives.
Figure 1: Some biologically active quinoxalinone derivatives.
α-Ketoglutaric acid (KGA) is an attractive precursor for medically oriented syntheses using multicomponent reactions due to its chemical structure as a dibasic keto acid and its role as an important component of numerous biochemical processes [47]. However, there are only a few studies in the literature on multicomponent reactions based on KGA. In 1992, Gein et al. [48] reported a multicomponent reaction involving KGA, aromatic aldehydes, and aromatic amines. This led to the formation of pyrrolone derivatives I, which showed anti-inflammatory activity [49,50] (Scheme 1, pathway A). In an earlier study by our research group, Sakhno et al. [51] described a three-component Doebner-type reaction involving KGA, aromatic aldehydes, and 3-amino-5-methylisoxazole, which led to the formation of [2-aryl-4-hydroxy-1-(5-methylisoxazol-3-yl)-5-oxo-2,5-dihydro-1H-pyrrol-3-yl]acetic acids II (Scheme 1, pathway B). Subsequently, a four-component Ugi reaction of compounds II with temperature-controlled formation of diastereomers of peptidomimetics III was also studied [52].
Scheme 1: Known multicomponent reactions of KGA.
Scheme 1: Known multicomponent reactions of KGA.
Taking into account all mentioned above, this work is dedicated to the synthesis of novel peptidomimetics using the four-component Ugi reaction and the study of a tandem Ugi/aza-Wittig combination based on α-ketoglutaric acid for the preparation of earlier unavailable quinoxalinone derivatives.
Results and Discussion
We began by studying the behavior of α-ketoglutaric acid in the four-component Ugi reaction with equimolar amounts of the reagents. It was found that stirring of aromatic aldehydes 2a–d, aromatic amines 3a–d, KGA (1) and tert-butyl isocyanide (4) (in a 1:1:1:1 molar ratio) in methanol for 24 hours at 45 °C resulted in the formation of 5-((aryl)(1-aryl-2-(tert-butylamino)-2-oxoethyl)amino)-4,5-dioxopentanoic acids 5a–l in 50–81% yields (Scheme 2, pathway A; Table 1). The compounds 5a–l were isolated easily by adding water to the reaction mass until it became cloudy, whereupon a precipitate of the product was formed and isolated (all experimental procedures can be found in Supporting Information File 1).
Table 1: Yields of compounds 5a–l.
| Aldehyde | R1 | Amine | R2 | Compound | Yield, % |
| 2a | 4-Cl | 3a | 4-Cl | 5a | 81 |
| 2a | 4-Cl | 3b | 4-CH3 | 5b | 64 |
| 2a | 4-Cl | 3c | 4-OCH3 | 5c | 68 |
| 2b | 4-OCH3 | 3a | 4-Cl | 5d | 50 |
| 2b | 4-OCH3 | 3b | 4-CH3 | 5e | 54 |
| 2b | 4-OCH3 | 3c | 4-OCH3 | 5f | 52 |
| 2c | 4-COOCH3 | 3a | 4-Cl | 5g | 57 |
| 2c | 4-COOCH3 | 3b | 4-CH3 | 5h | 72 |
| 2c | 4-COOCH3 | 3c | 4-OCH3 | 5i | 54 |
| 2d | 4-Br | 3a | 4-Cl | 5j | 77 |
| 2d | 4-Br | 3b | 4-CH3 | 5k | 73 |
| 2d | 4-Br | 3c | 4-OCH3 | 5l | 61 |
Since α-ketoglutaric acid is dibasic, increasing the stoichiometric amounts of the starting materials enables the Ugi reaction involving two carboxyl groups. Thus, mixing KGA (1), 4-chlorobenzaldehyde (2a), 4-chloroaniline (3a), and tert-butyl isocyanide (4) in a molar ratio of 1:2:2:2 in MeOH and subsequent stirring at a temperature of 45 °C for 24 hours led to the formation of N1,N5-bis(2-(tert-butylamino)-1-(4-chlorophenyl)-2-oxoethyl)-N1,N5-bis(4-chlorophenyl)-2-oxopentanediamide (6a) in 55% yield (Scheme 2, pathway B; Table 2).
Table 2: Yields of compounds 6a–d.
| Acid | Aldehyde | R3 | Amine | R4 | Compound | Yield, % |
| 5a | 2a | 4-Cl | 3a | 4-Cl | 6a | 45 (55)a |
| 2a | 4-Cl | 3c | 4-OCH3 | 6b | 41 | |
| 2a | 4-Cl | 3b | 4-CH3 | 6c | 61 | |
| 2c | 4-COOCH3 | 3b | 4-CH3 | 6d | 42 | |
aThe yield for the Ugi reaction on two carboxyl groups of KGA is given in parentheses.
Compounds 5 contain a free carboxyl group and thus can also be used as an acidic component in the Ugi reaction. Indeed, it was established that the reaction of 5-((2-(tert-butylamino)-1-(4-chlorophenyl)-2-oxoethyl)(4-chlorophenyl)amino)-4,5-dioxopentanoic acid (5a) with aldehydes 2a,c, amines 3a–c, and tert-butyl isocyanide (4) in a molar ratio of 1:1:1 at 45 °C for 48 hours gave N1,N5-diaryl-N1,N5-bis(1-aryl-2-(tert-butylamino)-2-oxoethyl)-2-oxopentanediamides 6a–d in 41–61% yields (Scheme 2, pathway C; Table 2). It is important to note that compounds 6a–d rapidly precipitated from the solution and did not require further purification after filtration.
The use of starting components containing highly reactive groups, for example, an azide group, in the Ugi reaction provides opportunities for various post-cyclizations to obtain nitrogen-containing heterocyclic systems with potential biological activity [18]. To prepare new quinoxalinone derivatives, we studied a tandem combination of Ugi/aza-Wittig reactions involving α-ketoglutaric acid and o-azidoanilines. First, 5-((2-arylazido)(1-aryl-2-(tert-butylamino)-2-oxoethyl)amino)-4,5-dioxopentanoic acids 8a,b,d,f–h were synthesized by the reaction of KGA (1), aldehydes 2, o-azidoanilines 7, and tert-butyl isocyanide (4) for 24 hours at 45 °C in 35–78% yields (Scheme 3; Table 3).
