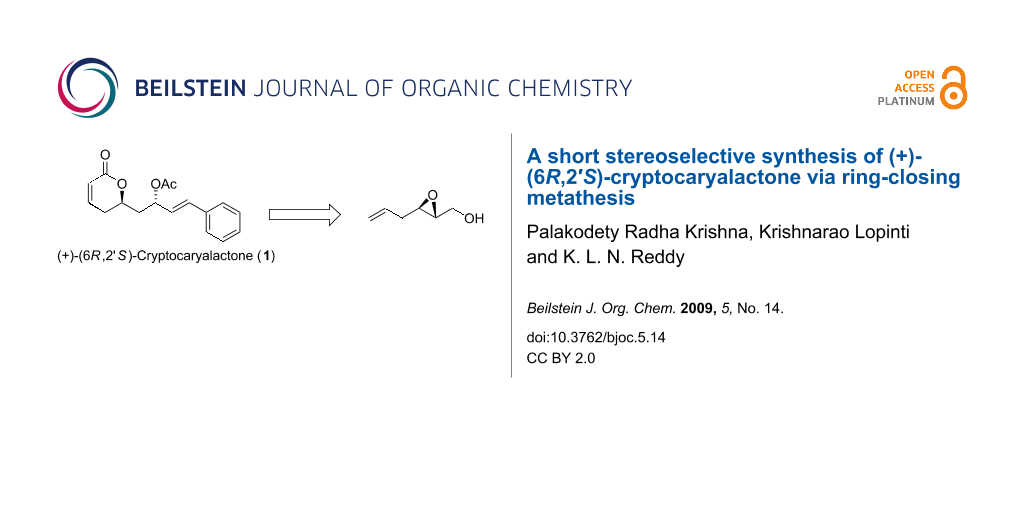
Abstract
A short stereoselective synthesis of (+)-(6R,2′S)-cryptocaryalactone was successfully completed. Key steps included the application of Carreira’s asymmetric alkynylation reaction to form a propargylic alcohol and subsequently lactone formation using the powerful ring-closing metathesis reaction.
Graphical Abstract
Introduction
Natural products play an important role in the development of drugs and mankind has always taken advantage of nature as pharmacy: approximately 40% of the drugs that have been approved over the last years are either natural products or derivatives and analogs thereof [1-3]. Indeed, 5,6-dihydropyran-2-ones of both natural and non-natural origin have been found to be cytotoxic. In addition to many other relevant pharmacological properties [4-7], they inhibit HIV protease [8,9], induce apoptosis [10-15], and have even proved to be antileukemic [16]. At least some of these pharmacological effects may be related to the presence of the conjugated double bond, which acts as a Michael acceptor [17-23].
One of the sub-classes of these 5,6-dihydro-2H-pyran-2-one compounds is the styryl lactones which possess a styryl moiety side chain. The styryl moiety of goniothalamin has been shown to be of importance for its cytotoxic effect on different cancer cells as well as its antimicrobial, larvicidal activity and anti-inflammatory activity [24]. The styryl-pyrone skeleton is often found in natural products from Equisetaceae and also from the primitive angiosperm families, such as Lauraceae, Piperaceae, Ranunculaceae and Zingiberaceae.
Cryptocaryalactone 1 [25,26], kurzilactone (2) [27], goniothalamin (3) [28], (+)-obolactone (4) [29] and (+)-cryptofoline (5) [30] (Figure 1) are some of the naturally occurring styryl lactones. (+)-(6R,2′S)-Cryptocaryalactone (1) first featured in the phytochemical literature when its isolation from Cryptocarya bourdilloni GAMB (Lauraceae) was reported in 1972 by Govindachari [31,32]. Its absolute stereochemistry was established by H. H. Meyer through stereoselective synthesis [25]. Recently Yadav et al. have synthesized (6R,2′S)-cryptocaryalactone and its epimer using stereoselective reduction of δ-hydroxy-β-keto ester [33]. All other possible isomers of cryptocaryalactone were also isolated from C. bourdilloni, C. moschata and C. myrtifolia and their absolute configuration was established [34]. These cryptocaryalactones are natural germination inhibitors with no effect on corn [35]. We were interested in synthesizing natural products containing 5,6-dihydro-2H-pyran-2-one moiety [36,37], and herein we describe a short and efficient synthesis of cryptocaryalactone 1.
