Abstract
A number of new ferrocene-π-extended-dithiafulvalenes were successfully synthesized as new electron donor compounds. The chemical structures and electrochemical behaviors of these compounds were investigated using several spectroscopic methods. The synthesis of these compounds was achieved using the modified Wittig–Horner cross-coupling reaction using n-BuLi/THF at temperature varies from −78 °C to 0 °C. These new classes of bis(1,3-dithiafulvalene)ferrocenes have the 1,3-dithiole ring system separated by ferrocene as conjugated spacer. The ferrocene-dithiafulvalenes derivatives 9 and 12 were prepared as side products during the synthesis of the targeted compounds as bis(1,3-dithiafulvalene)ferrocenes 8, 10 and 11 in variable yields. The redox properties of the compounds have been investigated by cyclic voltammetry at ambient temperature using tetra-n-butylammonium perchlorate (TBAP) as the supporting electrolyte compared to ferrocene and the derivative 9. In CH2Cl2 on a Pt working electrode and at ambient temperature, two oxidation waves associated with two reduction waves at scan rates 100 mV s−1 were observed for 9 and 12. In contrast the anodic peak potential of bis(1,3-dithiafulvalene)ferrocenes 8, 10 and 11 exhibited two and three oxidation waves associated with two reduction waves.
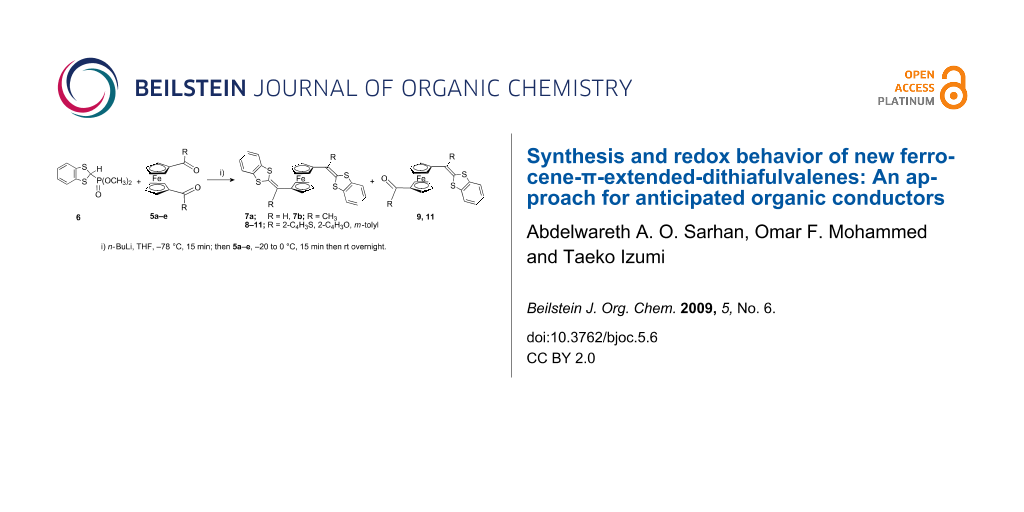
Graphical Abstract
Introduction
Electron-transfer reactions have found highly useful applications for organic synthesis, and therefore have encouraged scientists to synthesize a wide variety of molecules to develop new conducting charge transfer complexes [1-3]. As a result, we have turned our attention to the synthesis of a new series of tetrathiafulvalene derivatives. These are particularly intriguing molecules for several reasons: (i) they are chemically and photochemically stable in solution; (ii) they exhibit an extended conjugated structure which provides strong absorption in the UV/vis regions; (iii) they are considered as excellent building blocks for intramolecular charge and photoinduced electron-transfer processes [4]; (iv) they can be oxidized successively and reversibly (multistage redox states) to the cation radical and dication species. Related to this issue, Hudhomme and co-workers have synthesized and characterized a donor-acceptor dyad system involving tetrathiafulvalene (TTF) as electron donor attached by a flexible spacer to perylene derivatives as electron acceptor [5]. They have shown that the fluorescence of the tetrathiafulvalene–perylene derived dyad can be reversibly modulated by the transformation of the TTF unit into its radical cation and dication.