Scheme 3: Tandem Ugi/aza-Wittig combination involving KGA.
Scheme 3: Tandem Ugi/aza-Wittig combination involving KGA.
Table 3: Yields of compounds 8a,b,d,f–h and 9a–h.
| Aldehyde | R1 | Azidoaniline | R2 | Compound | Yield, % |
| 2a | 4-Cl | 7a | 4-Cl | 8a | 57 |
| 2a | 4-Cl | 7b | H | 8b | 54 |
| 2b | 4-OCH3 | 7b | H | 8d | 35 |
| 2e | H | 7a | 4-Cl | 8f | 78 |
| 2e | H | 7b | H | 8g | 71 |
| 2e | H | 7c | 4,6-CH3 | 8h | 64 |
| 2a | 4-Cl | 7a | 4-Cl | 9a | 82 |
| 2a | 4-Cl | 7b | H | 9b | 93 |
| 2a | 4-Cl | 7c | 4,6-CH3 | 9c | 46a |
| 2b | 4-OCH3 | 7b | H | 9d | 75 (51)a |
| 2b | 4-OCH3 | 7c | 4,6-CH3 | 9e | 35a |
| 2e | H | 7a | 4-Cl | 9f | 83 |
| 2e | H | 7b | H | 9g | 59 |
| 2e | H | 7c | 4,6-CH3 | 9h | 47 |
aThe yield for the one-pot Ugi/aza-Wittig combination in terms of KGA.
To isolate compounds 8a,b,d,f–h, the reaction mixture was poured onto ice and the resulting precipitate was filtered. These compounds also often had to be additionally purified by column chromatography.
Subsequently, the compounds 8a,b,d,f–h were dissolved in DCM and stirred in the presence of a stoichiometric amount of triphenylphosphine at 20 °C for 12 hours, resulting in the formation of 3-(4-(1-aryl-2-(tert-butylamino)-2-oxoethyl)-3-oxo-3,4-dihydroquinoxalin-2-yl)propanoic acids 9a,b,d,f–h in 33–93% yields (Scheme 3; Table 3). According to the literature [31,53], this reaction proceeds through the formation of an iminophosphorane intermediate (Scheme 3), the product of a Staudinger reaction, which, however, was not isolated because it easily undergoes intramolecular cyclization on a sufficiently electrophilic carbonyl carbon atom with the elimination of triphenylphosphine oxide via the aza-Wittig reaction.
It should be noted that the best method for the isolation of quinoxalinones 9 was column chromatography using an elution gradient of hexane/ethyl acetate 3:1 to hexane/ethyl acetate 1:2 with the addition of 0.1% formic acid. The use of other eluents such as acetonitrile and different ratios of hexane/ethyl acetate, dichloromethane/methanol as well as the application of methods for the isolation of triphenylphosphine oxide by complexation with calcium and magnesium salts [54,55] or precipitation from non-polar solvents such as hexane and toluene did not lead to a complete separation of quinoxalinones 9 and Ph3PO. We attribute this to the ability of Ph3PO to form a strong hydrogen bond between the phosphoryl oxygen and a proton from the donor group of the second molecule [56-58], in this case a carboxyl proton.
In order to avoid the step of isolation of the intermediate azide derivatives 8, we also studied a one-pot method for the synthesis of compounds 9. For this KGA 1, aldehydes 2a,b, azidoanilines 7b,c, and tert-butyl isocyanide (4) were stirred in methanol at 45 °C for 24 hours. The solvent was then evaporated, the residue dissolved in DCM, and stirred in the presence of Ph3P for 12 hours at 20 °C to give compounds 9c–e in 35–51% yields (Table 3).
Identification and structure determination of the compounds obtained was based on elemental analysis, mass spectrometry, 1H and 13C NMR spectroscopy, and by X-ray diffraction study (see Experimental part). The 1H NMR spectra of compounds 5 are characterized by the following signals: a broad carboxyl group proton singlet at 12.10–12.17 ppm, an NH group proton singlet at 7.74–8.02 ppm, an aromatic protons multiplet at 6.52–8.15 ppm, a CH group singlet at 5.93–6.12 ppm, triplets for two CH2 groups in the range of 2.04–2.89 ppm, a tert-butyl group singlet at 1.23–1.25 ppm, and signals for protons of the functional group. It should be noted that a doubling of the 1H NMR signals is observed for the azide derivatives 8 compared to compounds 5, which can be clearly seen in the spectrum of compound 8h with both substituted ortho-positions of the amine moiety. In our opinion, this indicates the presence of rotamerism [59,60] caused by the restricted rotation of the sterically hindered amide group.
The 1H NMR spectra of compounds 6 are marked by the following differences: the absence of the proton signal for the carboxyl group, the presence of two singlets for NH groups in the range of 7.61–7.96 ppm and two singlets for CH groups at 5.96–6.05 ppm, increased integral intensity of the aromatic multiplet in the range of 6.28–7.81 ppm, corresponding to 16 protons, multiplets for CH2 groups at 1.78–2.96 ppm, and two signals for tert-butyl groups protons at 1.21–1.24 ppm. The increase in signal multiplicity compared to compounds 5 could indicate the presence of diastereomerism and restricted amide rotation in compounds 6.
In the 1H NMR spectra of quinoxalinones 9, a shift of the methylene groups signal to a weaker field (2.90–3.14 ppm and 2.63–2.77 ppm) is observed in comparison to compounds 8 (2.60–3.06 ppm and 2.14–2.35 ppm). Furthermore, comparison of the 13C NMR spectra of compounds 8 and 9 shows a characteristic shift of the signal in the 198 ppm region, corresponding to the carbonyl carbon of compounds 8, to a stronger field in quinoxalinones 9, where cyclization of the keto group has occurred. The structure of quinoxalinones of type 9 was finally assigned based on X-ray diffraction analysis made for 3-(4-(2-(tert-butylamino)-1-(4-methoxyphenyl)-2-oxoethyl)-5,7-dimethyl-3-oxo-3,4-dihydroquinoxalin-2-yl)propanoic acid (9e) (Figure 2).