Figure 1: Some natural products containing styryl lactones.
Figure 1: Some natural products containing styryl lactones.
Results and Discussion
Retrosynthetic analysis
Retrosynthetic analysis (Figure 2) reveals that compound 1 could be synthesized from bis-olefin 6 by a ring-closing metathesis reaction, while the bis-olefin itself could be realised from the acryloylation of the corresponding homoallylic alcohol which in turn can be synthesized from 7. Chiral propargyl alcohol 7 was obtained by the Carreira asymmetric alkynylation reaction of the corresponding aldehyde, which was synthesized from the corresponding primary alcohol that was obtained from a regioselective ring-opening reaction of 2,3-epoxy alcohol 8.
The known 2,3-epoxy alcohol 8 was synthesized from the corresponding dienyl alcohol by the well-established Sharpless asymmetric epoxidation conditions in >94% ee as described in literature [38,39]. Compound 8 undergoes a reductive ring-opening reaction with Red-Al under standard reaction conditions to furnish the 1,3-diol 9 in 88% yield. Traces of 1,2-diol were oxidatively cleaved with NaIO4 in presence of catalytic amount of saturated NaHCO3 solution. Diol 9 was protected with anisaldehyde dimethylacetal in presence of PTSA to afford compound 10 in 95% yield, which was regioselectively opened with DIBAL-H to afford the primary alcohol 11 in 92% yield (Scheme 1).
Scheme 1: Synthesis of chiral propargyl secondary hydroxyl group.
Scheme 1: Synthesis of chiral propargyl secondary hydroxyl group.
The primary alcohol 11 was oxidized under Swern conditions and the crude aldehyde was exposed to the alkynylation reaction directly. Several base-mediated alkynylation conditions were examined to access the requisite propargyl alcohol 7 (Table 1).
Table 1: Asymmetric alkynylation with phenylacetylene.
| Reagents | Solvent | Temperature | dea |
|---|---|---|---|
| n-BuLi | THF | −78 °C | 28% |
| LDA | THF | −78 °C | 56% |
| LDA/HMPA | THF | −78 °C | 78% |
| Zn(OTf)2, Et3N, (−)-N-methylephedrine | toluene | 25 °C | 94% |
aThe diastereoselectivity was determined by NMR studies.
Amongst all of these, the Carreira asymmetric alkynylation gave excellent diastereomeric excess (>94% de) [40,41] and the propargyl alcohol 7 was obtained with the correct absolute configuration.
Confirmation of absolute configuration
The structure of compound 7 was confirmed by 1H NMR and 13C spectral analysis (Scheme 2). The absolute stereochemistry was assigned based on Rychnovsky’s analogy [42-44].
Scheme 2: Determination of the stereochemistry of the 1,3-anti diols.
Scheme 2: Determination of the stereochemistry of the 1,3-anti diols.
According to literature precedent, the relative configuration of a secondary 1,3-diol can be assigned from the chemical shift of acetonide carbon atoms in 13C NMR spectrum. So, upon deprotection of 7, diol 12 was obtained in good chemical yield (84%) and was further protected with 2,2-DMP, in presence of catalytic amount of PTSA, to furnish compound 13. The analytical data of acetonide 13 confirmed the anti configuration of the 1,3-diol. Since the first hydroxyl center was obtained through an unambiguous method, the stereochemistry of the newly created hydroxyl functionality could be confirmed as that depicted in Scheme 2.