After organic chemists had discovered organic metal tetrathiafulvalene-tetracyanoquinodimethane (TTF-TCNQ) charge transfer complexes, various synthetic approaches to tetrathiafulvalenes gained wide attention, and charge-transfer from TTF to TCNQ was extensively recognized [6,7]. For instance, π-extended-dithiafulvalenes have been successfully used as multi-electron donor moieties with high electrical conductivities in the preparation of new charge transfer (CT) complexes [8,9]. However, a number of modifications of the TTF framework have been added to improve their electrical conductance properties. For instance, TTFs containing two or more fused or covalently attached TTF units have been used for preparing superconducting salts [10,11]. Recently, a number of modifications have been performed even on the tetrathiafulvalene (TTF) skeleton 1 in the search of new molecular-based organic metals [12-16]. Furthermore, the extension of conjugation between the two 1,3-dithiole units of TTF and their conducting salts has been prepared studied [17-23].
The first compound belonging to the class of 1,1′-bis[(1,3-dithiol-2-ylidene)alkyl]ferrocenes (2) was shown to form 1:1 CT complexes with tetracyano-p-quinodimethane (TCNQ, 3) and dichlorodicyanoquinone (DDQ) 4, see Figure 1 [24-26]. Subsequently, a number of reports have appeared dealing with the electrochemical properties of these CT complexes of tetrathiafulvalenes having a ferrocene moiety [10,11].
These findings prompted us to synthesize and investigate the electrochemical properties of symmetrical 1,1′-bis[(1,3-dithiol-2-ylidene)heteroaryl/aryl]ferrocenes substituted with heterocyclic and aromatic ring moieties separated by ferrocene moiety as the conjugated spacer. Femtosecond time-resolved absorption and Raman studies are in progress to aid in the understanding of the photophysical properties of the bis(1,3-DTF)Fc-TCNQ charge transfer complexes, trying to pick up the transient of the mono- and dication and anion radical of these systems with TCNQ, respectively. From this study we can estimate the timescales of the charge separation and recombination of the produced radical ion pairs. Such laser spectroscopic studies will have a big impact in future, leading not only new synthetic applications, but also to the discovery of new photophysical properties of these systems [27].
Results and Discussion
In this work we synthesized a series of novel 1,1′-bis[(1,3-dithiol-2-ylidene)heteroaryl/aryl]ferrocene [1,1′-bis(1,3-DTF)Fc] derivatives as new electron donor compounds using the direct Wittig–Horner cross coupling reaction. The starting 1,1′-diacylferrocene derivatives 5a and 5b were synthesized cleanly and in high yields according to previously reported methods [12,13,15]. The 1,3-benzodithiole-2-phosphonate 6 was obtained in relatively high yield as described previously in literature [14,28,29].
Reaction of ferrocene-1,1′-dicarboxaldehyde (5a) or 1,1′-diacetylferrocene (5b) with 1,3-benzodithiole-2-phosphonate (6) in dry THF in the presence of n-BuLi at −78 °C following the Wittig–Horner reaction method afforded the corresponding 1,1′-bis[(1,3-dithiol-2-ylidene)methyl]ferrocenes 7a and 7b in good yields (Scheme 1). The 1,1′-bis(1,3-dithiafulvalene)ferrocene 7a was prepared following the procedures reported by A. Togni et al. [30] as dark red crystals in good yield, while 7b was obtained according to procedures similar to those of Sarhan et al. [31].
Scheme 1: Synthesis of π-extended dithiafulvalenes 7a and 7b. i) n-BuLi/THF, −78 °C, 15 min, then rt, overnight.
Scheme 1: Synthesis of π-extended dithiafulvalenes 7a and 7b. i) n-BuLi/THF, −78 °C, 15 min, then rt, overnig...
On application of the Wittig–Horner reaction (n-BuLi, −78 °C, THF) to 1,1′-bis(2-thienoyl)ferrocene (5c), with 1,3-benzodithiole 6 the unexpected ferrocene-dithiafulvalene (Fc-DTF) 9 was obtained as an orange-red oil in 33% yield. The targeted 1,1′-bis(1,3-DTF)Fc 8 was obtained in very low yield (<1%), could not be isolated in pure form and could only be detected by FAB mass spectra (M+ 678) (Scheme 2) [13]. However, upon reaction of the diacylferrocene 5c with the 1,3-benzodithiole-2-phosphonate 6 using a slight modification of the Wittig–Horner procedures, by carrying out the reaction at −20 to 0 °C and subsequently purifying by column chromatography using chloroform/hexane mixture, the 1,1′-bis(1,3-DTF)Fc 8 was obtained as dark red crystals in 31% yield followed by 9 in 12% yield in the second fraction.