![[1860-5397-21-157-2]](/bjoc/content/figures/1860-5397-21-157-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: Molecular structure of 3-(4-(2-(tert-butylamino)-1-(4-methoxyphenyl)-2-oxoethyl)-5,7-dimethyl-3-oxo-3,4-dihydroquinoxalin-2-yl)propanoic acid (9e) according to X-ray diffraction data.
Figure 2: Molecular structure of 3-(4-(2-(tert-butylamino)-1-(4-methoxyphenyl)-2-oxoethyl)-5,7-dimethyl-3-oxo...
Conclusion
To summarize, we have studied the Ugi reaction and its tandem combination with aza-Wittig reactions involving α-ketoglutaric acid. Depending on the stoichiometric ratio of the reagents, the Ugi reaction involves one or both carboxyl groups of KGA, leading to 5-((aryl)(1-aryl-2-(tert-butylamino)-2-oxoethyl)amino)-4,5-dioxopentanoic acids or N1,N5-diaryl-N1,N5-bis(1-aryl-2-(tert-butylamino)-2-oxoethyl)-2-oxopentanediamides, respectively. The tandem combination of Ugi and aza-Wittig reactions involving α-ketoglutaric acid and o-azidonanilines is an efficient method for the preparation of earlier unavailable quinoxalinone derivatives and can be carried out as a one-pot procedure without isolation of an intermediate azide-containing Ugi reaction product. Both quinoxalinones and Ugi reaction products may be valuable for organic synthesis and medicinal chemistry because the presence of a free carboxyl group makes them promising for use as building blocks for the construction of complex heterocyclic structures, including those using multicomponent reactions.
Supporting Information
| Supporting Information File 1: General synthetic procedures, characterization of compounds, 1H and 13C NMR spectra and X-ray data. | ||
| Format: PDF | Size: 2.7 MB | Download |
Data Availability Statement
Data generated and analyzed during this study is available from the corresponding author upon reasonable request.
References
-
Cores, Á.; Clerigué, J.; Orocio-Rodríguez, E.; Menéndez, J. C. Pharmaceuticals 2022, 15, 1009. doi:10.3390/ph15081009
Return to citation in text: [1] -
dos Santos, J. A.; de Castro, P. P.; de Oliveira, K. T.; Brocksom, T. J.; Amarante, G. W. Curr. Top. Med. Chem. 2023, 23, 990–1003. doi:10.2174/1568026623666230403102437
Return to citation in text: [1] -
Buskes, M. J.; Coffin, A.; Troast, D. M.; Stein, R.; Blanco, M.-J. ACS Med. Chem. Lett. 2023, 14, 376–385. doi:10.1021/acsmedchemlett.3c00012
Return to citation in text: [1] -
Chebanov, V. A.; Desenko, S. M.; Lipson, V. V.; Gorobets, N. Y. Multicomponent‐Switched Reactions in Synthesis of Heterocycles. Multicomponent Reactions towards Heterocycles; Wiley-VCH: Weinheim, Germany, 2022; pp 287–338. doi:10.1002/9783527832439.ch8
Return to citation in text: [1] -
Desenko, S. M.; Gorobets, M. Y.; Lipson, V. V.; Sakhno, Y. I.; Chebanov, V. A. Chem. Rec. 2024, 24, e202300244. doi:10.1002/tcr.202300244
Return to citation in text: [1] -
Ugi, I. Angew. Chem. 1959, 71, 386.
Return to citation in text: [1] -
Fotopoulou, E.; Anastasiou, P. K.; Tomza, C.; Neochoritis, C. G. Tetrahedron Green Chem 2024, 3, 100044. doi:10.1016/j.tgchem.2024.100044
Return to citation in text: [1] -
Murlykina, M. V.; Kolomiets, O. V.; Kornet, M. M.; Sakhno, Y. I.; Desenko, S. M.; Dyakonenko, V. V.; Shishkina, S. V.; Brazhko, O. A.; Musatov, V. I.; Tsygankov, A. V.; Van der Eycken, E. V.; Chebanov, V. A. Beilstein J. Org. Chem. 2019, 15, 1281–1288. doi:10.3762/bjoc.15.126
Return to citation in text: [1] [2] -
Kolomiiets, O. V.; Tsygankov, A. V.; Kornet, M. N.; Brazhko, A. A.; Musatov, V. I.; Chebanov, V. A. Beilstein J. Org. Chem. 2023, 19, 727–735. doi:10.3762/bjoc.19.53
Return to citation in text: [1] -
Tsygankov, A. V.; Vereshchak, V. O.; Savluk, T. O.; Desenko, S. M.; Ananieva, V. V.; Buravov, O. V.; Sakhno, Y. I.; Shishkina, S. V.; Chebanov, V. A. Beilstein J. Org. Chem. 2024, 20, 1773–1784. doi:10.3762/bjoc.20.156