The propargylic alcohol 7 was chemoselectively reduced with LiAlH4 in THF at 0 °C to give cinnamyl alcohol derivative 14 (87%, Scheme 3). Alcohol 14 was protected as its acetate under conventional reaction conditions. The PMB (p-methoxybenzyl protecting group) in compound 15 was selectively removed with DDQ in CH2Cl2/H2O (19:1) to afford homoallylic alcohol 16 (89%) without promoting the migration of the acetyl group. Finally, 16 was acrylated with acryloyl chloride/Et3N/CH2Cl2/0 °C to furnish the required bis-olefin 6 in 82% yield. Ring-closing metathesis of bis-olefin 6 with Grubbs’ 1st generation catalyst (I; 10 mol%) [45] gave the required (+)-(6R,2′S)-cryptocaryalactone (1) as a solid in 58% yield {m.p. 122–125 °C /lit. 126–127 °C and [α]D= +20.1 (c = 0.20)/lit. +19.0 (c = 0.67)} [25]. All the spectral data matched with the literature values.
Scheme 3: Synthesis of cryptocaryalactone by RCM.
Scheme 3: Synthesis of cryptocaryalactone by RCM.
Conclusion
In conclusion, a short stereoselective total synthesis of 1 has been accomplished by a convergent strategy wherein a chiral 2,3-epoxy alcohol was the starting material and Sharpless asymmetric epoxidation and Carreira asymmetric alkynylation were used as key steps for generating unambiguous assigned stereocenters. More importantly, the Grubbs’ ring-closing metathesis protocol was applied to construct the final 5,6-dihydropyrone ring of cryptocaryalactone. The advantage of this synthetic methodology is that one can in principle synthesize the other three diastereomers of cryptocaryalactone by altering the Sharpless epoxidation and Carreira’s conditions.
Supporting Information
| Supporting Information File 1: Experimental Data | ||
| Format: DOC | Size: 62.5 KB | Download |
References
-
Lee, K.-H. J. Nat. Prod. 2004, 67, 273–283. doi:10.1021/np030373o
Return to citation in text: [1] -
Butler, M. S. J. Nat. Prod. 2004, 67, 2141–2153. doi:10.1021/np040106y
Return to citation in text: [1] -
Clardy, J.; Walsh, C. Nature 2004, 432, 829–837. doi:10.1038/nature03194
Return to citation in text: [1] -
Larsen, A. K.; Escargueil, A. E.; Skladanowski, A. Pharmacol. Ther. 2003, 99, 167–181. doi:10.1016/S0163-7258(03)00058-5
Return to citation in text: [1] -
Richetti, A.; Cavallaro, A.; Ainis, T.; Fimiani, V. Immunopharmacol. Immunotoxicol. 2003, 25, 441–449. doi:10.1081/IPH-120024511
Return to citation in text: [1] -
Koizumi, F.; Ishiguro, H.; Ando, K.; Kondo, H.; Yoshida, M.; Matsuda, Y.; Nakanishi, S. J. Antibiot. 2003, 56, 603–609.
Return to citation in text: [1] -
Köster, M.; Lykke-Andersen, S.; Elnakady, Y. A.; Gerth, K.; Washausen, P.; Höfle, G.; Sasse, F.; Kjems, J.; Hauser, H. Exp. Cell Res. 2003, 286, 321–331. doi:10.1016/S0014-4827(03)00100-9
Return to citation in text: [1] -
Chrusciel, R. A.; Strohbach, J. W. Curr. Top. Med. Chem. 2004, 4, 1097–1114.
Return to citation in text: [1] -
Agrawal, V. K.; Singh, J.; Mishra, K. C.; Khadikar, P. V.; Jaliwala, Y. A. ARKIVOC 2006, No. ii, 162–177.