Scheme 2: Synthesis of π-extended dithiafulvalenes 8 and 9. i) 6, n-BuLi, THF, −78 °C, 15 min; then, 5c, −78 to 20 °C, overnight. ii) 6, n-BuLi, THF, −78 °C, 15 min; then 5c, −20 to 0 °C, 15 min, then rt overnight.
Scheme 2: Synthesis of π-extended dithiafulvalenes 8 and 9. i) 6, n-BuLi, THF, −78 °C, 15 min; then, 5c, −78 ...
Similarly, when 1,1′-bis(2-furoyl)ferrocene (5d) was subjected to reaction with 6 under the modified Wittig–Horner reaction at −20 to 0 °C in the presence of n-BuLi/THF followed by stirring the reaction mixture under nitrogen atmosphere overnight, the 1,1′-bis(1,3-DTF)Fc 10 was obtained as dark red crystals in 62% yield (Scheme 3).
Scheme 3: Synthesis of π-extended dithiafulvalenes 10. i) 6, n-BuLi, THF, −78 °C, 15 min; then 5d, −20 to 0 °C, 15 min, then rt overnight.
Scheme 3: Synthesis of π-extended dithiafulvalenes 10. i) 6, n-BuLi, THF, −78 °C, 15 min; then 5d, −20 to 0 °...
Moreover, when this reaction was carried out allowing 1,1′-bis(m-tolylcarbonyl)ferrocene (5e) to react with 6 following the same modified Wittig–Horner procedure, the product was identified as 1,1′-bis(1,3-DTF)Fc 11 in 24% yield in addition to the (1,3-DTF)Fc 12 as a major product (76% isolated yield) (Scheme 4).
Scheme 4: Synthesis of π-extended dithiafulvalenes 11 and dithiafulvalene 12. i) 6, n-BuLi, THF, −78 °C, 15 min; then 5e, −20 to 0 °C, 15 min, then rt overnight.
Scheme 4: Synthesis of π-extended dithiafulvalenes 11 and dithiafulvalene 12. i) 6, n-BuLi, THF, −78 °C, 15 m...
Electrochemistry
The electrochemical redox properties of the newly synthesized Fc-DTFs 9 and 12, the 1,1′-bis(1,3-DTF)Fc’s 8, 10, 11, and the acylferrocenes 5c–e were studied by cyclic voltammetry at room temperature in dry CH2Cl2 solutions, using Pt working electrode, Pt gauze as a counter electrode and Ag/AgCl as a reference electrode and tetra-n-butylammonium perchlorate (TBAP) as the supporting electrolyte. The electrochemical data for the investigated compounds were compared to those of ferrocene and summarized in Table 1, Table 2 and Table 3. The data show that compounds 5a–5e have more positive redox values than ferrocene.
Table 1: Cyclic voltammetric parameters of the compounds 5a–e (5 × 10−4 mol/L) and ferrocene Fc on a Pt working electrode, Pt gauze counter electrode and Ag/AgCl reference electrode in dry CH2Cl2 at ambient temperature using TBAP 0.1 mol L−1 concentration as the supporting electrolyte, scan rate 100 mV s−1
| Compound No. | Epc/mV | Epa/mV | E0′/mV | ΔEp/mV |
|---|---|---|---|---|
| Fc | 481 | 554 | 518 | 73 |
| 5a | 1047 | 1133 | 1090 | 86 |
| 5b | 985 | 1075 | 1030 | 90 |
| 5c | 960 | 1094 | 1027 | 134 |
| 5d | 901 | 1015 | 958 | 114 |
| 5e | 913 | 1028 | 972 | 115 |
Table 2: Cyclic voltammetric parameters of the compounds Fc-DTF 9 and 12 (5 × 10−4 mol/L) on a Pt working electrode, Pt gauze counter electrode and Ag/AgCl reference electrode in dry CH2Cl2 at ambient temperature using TBAP 0.1 mol L−1 concentration as the supporting electrolyte, scan rate 100 mV s−1
| Compound No. | Epc/mV | Epa/mV | E0′/mV | ΔEp/mV | ||||
|---|---|---|---|---|---|---|---|---|
| P1 | P2 | P1 | P2 | P1 | P2 | P1 | P2 | |
| Fc-DTF 9a | 653 | 982 | 725 | 1119 | 689 | 1051 | 72 | 137 |
| Fc-DTF 12 | 600 | 977 | 698 | 1158 | 649 | 1068 | 98 | 181 |
aFc-DTF 9 was measured at scan rate 20 mV s−1.