Return to citation in text: [1] -
Liu, C.; Voskressensky, L. G.; Van der Eycken, E. V. Chem. – Eur. J. 2024, 30, e202303597. doi:10.1002/chem.202303597
Return to citation in text: [1] -
Ahmadi Shourkaei, F.; Rashidi Ranjbar, P.; Taslimi, P.; Mahdavi, M. Chem. Biodiversity 2024, 21, e202301292. doi:10.1002/cbdv.202301292
Return to citation in text: [1] -
Morales-Salazar, I.; Montes-Enríquez, F. P.; Garduño-Albino, C. E.; García-Sánchez, M. A.; Ibarra, I. A.; Rojas-Aguirre, Y.; García-Hernández, M. E.; Sarmiento-Silva, R. E.; Alcaraz-Estrada, S. L.; Díaz-Cervantes, E.; González-Zamora, E.; Islas-Jácome, A. RSC Med. Chem. 2023, 14, 154–165. doi:10.1039/d2md00350c
Return to citation in text: [1] -
Zeng, Y.; Li, Y.; Liu, G.; Wei, Y.; Wu, Y.; Tao, L. ACS Appl. Polym. Mater. 2020, 2, 404–410. doi:10.1021/acsapm.9b00874
Return to citation in text: [1] -
Valdes, O.; Ali, A.; Carrasco-Sánchez, V.; Cabrera-Barjas, G.; Duran-Lara, E.; Ibrahim, M.; Ahmad, S.; Moreno, R.; Concepción, O.; de la Torre, A. F.; Abrar, M.; Morales-Quintana, L.; Abril, D. Comput. Biol. Chem. 2023, 106, 107932. doi:10.1016/j.compbiolchem.2023.107932
Return to citation in text: [1] -
Tomohara, K.; Maneenet, J.; Ohashi, N.; Nose, T.; Fujii, R.; Kim, M. J.; Sun, S.; Awale, S. Biol. Pharm. Bull. 2023, 46, 1412–1420. doi:10.1248/bpb.b23-00224
Return to citation in text: [1] -
Li, Y.; He, L.; Qin, H.; Liu, Y.; Yang, B.; Xu, Z.; Yang, D. Molecules 2024, 29, 464. doi:10.3390/molecules29020464
Return to citation in text: [1] -
Bariwal, J.; Kaur, R.; Voskressensky, L. G.; Van der Eycken, E. V. Front. Chem. (Lausanne, Switz.) 2018, 6, 557. doi:10.3389/fchem.2018.00557
Return to citation in text: [1] [2] -
Mazur, M. O.; Zhelavskyi, O. S.; Zviagin, E. M.; Shishkina, S. V.; Musatov, V. I.; Kolosov, M. A.; Shvets, E. H.; Andryushchenko, A. Y.; Chebanov, V. A. Beilstein J. Org. Chem. 2021, 17, 678–687. doi:10.3762/bjoc.17.57
Return to citation in text: [1] -
Botsula, I.; Sсhavikin, J.; Heinämäki, J.; Laidmäe, I.; Mazur, M.; Raal, A.; Koshovyi, O.; Kireyev, I.; Chebanov, V. Eur. J. Pharm. Sci. 2024, 195, 106712. doi:10.1016/j.ejps.2024.106712
Return to citation in text: [1] -
Botsula, I. V.; Kireyev, I. V.; Mazur, M. O.; Chebanov, V. A. Visn. Farm. 2024, 107, 136–143. doi:10.24959/nphj.24.144
Return to citation in text: [1] -
Botsula, I. V.; Kireyev, I. V.; Koshovyi, O. M.; Chebanov, V. A. Curr. Issues Pharm. Med.: Sci. Pract. 2023, 16, 217–222. doi:10.14739/2409-2932.2023.3.287999
Return to citation in text: [1] -
Botsula, I. V.; Kireyev, I. V.; Koshovyi, O. M.; Mazur, M. O.; Chebanov, V. A. Farm. Chasopic 2023, 70–77. doi:10.11603/2312-0967.2023.4.14297
Return to citation in text: [1] -
Botsula, I.; Kireyev, I.; Koshovyi, O.; Heinämäki, J.; Raal, A.; Mazur, M.; Chebanov, V. ScienceRise: Pharm. Sci. 2024, 1, 40–48. doi:10.15587/2519-4852.2024.299205
Return to citation in text: [1] -
Xiong, J.; Wei, X.; Wan, Y.-C.; Ding, M.-W. Tetrahedron 2019, 75, 1072–1078. doi:10.1016/j.tet.2019.01.014
Return to citation in text: [1] [2] [3] -
Hollanders, C.; Elsocht, M.; Van der Poorten, O.; Jida, M.; Renders, E.; Maes, B. U. W.; Ballet, S. Chem. Commun. 2021, 57, 6863–6866. doi:10.1039/d1cc01701b
Return to citation in text: [1] -
Yan, Y.-M.; Li, H.-Y.; Zhang, M.; Wang, R.-X.; Zhou, C.-G.; Ren, Z.-X.; Ding, M.-W. Synlett 2020, 31, 73–76. doi:10.1055/s-0037-1610737
Return to citation in text: [1] -
Barlow, T. M. A.; Jida, M.; Guillemyn, K.; Tourwé, D.; Caveliers, V.; Ballet, S. Org. Biomol. Chem. 2016, 14, 4669–4677. doi:10.1039/c6ob00438e
Return to citation in text: [1] -
Welsch, S. J.; Umkehrer, M.; Kalinski, C.; Ross, G.; Burdack, C.; Kolb, J.; Wild, M.; Ehrlich, A.; Wessjohann, L. A. Tetrahedron Lett. 2015, 56, 1025–1029. doi:10.1016/j.tetlet.2015.01.043