Return to citation in text: [1] -
Inayat-Hussain, S. H.; Annuar, B. O.; Din, L. B.; Taniguchi, N. Toxicol. Lett. 2002, 131, 153–159. doi:10.1016/S0378-4274(02)00025-5
Return to citation in text: [1] -
Inayat-Hussain, S. H.; Annuar, B. O.; Din, L. B.; Ali, A. M.; Ross, D. Toxicol. in Vitro 2003, 17, 433–439. doi:10.1016/S0887-2333(03)00051-1
Return to citation in text: [1] -
Chan, K. M.; Rajab, N. F.; Ishak, M. H. A.; Ali, A. M.; Yusoff, K.; Din, L. B.; Inayat-Hussain, S. H. Chem.-Biol. Interact. 2006, 159, 129–135. doi:10.1016/j.cbi.2005.10.107
Return to citation in text: [1] -
Blatt, N. B.; Glick, G. D. Bioorg. Med. Chem. 2001, 9, 1371–1384. doi:10.1016/S0968-0896(01)00041-4
Return to citation in text: [1] -
Huang, Z. Chem. Biol. 2002, 9, 1059–1072. doi:10.1016/S1074-5521(02)00247-8
Return to citation in text: [1] -
Newmeyer, D. D.; Ferguson-Miller, S. Cell 2003, 112, 481–490. doi:10.1016/S0092-8674(03)00116-8
Return to citation in text: [1] -
Kikuchi, H.; Sasaki, K.; Sekiya, J.; Maeda, Y.; Amagai, A.; Kubohara, Y.; Oshima, Y. Bioorg. Med. Chem. 2004, 12, 3203–3214. doi:10.1016/j.bmc.2004.04.001
Return to citation in text: [1] -
Hoffmann, H. M. R.; Rabe, J. Angew. Chem., Int. Ed. Engl. 1985, 24, 94–110. doi:10.1002/anie.198500941
Return to citation in text: [1] -
Negishi, E.-i.; Kotora, M. Tetrahedron 1997, 53, 6707–6738. doi:10.1016/S0040-4020(97)00199-3
Return to citation in text: [1] -
Collins, I. J. Chem. Soc., Perkin Trans. 1 1999, 1377–1395. doi:10.1039/a808137i
Return to citation in text: [1] -
Carter, N. B.; Nadany, A. E.; Sweeney, J. B. J. Chem. Soc., Perkin Trans. 1 2002, 2324–2342. doi:10.1039/b007664n
Return to citation in text: [1] -
Bialy, L.; Waldmann, H. Chem.–Eur. J. 2004, 10, 2759–2780. doi:10.1002/chem.200305543
Return to citation in text: [1] -
Njardarson, J. T.; Gaul, C.; Shan, D.; Huang, X.-Y.; Danishefsky, S. J. J. Am. Chem. Soc. 2004, 126, 1038–1040. doi:10.1021/ja039714a
Return to citation in text: [1] -
Shaw, S. J.; Sundermann, K. F.; Burlingame, M. A.; Myles, D. C.; Freeze, B. S.; Xian, M.; Brouard, I.; Smith, A. B., III. J. Am. Chem. Soc. 2005, 127, 6532–6533. doi:10.1021/ja051185i
Return to citation in text: [1] -
de Fátima, A.; Marquissolo, C.; de Albuquerque, S.; Carraro-Abrahão, A. A.; Pilli, R. A. Eur. J. Med. Chem. 2006, 41, 1210–1213. doi:10.1016/j.ejmech.2006.05.010
Return to citation in text: [1] -
Meyer, H. H. Liebigs Ann. Chem. 1984, 977–981. doi:10.1002/jlac.198419840515
Return to citation in text: [1] [2] [3] -
Mori, Y.; Furukawa, H. Chem. Pharm. Bull. 1994, 42, 2161–2163.
Return to citation in text: [1] -
Fu, X.; Sévenet, T.; Hamid, A.; Hadi, A.; Remy, F.; Païs, M. Phytochemistry 1993, 33, 1272–1274. doi:10.1016/0031-9422(93)85065-Y
Return to citation in text: [1] -
Blázquez, M. A.; Bermejo, A.; Zafra-Polo, M. C.; Cortes, D. Phytochem. Anal. 1999, 10, 161–170. doi:10.1002/(SICI)1099-1565(199907/08)10:4<161::AID-PCA453>3.0.CO;2-2
Return to citation in text: [1] -
Matsuoka, Y.; Aikawa, K.; Irie, R.; Katsuki, T. Heterocycles 2005, 66, 187–194. doi:10.3987/COM-05-S(K)73
Return to citation in text: [1] -
Zhang, J.; Li, Y.; Wang, W.; She, X.; Pan, X. J. Org. Chem. 2006, 71, 2918–2921. doi:10.1021/jo060028e
Return to citation in text: [1] -
Govindachari, T. R.; Parthasarathy, P. C.; Modi, J. D. Indian J. Chem. 1972, 10, 149–151.