Table 3: Cyclic voltammetric parameters of the compounds 8, 10, 11 (5 × 10−4 mol/L) on a Pt working electrode, Pt gauze counter electrode and Ag/AgCl reference electrode in dry CH2Cl2 at ambient temperature using TBAP 0.1 mol L−1 concentration as the supporting electrolyte, scan rate 100 mV s−1
| Compound No. | Epc/mV | Epa/mV | E0′/mV | ΔEp/mV | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | P2 | P3 | P1 | P2 | P3 | P1 | P2 | P3 | P1 | P2 | P3 | |
| 8 (SR = 20 mV) | 414 | 925 | 501 | 1038 | 458 | 982 | 87 | 113 | ||||
| 8 (SR = 100 mV) | 394 | 832 | 570 | 905 | 1043 | 482 | 869 | 938 | 176 | 73 | 211 | |
| 10 | 449 | 721 | 992 | 519 | 889 | 1067 | 484 | 805 | 1030 | 70 | 168 | 75 |
| 11 | 258 | 859 | 343 | 1022 | 258 | 941 | 85 | 163 | ||||
These results indicate that the separation of the anodic and the cathodic peak potentials, ΔEp, are almost the same for compounds 5d and 5e, while a small positive shift in the formal potential, E0′, (60 mV) for compound 5a was observed compared to compound 5b. This shift can be attributed to the replacement of hydrogen atom in 5a by the electron donating methyl group in 5b, which facilitates the redox process of compound 5b [32]. On the other hand, on replacement of the hydrogen atom in 5a with conjugated ring system, e.g. thiophene, furyl or m-tolyl, a decrease in the formal potential, E0′, at 63, 118 and 133 mV is observed for compounds 5c, 5e and 5d, respectively. This indicates that the substitution of the hydrogen atom with a conjugated ring system leads to a decrease in the formal potential, E0′. The ΔEp values are 86, 5a; 90, 5b; 134, 5c; 114, 5d; 115, 5e; mV compared to ferrocene 73 mV. The cyclic voltammetric behavior of compounds 5d and 5e is almost the same, in which ΔEp values are 114 mV and 115 mV for 5d and 5e respectively, whereas a positive shift of 14 mV in the E0′ was observed for compounds 5e compared to compound 5d. This confirms that the substituted tolyl ring in compound 5e enhances the electron transfer process and consequently its redox behavior. Compared to the parent ferrocene a large positive shift in the formal potential, E0′, (572, 512, 509, 440, 454 mV) was observed compared to compounds 5a–e respectively.
The electrochemical behaviors of diacylferrocene derivatives 5a–e are markedly affected by the scan rate, in which at higher scan rate (ν ≥ 600 mV s−1), broadening of ΔEp was observed (ΔEp > 230 mV), indicating that the irreversibility of the electron-transfer process was maintained under these conditions, possibly due to the onset of kinetic complications. The electrochemical redox properties of compounds 5a–e were studied by cyclic voltammetry and the data are listed in Table 1. One oxidation wave potential associated with one reduction wave for these ferrocenyl diketones was observed in the potential range ca 1015–1100 mV at lower scan rate (10–600 mV s−1) (Table 1).
Compounds 9 and 12 also showed some common features depending the solvent and scan rate effects. In CH2Cl2 on a Pt electrode and at ambient temperature, compounds 9 and 12 showed two oxidation waves with peak potentials of 725, 1119 mV for 9 and 698, 1158 mV for 12 at scan rates 100 mV s−1. For 9 and 12, such a process is electrochemically reversible or quasi-reversible (ΔEp1 72 mV and ΔEp2 137 mV) for 9 and (ΔEp1 98 mV and ΔEp2 181 mV) for 12. The ΔEp2 was not observed with an increase of scan rate and the second wave was distorted when increasing the scan rate more than 100–400 mV s−1 (Table 2).
The 1,1′-bis(1,3-DTF)Fc’s 8, 10 and 11 showed three couples of redox waves observed clearly in the cyclic voltammograms at the potential range, Epa ca 340–1070 mV at lower scan rate (10–100 mV s−1). The first couple of redox waves in the potential range ca 340–725 mV is due to the redox process of the DTF/DTF+ system, whereas the second couple of redox waves in the potential range ca. 900–1022 mV is attributed to the Fc/Fc+ redox process. The third couple of redox waves which attributed to the DTF+/DTF++ appeared at 1040–1070 mV, Table 3.