Return to citation in text: [1] -
Yan, Y.-M.; Li, H.-Y.; Ren, J.; Wang, S.; Ding, M.-W. Synlett 2018, 29, 1447–1450. doi:10.1055/s-0037-1609846
Return to citation in text: [1] -
Yan, Y.-M.; Gao, Y.; Ding, M.-W. Tetrahedron 2016, 72, 5548–5557. doi:10.1016/j.tet.2016.07.048
Return to citation in text: [1] [2] [3] -
Yang, M.-L.; Chen, H.-R.; Zhao, L.; Ding, M.-W. Synthesis 2023, 55, 465–472. doi:10.1055/a-1932-5811
Return to citation in text: [1] -
Yang, M.-L.; Zhao, L.; Chen, H.-R.; Ding, M.-W. J. Org. Chem. 2023, 88, 16424–16434. doi:10.1021/acs.joc.3c01955
Return to citation in text: [1] -
Yang, M.-L.; Zhao, L.; Chen, H.-R.; Ding, M.-W. J. Org. Chem. 2023, 88, 1898–1906. doi:10.1021/acs.joc.2c02621
Return to citation in text: [1] -
Xu, Y.; Yuan, D.; Zhang, Y.; Zhang, Y.; He, P.; Ren, Z.-L. Tetrahedron Lett. 2024, 140, 155046. doi:10.1016/j.tetlet.2024.155046
Return to citation in text: [1] -
Sun, M.; Zeng, C.-Y.; Zhang, E.-S.; Zhang, Q.; Chen, K.; Xu, Y.-G.; Fan, M.-X.; He, M.-R.; Shi, J.-H.; Ding, M.-W. Asian J. Org. Chem. 2024, 13, e202400036. doi:10.1002/ajoc.202400036
Return to citation in text: [1] -
Jiang, X.; Wu, K.; Bai, R.; Zhang, P.; Zhang, Y. Eur. J. Med. Chem. 2022, 229, 114085. doi:10.1016/j.ejmech.2021.114085
Return to citation in text: [1] -
Ayoup, M. S.; Rabee, A. R.; Abdel-Hamid, H.; Amer, A.; Abu-Serie, M. M.; Ashraf, S.; Ghareeb, D. A.; Ibrahim, R. S.; Hawsawi, M. B.; Negm, A.; Ismail, M. M. F. ACS Omega 2024, 9, 24643–24653. doi:10.1021/acsomega.4c01075
Return to citation in text: [1] -
Odusami, J. A.; Ikhile, M. I.; Olasupo, I. A.; Fotsing, M. C. D.; Asekun, O. T.; Ndinteh, D. T.; Familoni, O. B. Sci. Afr. 2024, 25, e02317. doi:10.1016/j.sciaf.2024.e02317
Return to citation in text: [1] -
El Bakri, Y.; Saravanan, K.; Ahmad, S.; Mague, J. T. J. Biomol. Struct. Dyn. 2023, 41, 5277–5290. doi:10.1080/07391102.2022.2084456
Return to citation in text: [1] -
Suthar, S. K.; Chundawat, N. S.; Singh, G. P.; Padrón, J. M.; Jhala, Y. K. Eur. J. Med. Chem. Rep. 2022, 5, 100040. doi:10.1016/j.ejmcr.2022.100040
Return to citation in text: [1] -
Meka, G.; Chintakunta, R. Results Chem. 2023, 5, 100783. doi:10.1016/j.rechem.2023.100783
Return to citation in text: [1] -
Hussain, S.; Parveen, S.; Hao, X.; Zhang, S.; Wang, W.; Qin, X.; Yang, Y.; Chen, X.; Zhu, S.; Zhu, C.; Ma, B. Eur. J. Med. Chem. 2014, 80, 383–392. doi:10.1016/j.ejmech.2014.04.047
Return to citation in text: [1] -
Missioui, M.; Mortada, S.; Guerrab, W.; Serdaroğlu, G.; Kaya, S.; Mague, J. T.; Essassi, E. M.; Faouzi, M. E. A.; Ramli, Y. J. Mol. Struct. 2021, 1239, 130484. doi:10.1016/j.molstruc.2021.130484
Return to citation in text: [1] -
Koltun, D. O.; Parkhill, E. Q.; Vasilevich, N. I.; Glushkov, A. I.; Zilbershtein, T. M.; Ivanov, A. V.; Cole, A. G.; Henderson, I.; Zautke, N. A.; Brunn, S. A.; Mollova, N.; Leung, K.; Chisholm, J. W.; Zablocki, J. Bioorg. Med. Chem. Lett. 2009, 19, 2048–2052. doi:10.1016/j.bmcl.2009.02.019
Return to citation in text: [1] -
Dudash, J., Jr.; Zhang, Y.; Moore, J. B.; Look, R.; Liang, Y.; Beavers, M. P.; Conway, B. R.; Rybczynski, P. J.; Demarest, K. T. Bioorg. Med. Chem. Lett. 2005, 15, 4790–4793. doi:10.1016/j.bmcl.2005.07.021
Return to citation in text: [1] -
Bayliak, M. M.; Lushchak, V. I. Ageing Res. Rev. 2021, 66, 101237. doi:10.1016/j.arr.2020.101237
Return to citation in text: [1] -
Gein, V. L.; Popov, A. V.; Andreichikov, Y. S. J. Gen. Chem. USSR 1992, 62, 1378.
Return to citation in text: [1] -
Gein, V. L.; Popov, A. V.; Kolla, V. É.; Popova, N. A.; Potemkin, K. D. Pharm. Chem. J. 1993, 27, 343–346. doi:10.1007/bf00819965
Return to citation in text: [1] -
Gein, V. L.; Popov, A. V.; Kolla, W. E.; Popova, N. A. Pharmazie 1993, 48, 107–109.
