Abstract
A new process for the preparation of 1,2-benzisoxazole-3-methanesulfonates and 4-oximino-2,3-dihydrobenzoxathiin-2,2-dioxides (sultone oximes) is described. These compounds are important intermediates for the preparation of zonisamide, an anti-convulsant drug.
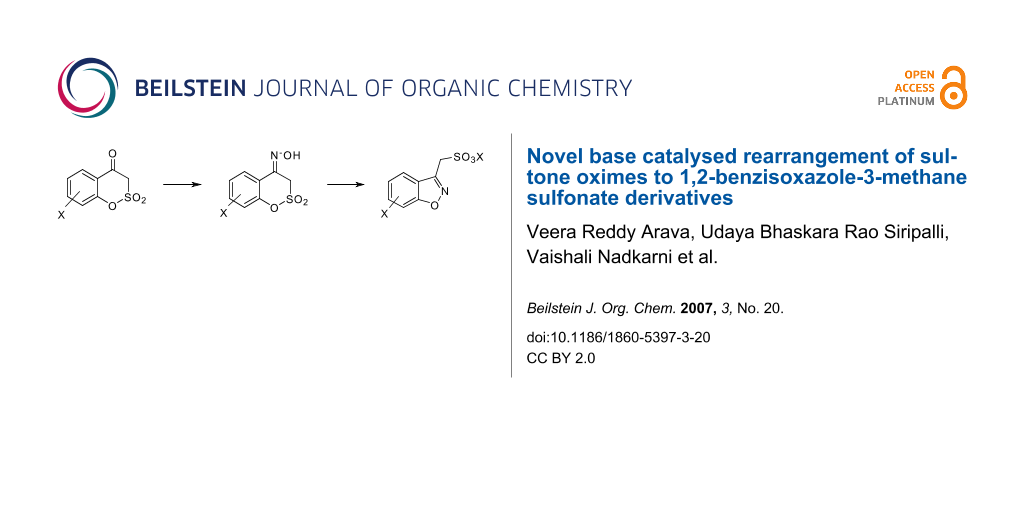
Graphical Abstract
Background
3-Alkyl-1,2-benzisoxazole derivatives are known to have important biological activities and are useful in different therapies. The 1,2-benzisoxazole moieties are isosteric with indoles and can mimic/bind to biologically important enzymes in a manner similar to indole derivatives. Out of many biologically active compounds, zonisamide is widely prescribed as an anti-epileptic drug. It was developed by Dianippon of Japan.
Compounds 1 were originally prepared from the rearrangement of coumarin-4-one oximes 3 as shown in Scheme 1.
Scheme 1: Conversion of coumarin-4-one oximes to 3-alkyl-1,2-benzisoxazole derivatives.
Scheme 1: Conversion of coumarin-4-one oximes to 3-alkyl-1,2-benzisoxazole derivatives.
The 1,2-benzisoxazole-3-acetic acids were converted into halogenated compounds, then reacted with various different nucleophiles to generate the compounds 1 as shown in Scheme 2.
Scheme 2: Reactions of 3-halomethyl derivatives with other nucleophiles.
Scheme 2: Reactions of 3-halomethyl derivatives with other nucleophiles.
Although the reactions in Scheme 2 were successfully implemented on large scale (~100 kgs), the lachrymatric nature of compound 4 (X = Br) created significant handling problems to the operating personnel. In our efforts to prepare the benzisoxazoles 10 without involving halogenated intermediates, we considered the replacement of the lactone carbonyl group in 3 with the SO2 group and studied the feasibility of the rearrangement of 5 to directly generate 10a.
Scheme 3: Sultone oximes and its precursor ketones.
Scheme 3: Sultone oximes and its precursor ketones.
The synthesis of compounds 5 has not been reported in the literature. Synthesis of compound 6 (1,2-benzoxathiin-4(3H)-one-2,2-dioxide Y = H), an obvious precursor for 5, was reported by Timoney et al., in an overall yield of ~30% [1] via the cyclisation of the methanesulfonate of salicylaldehyde followed by oxidation (Scheme 4).
Scheme 4: Synthesis of 1,2-benzoxathiin-4(3H)-one-2,2-dioxide 6 from methanesulfonate of salicylaldehyde.
Scheme 4: Synthesis of 1,2-benzoxathiin-4(3H)-one-2,2-dioxide 6 from methanesulfonate of salicylaldehyde.