Return to citation in text: [1] -
Govindachari, T. R.; Parthasarathy, P. C. Tetrahedron Lett. 1971, 3401–3402. doi:10.1016/S0040-4039(01)97189-8
Return to citation in text: [1] -
Sabitha, G.; Bhaskar, V.; Reddy, S. S. S.; Yadav, J. S. Tetrahedron 2008, 64, 10207–10213. doi:10.1016/j.tet.2008.08.032
Return to citation in text: [1] -
Spencer, G. F.; England, R. E.; Wolf, R. B. Phytochemistry 1984, 23, 2499–2500. doi:10.1016/S0031-9422(00)84083-2
Return to citation in text: [1] -
Drewes, S. E.; Horn, M. M.; Ramesar, N. S.; Ferreira, D.; Nel, R. J. J.; Hutchings, A. Phytochemistry 1998, 49, 1683–1687. doi:10.1016/S0031-9422(98)00328-8
Return to citation in text: [1] -
Radha Krishna, P.; Srinivas, R. Tetrahedron Lett. 2007, 48, 2013–2015. doi:10.1016/j.tetlet.2007.01.056
Return to citation in text: [1] -
Radha Krishna, P.; Ramana Reddy, V. V. Tetrahedron Lett. 2005, 46, 3905–3907. doi:10.1016/j.tetlet.2005.03.202
Return to citation in text: [1] -
Katsuki, T.; Martin, V. S. Asymmetric Epoxidation of Allylic Alcohols: The Katsuki–Sharpless Epoxidation Reaction. In Organic Reactions; Paquette, L. A., Ed.; John Wiley & Sons Inc.: New York, 1996; Vol. 48, pp 1–299.
Return to citation in text: [1] -
Ma, S.; Ni, B. Chem.–Eur. J. 2004, 10, 3286–3300. doi:10.1002/chem.200305581
Return to citation in text: [1] -
García-Fortanet, J.; Murga, J.; Carda, M.; Marco, J. A. Tetrahedron 2004, 60, 12261–12267. doi:10.1016/j.tet.2004.10.010
Return to citation in text: [1] -
Frantz, D. E.; Fässler, R.; Carreira, E. M. J. Am. Chem. Soc. 2000, 122, 1806–1807. doi:10.1021/ja993838z
Return to citation in text: [1] -
Rychnovsky, S. D.; Skalitzky, D. J. Tetrahedron Lett. 1990, 31, 945–948. doi:10.1016/S0040-4039(00)94399-5
Return to citation in text: [1] -
Rychnovsky, S. D.; Rogers, B.; Yang, G. J. Org. Chem. 1993, 58, 3511–3515. doi:10.1021/jo00065a011
Return to citation in text: [1] -
Evans, D. A.; Hoveyda, A. H. J. Am. Chem. Soc. 1990, 112, 6447–6449. doi:10.1021/ja00173a071
Return to citation in text: [1] -
Deiters, A.; Martin, S. F. Chem. Rev. 2004, 104, 2199–2238. doi:10.1021/cr0200872
Return to citation in text: [1]
| 38. | Katsuki, T.; Martin, V. S. Asymmetric Epoxidation of Allylic Alcohols: The Katsuki–Sharpless Epoxidation Reaction. In Organic Reactions; Paquette, L. A., Ed.; John Wiley & Sons Inc.: New York, 1996; Vol. 48, pp 1–299. |
| 39. | Ma, S.; Ni, B. Chem.–Eur. J. 2004, 10, 3286–3300. doi:10.1002/chem.200305581 |
| 35. | Drewes, S. E.; Horn, M. M.; Ramesar, N. S.; Ferreira, D.; Nel, R. J. J.; Hutchings, A. Phytochemistry 1998, 49, 1683–1687. doi:10.1016/S0031-9422(98)00328-8 |