Return to citation in text: [1] -
Sakhno, Y. I.; Radchenko, O. V.; Muravyova, E. A.; Sirko, S. M.; Shishkina, S. V.; Musatov, V. I.; Desenko, S. M.; Chebanov, V. A. Chem. Heterocycl. Compd. 2021, 57, 261–265. doi:10.1007/s10593-021-02902-w
Return to citation in text: [1] -
Sakhno, Y.; Radchenko, O.; Saraev, V.; Shliapkina, Y.; Kaidash, M.; Shyshkina, M.; Shishkina, S.; Musatov, V.; Desenko, S.; Chebanov, V. SynOpen 2023, 7, 258–266. doi:10.1055/a-2091-7934
Return to citation in text: [1] -
Pedrood, K.; Montazer, M. N.; Larijani, B.; Mahdavi, M. Synthesis 2021, 53, 2342–2366. doi:10.1055/a-1394-7511
Return to citation in text: [1] -
Moschetta, E. G.; Cardinal-David, B.; Dunn, T. B.; Diwan, M. Org. Process Res. Dev. 2024, 28, 2677–2682. doi:10.1021/acs.oprd.4c00071
Return to citation in text: [1] -
Hergueta, A. R. Org. Process Res. Dev. 2022, 26, 1845–1853. doi:10.1021/acs.oprd.2c00104
Return to citation in text: [1] -
Smith, G.; Lynch, D. E.; Byriel, K. A.; Kennard, C. H. L. Z. Kristallogr. 1997, 212, 130–134. doi:10.1524/zkri.1997.212.2.130
Return to citation in text: [1] -
Yenikaya, C.; Öğretir, C. J. Mol. Struct.: THEOCHEM 2005, 731, 1–5. doi:10.1016/j.theochem.2004.09.063
Return to citation in text: [1] -
Etter, M. C.; Baures, P. W. J. Am. Chem. Soc. 1988, 110, 639–640. doi:10.1021/ja00210a076
Return to citation in text: [1] -
Ototake, N.; Nakamura, M.; Dobashi, Y.; Fukaya, H.; Kitagawa, O. Chem. – Eur. J. 2009, 15, 5090–5095. doi:10.1002/chem.200802627
Return to citation in text: [1] -
Watanabe, Y.; Take, U.; Senda, R.; Sato, A.; Kitagawa, O. J. Org. Chem. 2025, 90, 784–793. doi:10.1021/acs.joc.4c02772
Return to citation in text: [1]
| 44. | Missioui, M.; Mortada, S.; Guerrab, W.; Serdaroğlu, G.; Kaya, S.; Mague, J. T.; Essassi, E. M.; Faouzi, M. E. A.; Ramli, Y. J. Mol. Struct. 2021, 1239, 130484. doi:10.1016/j.molstruc.2021.130484 |
| 45. | Koltun, D. O.; Parkhill, E. Q.; Vasilevich, N. I.; Glushkov, A. I.; Zilbershtein, T. M.; Ivanov, A. V.; Cole, A. G.; Henderson, I.; Zautke, N. A.; Brunn, S. A.; Mollova, N.; Leung, K.; Chisholm, J. W.; Zablocki, J. Bioorg. Med. Chem. Lett. 2009, 19, 2048–2052. doi:10.1016/j.bmcl.2009.02.019 |
| 46. | Dudash, J., Jr.; Zhang, Y.; Moore, J. B.; Look, R.; Liang, Y.; Beavers, M. P.; Conway, B. R.; Rybczynski, P. J.; Demarest, K. T. Bioorg. Med. Chem. Lett. 2005, 15, 4790–4793. doi:10.1016/j.bmcl.2005.07.021 |
| 1. | Cores, Á.; Clerigué, J.; Orocio-Rodríguez, E.; Menéndez, J. C. Pharmaceuticals 2022, 15, 1009. doi:10.3390/ph15081009 |
| 2. | dos Santos, J. A.; de Castro, P. P.; de Oliveira, K. T.; Brocksom, T. J.; Amarante, G. W. Curr. Top. Med. Chem. 2023, 23, 990–1003. doi:10.2174/1568026623666230403102437 |
| 3. | Buskes, M. J.; Coffin, A.; Troast, D. M.; Stein, R.; Blanco, M.-J. ACS Med. Chem. Lett. 2023, 14, 376–385. doi:10.1021/acsmedchemlett.3c00012 |
| 4. | Chebanov, V. A.; Desenko, S. M.; Lipson, V. V.; Gorobets, N. Y. Multicomponent‐Switched Reactions in Synthesis of Heterocycles. Multicomponent Reactions towards Heterocycles; Wiley-VCH: Weinheim, Germany, 2022; pp 287–338. doi:10.1002/9783527832439.ch8 |
| 5. | Desenko, S. M.; Gorobets, M. Y.; Lipson, V. V.; Sakhno, Y. I.; Chebanov, V. A. Chem. Rec. 2024, 24, e202300244. doi:10.1002/tcr.202300244 |
| 11. | Liu, C.; Voskressensky, L. G.; Van der Eycken, E. V. Chem. – Eur. J. 2024, 30, e202303597. doi:10.1002/chem.202303597 |
| 27. | Yan, Y.-M.; Li, H.-Y.; Zhang, M.; Wang, R.-X.; Zhou, C.-G.; Ren, Z.-X.; Ding, M.-W. Synlett 2020, 31, 73–76. doi:10.1055/s-0037-1610737 |
| 31. | Yan, Y.-M.; Gao, Y.; Ding, M.-W. Tetrahedron 2016, 72, 5548–5557. doi:10.1016/j.tet.2016.07.048 |
| 53. | Pedrood, K.; Montazer, M. N.; Larijani, B.; Mahdavi, M. Synthesis 2021, 53, 2342–2366. doi:10.1055/a-1394-7511 |
| 8. | Murlykina, M. V.; Kolomiets, O. V.; Kornet, M. M.; Sakhno, Y. I.; Desenko, S. M.; Dyakonenko, V. V.; Shishkina, S. V.; Brazhko, O. A.; Musatov, V. I.; Tsygankov, A. V.; Van der Eycken, E. V.; Chebanov, V. A. Beilstein J. Org. Chem. 2019, 15, 1281–1288. doi:10.3762/bjoc.15.126 |