The redox behavior of compound 8 was studied using different scan rates (10, 20, 50, 200 and 300 mV s−1) at ambient temperature, and showed only the appearance of two quasi-reversible oxidation processes associated with two reduction waves (SR = 20 mV; Ep1 = 414–501 mV, Ep2 = 925–1035 mV) depending on the scan rates, Table 3. At scan rate SR = 100 mV (Ep1 = 390–570 mV, Ep2 = 832–905 mV and Ep3 = 1043 mV) three oxidation processes with two associated reduction peaks could be observed. Controlled potential coulometry in correspondence to the first anodic step (EOx1 = +0.57 V) consumes one electron/molecule typical of ferrocenium species, showing a cyclic voltammetric response quite complementary to the first step illustrated in Figure 2. The second oxidation step, although displaying a good extent of chemical reversibility in cyclic voltammetry and consuming one electron/molecule (EOx2 = +0.905 V), affords a dirty green solution which no longer exhibits a cyclic voltammetric profile attributable to the generation of redox congeners of 8. This proves that the primarily electro-generated dication [8]+2 is a transient species. This third anodic step is an irreversible oxidation at high-potential values (Ep = +0.938 V). These voltammetric data are in agreement with those previously reported [27,30] for the redox behavior of the compound 8 in which the third oxidation peak was observed as irreversible at a more positive potential value (Ep3 = 1043 mV), Table 3 and Figure 2.
Figure 2: Cyclic voltammetry (CV) of compounds 8, 10 and 11 in CH2Cl2 at scan rate 100 mV.
Figure 2: Cyclic voltammetry (CV) of compounds 8, 10 and 11 in CH2Cl2 at scan rate 100 mV.
The aforementioned electrochemical results of compounds 7a, 7b, 8, 10 and 11, where ferrocene is a spacer between the two 1,3-dithiole units, indicate that the interaction of ferrocene directly with the DTF rings causes significant changes in electron donating properties and consequently the electrochemical behavior of these compounds. Comparing the data of compounds 8, 10 and 11 with those previously prepared by Togni et al. [30] and Sarhan et al. [31] we found that the CV data obtained here was in agreement with that reported for 7a and 7b, while for compounds 8, 10 and 11 three reversible oxidation processes associated with three reduction waves were clearly resolved. Controlled potential coulometry corresponding to the first anodic step for 8 (E = 570 mV) consumed one electron/molecule. We also found that the third anodic process is irreversible in character. Analysis of the cyclic voltammograms relevant to the first oxidation process with scan rates varying from 20 mV s−1 to 400 mV s−1 shows that the peak-to-peak separation progressively increases and the 100 mV scan rate is ideal in most cases. Introducing the conjugated ring system such as 2-thienyl, 2-furyl and C6H4CH3-m leads to the formation of good donor ability highly expected to form stable conducting materials, opening a very interesting research area in the ET reaction and intermolecular CT complexes.
Conclusion
In conclusion, a number of 1,1′-substituted diacylferrocenes 5a–e were synthesized by Friedel–Crafts reaction. Their structures were confirmed by spectral analyses and were in satisfactory agreements with those reported in literature. Some new 1,1′-bis(1,3-DTF)Fc’s and Fc-DTFs were made as multicomponent electron donor systems by the Wittig–Horner reaction of the respective phosphonate ester 6 with different ferreocenylketones using the modifications introduced onto the reaction. The redox chemistry of the ferreocenylketones and these new π-conjugated hybrids 8, 10 and 11 has been studied using cyclic voltammetry at ambient temperature on a Pt working electrode, using TBAP as the supporting electrolyte. The CV exhibited good donor properties, showing a one-electron quasireversible oxidation potential. The oxidation potential values, and to a larger extent the reduction potential values, are strongly influenced by the scan rate. Increasing the scan rate from 20 to 600 mV s−1 leads to an increase of the oxidation potential values. The Fc-DTF derivatives 9 and 12 were prepared as side products during the synthesis of the targeted compounds as 1,1′-bis(1,3-DTF)Fc’s 8, 10 and 11 in variable yields. Their electrochemistry was studied and compared to the previously reported derivative 9. In CH2Cl2 on a Pt electrode and at ambient temperature, compound 12 showed two oxidation waves associated with two reduction waves with peak potentials of 698, 1158 mV at scan rates 100 mV s−1. In contrast the anodic peak potential (E1pa) in compounds 9 and 12 is higher than that of ferrocene by 171 mV for 9 and 144 mV for 12, respectively.