| 36. | Radha Krishna, P.; Srinivas, R. Tetrahedron Lett. 2007, 48, 2013–2015. doi:10.1016/j.tetlet.2007.01.056 |
| 37. | Radha Krishna, P.; Ramana Reddy, V. V. Tetrahedron Lett. 2005, 46, 3905–3907. doi:10.1016/j.tetlet.2005.03.202 |
| 1. | Lee, K.-H. J. Nat. Prod. 2004, 67, 273–283. doi:10.1021/np030373o |
| 2. | Butler, M. S. J. Nat. Prod. 2004, 67, 2141–2153. doi:10.1021/np040106y |
| 3. | Clardy, J.; Walsh, C. Nature 2004, 432, 829–837. doi:10.1038/nature03194 |
| 16. | Kikuchi, H.; Sasaki, K.; Sekiya, J.; Maeda, Y.; Amagai, A.; Kubohara, Y.; Oshima, Y. Bioorg. Med. Chem. 2004, 12, 3203–3214. doi:10.1016/j.bmc.2004.04.001 |
| 33. | Sabitha, G.; Bhaskar, V.; Reddy, S. S. S.; Yadav, J. S. Tetrahedron 2008, 64, 10207–10213. doi:10.1016/j.tet.2008.08.032 |
| 10. | Inayat-Hussain, S. H.; Annuar, B. O.; Din, L. B.; Taniguchi, N. Toxicol. Lett. 2002, 131, 153–159. doi:10.1016/S0378-4274(02)00025-5 |
| 11. | Inayat-Hussain, S. H.; Annuar, B. O.; Din, L. B.; Ali, A. M.; Ross, D. Toxicol. in Vitro 2003, 17, 433–439. doi:10.1016/S0887-2333(03)00051-1 |
| 12. | Chan, K. M.; Rajab, N. F.; Ishak, M. H. A.; Ali, A. M.; Yusoff, K.; Din, L. B.; Inayat-Hussain, S. H. Chem.-Biol. Interact. 2006, 159, 129–135. doi:10.1016/j.cbi.2005.10.107 |
| 13. | Blatt, N. B.; Glick, G. D. Bioorg. Med. Chem. 2001, 9, 1371–1384. doi:10.1016/S0968-0896(01)00041-4 |
| 14. | Huang, Z. Chem. Biol. 2002, 9, 1059–1072. doi:10.1016/S1074-5521(02)00247-8 |
| 15. | Newmeyer, D. D.; Ferguson-Miller, S. Cell 2003, 112, 481–490. doi:10.1016/S0092-8674(03)00116-8 |
| 34. | Spencer, G. F.; England, R. E.; Wolf, R. B. Phytochemistry 1984, 23, 2499–2500. doi:10.1016/S0031-9422(00)84083-2 |
| 8. | Chrusciel, R. A.; Strohbach, J. W. Curr. Top. Med. Chem. 2004, 4, 1097–1114. |
| 9. | Agrawal, V. K.; Singh, J.; Mishra, K. C.; Khadikar, P. V.; Jaliwala, Y. A. ARKIVOC 2006, No. ii, 162–177. |
| 31. | Govindachari, T. R.; Parthasarathy, P. C.; Modi, J. D. Indian J. Chem. 1972, 10, 149–151. |
| 32. | Govindachari, T. R.; Parthasarathy, P. C. Tetrahedron Lett. 1971, 3401–3402. doi:10.1016/S0040-4039(01)97189-8 |
| 4. | Larsen, A. K.; Escargueil, A. E.; Skladanowski, A. Pharmacol. Ther. 2003, 99, 167–181. doi:10.1016/S0163-7258(03)00058-5 |
| 5. | Richetti, A.; Cavallaro, A.; Ainis, T.; Fimiani, V. Immunopharmacol. Immunotoxicol. 2003, 25, 441–449. doi:10.1081/IPH-120024511 |
| 6. | Koizumi, F.; Ishiguro, H.; Ando, K.; Kondo, H.; Yoshida, M.; Matsuda, Y.; Nakanishi, S. J. Antibiot. 2003, 56, 603–609. |