| 9. | Kolomiiets, O. V.; Tsygankov, A. V.; Kornet, M. N.; Brazhko, A. A.; Musatov, V. I.; Chebanov, V. A. Beilstein J. Org. Chem. 2023, 19, 727–735. doi:10.3762/bjoc.19.53 |
| 10. | Tsygankov, A. V.; Vereshchak, V. O.; Savluk, T. O.; Desenko, S. M.; Ananieva, V. V.; Buravov, O. V.; Sakhno, Y. I.; Shishkina, S. V.; Chebanov, V. A. Beilstein J. Org. Chem. 2024, 20, 1773–1784. doi:10.3762/bjoc.20.156 |
| 28. | Barlow, T. M. A.; Jida, M.; Guillemyn, K.; Tourwé, D.; Caveliers, V.; Ballet, S. Org. Biomol. Chem. 2016, 14, 4669–4677. doi:10.1039/c6ob00438e |
| 54. | Moschetta, E. G.; Cardinal-David, B.; Dunn, T. B.; Diwan, M. Org. Process Res. Dev. 2024, 28, 2677–2682. doi:10.1021/acs.oprd.4c00071 |
| 55. | Hergueta, A. R. Org. Process Res. Dev. 2022, 26, 1845–1853. doi:10.1021/acs.oprd.2c00104 |
| 7. | Fotopoulou, E.; Anastasiou, P. K.; Tomza, C.; Neochoritis, C. G. Tetrahedron Green Chem 2024, 3, 100044. doi:10.1016/j.tgchem.2024.100044 |
| 25. | Xiong, J.; Wei, X.; Wan, Y.-C.; Ding, M.-W. Tetrahedron 2019, 75, 1072–1078. doi:10.1016/j.tet.2019.01.014 |
| 52. | Sakhno, Y.; Radchenko, O.; Saraev, V.; Shliapkina, Y.; Kaidash, M.; Shyshkina, M.; Shishkina, S.; Musatov, V.; Desenko, S.; Chebanov, V. SynOpen 2023, 7, 258–266. doi:10.1055/a-2091-7934 |
| 25. | Xiong, J.; Wei, X.; Wan, Y.-C.; Ding, M.-W. Tetrahedron 2019, 75, 1072–1078. doi:10.1016/j.tet.2019.01.014 |
| 26. | Hollanders, C.; Elsocht, M.; Van der Poorten, O.; Jida, M.; Renders, E.; Maes, B. U. W.; Ballet, S. Chem. Commun. 2021, 57, 6863–6866. doi:10.1039/d1cc01701b |
| 18. | Bariwal, J.; Kaur, R.; Voskressensky, L. G.; Van der Eycken, E. V. Front. Chem. (Lausanne, Switz.) 2018, 6, 557. doi:10.3389/fchem.2018.00557 |
| 16. | Tomohara, K.; Maneenet, J.; Ohashi, N.; Nose, T.; Fujii, R.; Kim, M. J.; Sun, S.; Awale, S. Biol. Pharm. Bull. 2023, 46, 1412–1420. doi:10.1248/bpb.b23-00224 |
| 17. | Li, Y.; He, L.; Qin, H.; Liu, Y.; Yang, B.; Xu, Z.; Yang, D. Molecules 2024, 29, 464. doi:10.3390/molecules29020464 |
| 19. | Mazur, M. O.; Zhelavskyi, O. S.; Zviagin, E. M.; Shishkina, S. V.; Musatov, V. I.; Kolosov, M. A.; Shvets, E. H.; Andryushchenko, A. Y.; Chebanov, V. A. Beilstein J. Org. Chem. 2021, 17, 678–687. doi:10.3762/bjoc.17.57 |
| 49. | Gein, V. L.; Popov, A. V.; Kolla, V. É.; Popova, N. A.; Potemkin, K. D. Pharm. Chem. J. 1993, 27, 343–346. doi:10.1007/bf00819965 |
| 50. | Gein, V. L.; Popov, A. V.; Kolla, W. E.; Popova, N. A. Pharmazie 1993, 48, 107–109. |
| 8. | Murlykina, M. V.; Kolomiets, O. V.; Kornet, M. M.; Sakhno, Y. I.; Desenko, S. M.; Dyakonenko, V. V.; Shishkina, S. V.; Brazhko, O. A.; Musatov, V. I.; Tsygankov, A. V.; Van der Eycken, E. V.; Chebanov, V. A. Beilstein J. Org. Chem. 2019, 15, 1281–1288. doi:10.3762/bjoc.15.126 |
| 14. | Zeng, Y.; Li, Y.; Liu, G.; Wei, Y.; Wu, Y.; Tao, L. ACS Appl. Polym. Mater. 2020, 2, 404–410. doi:10.1021/acsapm.9b00874 |
| 15. | Valdes, O.; Ali, A.; Carrasco-Sánchez, V.; Cabrera-Barjas, G.; Duran-Lara, E.; Ibrahim, M.; Ahmad, S.; Moreno, R.; Concepción, O.; de la Torre, A. F.; Abrar, M.; Morales-Quintana, L.; Abril, D. Comput. Biol. Chem. 2023, 106, 107932. doi:10.1016/j.compbiolchem.2023.107932 |
| 20. | Botsula, I.; Sсhavikin, J.; Heinämäki, J.; Laidmäe, I.; Mazur, M.; Raal, A.; Koshovyi, O.; Kireyev, I.; Chebanov, V. Eur. J. Pharm. Sci. 2024, 195, 106712. doi:10.1016/j.ejps.2024.106712 |
| 21. | Botsula, I. V.; Kireyev, I. V.; Mazur, M. O.; Chebanov, V. A. Visn. Farm. 2024, 107, 136–143. doi:10.24959/nphj.24.144 |
| 22. | Botsula, I. V.; Kireyev, I. V.; Koshovyi, O. M.; Chebanov, V. A. Curr. Issues Pharm. Med.: Sci. Pract. 2023, 16, 217–222. doi:10.14739/2409-2932.2023.3.287999 |
| 23. | Botsula, I. V.; Kireyev, I. V.; Koshovyi, O. M.; Mazur, M. O.; Chebanov, V. A. Farm. Chasopic 2023, 70–77. doi:10.11603/2312-0967.2023.4.14297 |
| 24. | Botsula, I.; Kireyev, I.; Koshovyi, O.; Heinämäki, J.; Raal, A.; Mazur, M.; Chebanov, V. ScienceRise: Pharm. Sci. 2024, 1, 40–48. doi:10.15587/2519-4852.2024.299205 |
| 51. | Sakhno, Y. I.; Radchenko, O. V.; Muravyova, E. A.; Sirko, S. M.; Shishkina, S. V.; Musatov, V. I.; Desenko, S. M.; Chebanov, V. A. Chem. Heterocycl. Compd. 2021, 57, 261–265. doi:10.1007/s10593-021-02902-w |