Results and discussion
In order to prepare 6 in better yield, we choose methyl salicylate as the starting material and reacted it with methanesulfonyl chloride to provide the methanesulfonate derivative 9. After exploring different reaction conditions with NaOH and KOH as bases, it was found that NaH in DMSO was optimal for the cyclisation. Other strong bases also gave good yields of 6. The generality of the methodology has been established for several derivatives (Table 1). It was found that the reaction was efficient when groups such as Br, Cl, OMe & Me are present on the aromatic ring. On the other hand, the presence of nitro groups on the aromatic ring (3, 5 or 3,5-dinitro) failed to produce the cyclised products and gave only salicylic acid derivatives. All the 1,2-benzoxathiin-4(3H)-one-2,2-dioxides 6 were characterised from their analytical and spectral data.
Scheme 5: Preparation of 1,2-benzoxathiin-4(3H)-one-2,2-dioxides.
Scheme 5: Preparation of 1,2-benzoxathiin-4(3H)-one-2,2-dioxides.
Conversion of ketones 6 into the corresponding oximes 5 proceeded uneventfully, and oximes 5 were characterised from the spectral data and physical properties (Table 2).
Although conversion of sultone oxime 5 into 3-alkyl-1,2-benzisoxazole derivatives [2,3] proved to be difficult using NaOH and KOH under different conditions, it was found that the rearrangement proceeded smoothly with either NaOH in ethylene glycol or with a solution of sodium methoxide in methanol. The yields are depicted in Table 3.
Scheme 7: Preparation of 3-alkyl-1,2-benzisoxazole derivatives.
Scheme 7: Preparation of 3-alkyl-1,2-benzisoxazole derivatives.
The unsubstituted sodium 1,2-benzisoxazole-3-methane-sulfonate (10a) was converted into zonisamide employing the literature procedure (Scheme 8). [4]
Scheme 8: Synthesis of zonisamide from 1,2-benzisoxazole-3-methane-sulfonate.
Scheme 8: Synthesis of zonisamide from 1,2-benzisoxazole-3-methane-sulfonate.
Conclusion
In summary, we have developed a simple and economical process for the preparation of 1,2-benzisoxazole-3-methanesulfonates from sultone oximes, which can be employed for the production of zonisamide and its derivatives. The 1,2-benzisoxazole-3-methanesulfonic acid sodium salt preparation has been filed as a PCT application. [5,6]
Supporting Information
| Supporting Information File 1: The experimental section. The experimental data and the results of analysis. | ||
| Format: DOC | Size: 54.0 KB | Download |
References
-
Claney, J. M.; Alice, G.; Timoney, R. G. Int. J. Sulfur Chem., Part A 1972, 2, 249–255.
Return to citation in text: [1] -
Posner, T.; Hess, R. Ber. Dtsch. Chem. Ges. 1913, 46, 3816–3833.
Return to citation in text: [1] -
Posner, T. Ber. Dtsch. Chem. Ges. 1909, 42, 2523–2532.
Return to citation in text: [1] -
Kurokawa, M.; Masuda, Y.; Uno, H. Methane-sulfonamide derivatives, the preparation thereof and composition comprising the same. U.S. Patent 4,172,896, Oct 30, 1979.
Return to citation in text: [1] -
Reddy, A. V.; Rajendiran, C.; Vaishali, N.; Jasti, V. Improved process for the preparation of intermediates useful for the preparation of zonisamide. WO2005/030738 A1, April 7, 2005.
Return to citation in text: [1] -
Razzetti, G.; Mantegazza, S.; Castaldi, G.; Allegrini, P.; Lucchini, V.; Bologna, A. A process for the preparation of benzo[d]isoxazol-3-yl-methanesulfonic acid and the intermediates thereof. WO2004/063173 A1, July 29, 2004.
Return to citation in text: [1]
| 1. | Claney, J. M.; Alice, G.; Timoney, R. G. Int. J. Sulfur Chem., Part A 1972, 2, 249–255. |
| 5. | Reddy, A. V.; Rajendiran, C.; Vaishali, N.; Jasti, V. Improved process for the preparation of intermediates useful for the preparation of zonisamide. WO2005/030738 A1, April 7, 2005. |
| 6. | Razzetti, G.; Mantegazza, S.; Castaldi, G.; Allegrini, P.; Lucchini, V.; Bologna, A. A process for the preparation of benzo[d]isoxazol-3-yl-methanesulfonic acid and the intermediates thereof. WO2004/063173 A1, July 29, 2004. |
| 4. | Kurokawa, M.; Masuda, Y.; Uno, H. Methane-sulfonamide derivatives, the preparation thereof and composition comprising the same. U.S. Patent 4,172,896, Oct 30, 1979. |
| 2. | Posner, T.; Hess, R. Ber. Dtsch. Chem. Ges. 1913, 46, 3816–3833. |
| 3. | Posner, T. Ber. Dtsch. Chem. Ges. 1909, 42, 2523–2532. |
© 2007 Arava et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)