| 7. | Köster, M.; Lykke-Andersen, S.; Elnakady, Y. A.; Gerth, K.; Washausen, P.; Höfle, G.; Sasse, F.; Kjems, J.; Hauser, H. Exp. Cell Res. 2003, 286, 321–331. doi:10.1016/S0014-4827(03)00100-9 |
| 27. | Fu, X.; Sévenet, T.; Hamid, A.; Hadi, A.; Remy, F.; Païs, M. Phytochemistry 1993, 33, 1272–1274. doi:10.1016/0031-9422(93)85065-Y |
| 29. | Matsuoka, Y.; Aikawa, K.; Irie, R.; Katsuki, T. Heterocycles 2005, 66, 187–194. doi:10.3987/COM-05-S(K)73 |
| 45. | Deiters, A.; Martin, S. F. Chem. Rev. 2004, 104, 2199–2238. doi:10.1021/cr0200872 |
| 25. | Meyer, H. H. Liebigs Ann. Chem. 1984, 977–981. doi:10.1002/jlac.198419840515 |
| 26. | Mori, Y.; Furukawa, H. Chem. Pharm. Bull. 1994, 42, 2161–2163. |
| 30. | Zhang, J.; Li, Y.; Wang, W.; She, X.; Pan, X. J. Org. Chem. 2006, 71, 2918–2921. doi:10.1021/jo060028e |
| 24. | de Fátima, A.; Marquissolo, C.; de Albuquerque, S.; Carraro-Abrahão, A. A.; Pilli, R. A. Eur. J. Med. Chem. 2006, 41, 1210–1213. doi:10.1016/j.ejmech.2006.05.010 |
| 40. | García-Fortanet, J.; Murga, J.; Carda, M.; Marco, J. A. Tetrahedron 2004, 60, 12261–12267. doi:10.1016/j.tet.2004.10.010 |
| 41. | Frantz, D. E.; Fässler, R.; Carreira, E. M. J. Am. Chem. Soc. 2000, 122, 1806–1807. doi:10.1021/ja993838z |
| 17. | Hoffmann, H. M. R.; Rabe, J. Angew. Chem., Int. Ed. Engl. 1985, 24, 94–110. doi:10.1002/anie.198500941 |
| 18. | Negishi, E.-i.; Kotora, M. Tetrahedron 1997, 53, 6707–6738. doi:10.1016/S0040-4020(97)00199-3 |
| 19. | Collins, I. J. Chem. Soc., Perkin Trans. 1 1999, 1377–1395. doi:10.1039/a808137i |
| 20. | Carter, N. B.; Nadany, A. E.; Sweeney, J. B. J. Chem. Soc., Perkin Trans. 1 2002, 2324–2342. doi:10.1039/b007664n |
| 21. | Bialy, L.; Waldmann, H. Chem.–Eur. J. 2004, 10, 2759–2780. doi:10.1002/chem.200305543 |
| 22. | Njardarson, J. T.; Gaul, C.; Shan, D.; Huang, X.-Y.; Danishefsky, S. J. J. Am. Chem. Soc. 2004, 126, 1038–1040. doi:10.1021/ja039714a |
| 23. | Shaw, S. J.; Sundermann, K. F.; Burlingame, M. A.; Myles, D. C.; Freeze, B. S.; Xian, M.; Brouard, I.; Smith, A. B., III. J. Am. Chem. Soc. 2005, 127, 6532–6533. doi:10.1021/ja051185i |
| 28. | Blázquez, M. A.; Bermejo, A.; Zafra-Polo, M. C.; Cortes, D. Phytochem. Anal. 1999, 10, 161–170. doi:10.1002/(SICI)1099-1565(199907/08)10:4<161::AID-PCA453>3.0.CO;2-2 |
| 42. | Rychnovsky, S. D.; Skalitzky, D. J. Tetrahedron Lett. 1990, 31, 945–948. doi:10.1016/S0040-4039(00)94399-5 |
| 43. | Rychnovsky, S. D.; Rogers, B.; Yang, G. J. Org. Chem. 1993, 58, 3511–3515. doi:10.1021/jo00065a011 |
| 44. | Evans, D. A.; Hoveyda, A. H. J. Am. Chem. Soc. 1990, 112, 6447–6449. doi:10.1021/ja00173a071 |
© 2009 Radha Krishna et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)