| 13. | Morales-Salazar, I.; Montes-Enríquez, F. P.; Garduño-Albino, C. E.; García-Sánchez, M. A.; Ibarra, I. A.; Rojas-Aguirre, Y.; García-Hernández, M. E.; Sarmiento-Silva, R. E.; Alcaraz-Estrada, S. L.; Díaz-Cervantes, E.; González-Zamora, E.; Islas-Jácome, A. RSC Med. Chem. 2023, 14, 154–165. doi:10.1039/d2md00350c |
| 47. | Bayliak, M. M.; Lushchak, V. I. Ageing Res. Rev. 2021, 66, 101237. doi:10.1016/j.arr.2020.101237 |
| 12. | Ahmadi Shourkaei, F.; Rashidi Ranjbar, P.; Taslimi, P.; Mahdavi, M. Chem. Biodiversity 2024, 21, e202301292. doi:10.1002/cbdv.202301292 |
| 18. | Bariwal, J.; Kaur, R.; Voskressensky, L. G.; Van der Eycken, E. V. Front. Chem. (Lausanne, Switz.) 2018, 6, 557. doi:10.3389/fchem.2018.00557 |
| 48. | Gein, V. L.; Popov, A. V.; Andreichikov, Y. S. J. Gen. Chem. USSR 1992, 62, 1378. |
| 32. | Yang, M.-L.; Chen, H.-R.; Zhao, L.; Ding, M.-W. Synthesis 2023, 55, 465–472. doi:10.1055/a-1932-5811 |
| 33. | Yang, M.-L.; Zhao, L.; Chen, H.-R.; Ding, M.-W. J. Org. Chem. 2023, 88, 16424–16434. doi:10.1021/acs.joc.3c01955 |
| 34. | Yang, M.-L.; Zhao, L.; Chen, H.-R.; Ding, M.-W. J. Org. Chem. 2023, 88, 1898–1906. doi:10.1021/acs.joc.2c02621 |
| 35. | Xu, Y.; Yuan, D.; Zhang, Y.; Zhang, Y.; He, P.; Ren, Z.-L. Tetrahedron Lett. 2024, 140, 155046. doi:10.1016/j.tetlet.2024.155046 |
| 36. | Sun, M.; Zeng, C.-Y.; Zhang, E.-S.; Zhang, Q.; Chen, K.; Xu, Y.-G.; Fan, M.-X.; He, M.-R.; Shi, J.-H.; Ding, M.-W. Asian J. Org. Chem. 2024, 13, e202400036. doi:10.1002/ajoc.202400036 |
| 29. | Welsch, S. J.; Umkehrer, M.; Kalinski, C.; Ross, G.; Burdack, C.; Kolb, J.; Wild, M.; Ehrlich, A.; Wessjohann, L. A. Tetrahedron Lett. 2015, 56, 1025–1029. doi:10.1016/j.tetlet.2015.01.043 |
| 56. | Smith, G.; Lynch, D. E.; Byriel, K. A.; Kennard, C. H. L. Z. Kristallogr. 1997, 212, 130–134. doi:10.1524/zkri.1997.212.2.130 |
| 57. | Yenikaya, C.; Öğretir, C. J. Mol. Struct.: THEOCHEM 2005, 731, 1–5. doi:10.1016/j.theochem.2004.09.063 |
| 58. | Etter, M. C.; Baures, P. W. J. Am. Chem. Soc. 1988, 110, 639–640. doi:10.1021/ja00210a076 |
| 25. | Xiong, J.; Wei, X.; Wan, Y.-C.; Ding, M.-W. Tetrahedron 2019, 75, 1072–1078. doi:10.1016/j.tet.2019.01.014 |
| 30. | Yan, Y.-M.; Li, H.-Y.; Ren, J.; Wang, S.; Ding, M.-W. Synlett 2018, 29, 1447–1450. doi:10.1055/s-0037-1609846 |
| 31. | Yan, Y.-M.; Gao, Y.; Ding, M.-W. Tetrahedron 2016, 72, 5548–5557. doi:10.1016/j.tet.2016.07.048 |
| 59. | Ototake, N.; Nakamura, M.; Dobashi, Y.; Fukaya, H.; Kitagawa, O. Chem. – Eur. J. 2009, 15, 5090–5095. doi:10.1002/chem.200802627 |
| 60. | Watanabe, Y.; Take, U.; Senda, R.; Sato, A.; Kitagawa, O. J. Org. Chem. 2025, 90, 784–793. doi:10.1021/acs.joc.4c02772 |
| 42. | Meka, G.; Chintakunta, R. Results Chem. 2023, 5, 100783. doi:10.1016/j.rechem.2023.100783 |
| 43. | Hussain, S.; Parveen, S.; Hao, X.; Zhang, S.; Wang, W.; Qin, X.; Yang, Y.; Chen, X.; Zhu, S.; Zhu, C.; Ma, B. Eur. J. Med. Chem. 2014, 80, 383–392. doi:10.1016/j.ejmech.2014.04.047 |
| 40. | El Bakri, Y.; Saravanan, K.; Ahmad, S.; Mague, J. T. J. Biomol. Struct. Dyn. 2023, 41, 5277–5290. doi:10.1080/07391102.2022.2084456 |
| 41. | Suthar, S. K.; Chundawat, N. S.; Singh, G. P.; Padrón, J. M.; Jhala, Y. K. Eur. J. Med. Chem. Rep. 2022, 5, 100040. doi:10.1016/j.ejmcr.2022.100040 |
| 38. | Ayoup, M. S.; Rabee, A. R.; Abdel-Hamid, H.; Amer, A.; Abu-Serie, M. M.; Ashraf, S.; Ghareeb, D. A.; Ibrahim, R. S.; Hawsawi, M. B.; Negm, A.; Ismail, M. M. F. ACS Omega 2024, 9, 24643–24653. doi:10.1021/acsomega.4c01075 |
| 39. | Odusami, J. A.; Ikhile, M. I.; Olasupo, I. A.; Fotsing, M. C. D.; Asekun, O. T.; Ndinteh, D. T.; Familoni, O. B. Sci. Afr. 2024, 25, e02317. doi:10.1016/j.sciaf.2024.e02317 |
| 31. | Yan, Y.-M.; Gao, Y.; Ding, M.-W. Tetrahedron 2016, 72, 5548–5557. doi:10.1016/j.tet.2016.07.048 |
| 37. | Jiang, X.; Wu, K.; Bai, R.; Zhang, P.; Zhang, Y. Eur. J. Med. Chem. 2022, 229, 114085. doi:10.1016/j.ejmech.2021.114085 |
© 2025 Honcharov et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.