Experimental
Melting points were recorded on a Gallenkamp melting point apparatus and are uncorrected. Infrared spectra (IR) were measured on a Hitachi 260-10 spectrometer. 1H NMR and 13C NMR spectra were recorded at room temperature on a Varian Nuclear Magnetic Resonance Spectrometer (500 MHz). Chemical shifts are denoted in δ units (ppm), relative to tetramethylsilane (TMS) as internal standard, J values are given in Hz. MS and FAB-MS spectra were obtained using a JEOL JMS-AX505HA. CV was measured on a cyclic voltammeter (Model CS-1090/Model CS-1087). Column chromatography was performed on silica gel 60 (230–400 Mesh ASTM). Solvents were distilled before use. Acylferrocenes were prepared according to the methods that previously described in literature.
Synthesis of 1,1′-bis(2-furoyl)ferrocene (5d). In a dried three-necked flask, a mixture of ferrocene (3.72 g, 0.02 mol) and 2-furoyl chloride (0.02 mol) was stirred in dry CH2Cl2 (100 ml) at 0 °C for 10 min. Anhydrous aluminium chloride (2.8 g, 0.021 mol) was added at such a rate that the reaction mixture remained below 5 °C. The appearance of a blue color indicates that the reaction is occurring. This addition required ca. 20 min, and after its completion stirring was continued for 30 min with ice cooling and for a further 2 h at room temperature. The reaction mixture was cooled again in ice, 50 ml of water was added cautiously, and the resulting two phases were stirred vigorously for 30 min. After transferring the mixture to a separator funnel, the layers were separated, and the aqueous layer was extracted with two 50 ml portions of dichloromethane. The combined dichloromethane extracts were washed once with 50 ml of water, twice with 50 ml portions of 10% aqueous sodium hydroxide and dried over sodium sulfate. The dichloromethane was removed under vacuum and the residue was collected and chromatographed on silica gel using chloroform to give 0.4 g of yellow crystals of ferrocene from the early fractions followed by dark red crystals of 1,1′-bis(2-furoyl)ferrocene (5d) in 21% yield, mp 104–106 °C. IR (KBr) ν 3100s, 1730m, 1619s, 1606s, 1563s, 1479s, 1442s, 1375s, 1334s, 1295s, 1220s, 1155s, 1079s, 1051s, 1020s, 975s, 912s, 881s, 813s, 767s, 599s, 501s cm−l. 1H NMR (CDCl3) δ 7.50 (s, 2H, furan-H), 7.27 (s, 2H, furan-H), 6.54 (d, J = 1 Hz, J = 2 Hz, 2H, furan-H), 5.16 (d, J = 2 Hz, 4H, ferrocene-H), 4.55 (d, J = 2 Hz, 4H, ferrocene-H). 13C NMR (CDCl3) δ 183.60 (2 CO), 153.41 (furan, C-2, C-2′), 145.64 (furan, C-5, C-5′), 117.15, 112.13 (furan-C-3, C-4, C-3′, C-4′), 79.10 (ferrocene-C-1, C-1′), 74.23, 72.45 (ferrocene-CH). FAB-MS m/z (%) [M+ 374 (54)]. Elemental analysis for C20H14FeO4 (374.17), Calcd: C; 64.20, H; 3.77%. Found: C; 64.18, H; 3.72%.
1,1′-Bis(m-tolylcarbonyl)ferrocene (5e): Similar to 5d this was obtained as dark red crystals in 67% yield. IR (KBr) ν 3099s, 2362m, 2919m, 1639s, 1600s, 1580s, 1448s, 1398m, 1373s, 1336m, 1294s, 1224s, 1145s, 1095m, 1060s, 1031s, 890s, 836s, 781s, 752s, 669s cm−1. 1H NMR (CDCl3) δ 7.57 (d, J = 0.5 Hz, 4H, aromatic-H), 7.34 (d, J = 0.5 Hz, 2H, aromatic-H), 7.31 (d, J = 8 Hz, 2H, aromatic-H), 4.91 (t, 4H, ferrocene-H), 4.56 (s, 4H, ferrocene-H), 6.18 (s, 6H, 2 CH3). 13C NMR (CDCl3) δ 228.48 (CO), 138.80, 133.36, 129.30, 128.75, 126.02 (aromatic-H), 80.00 (ferrocene-C), 75.32, 73.78 (ferrocene-CH). FAB MS m/z (%) [M+ 422 (100)]. Elemental analysis for C26H22FeO2 (422.10), Calcd: C; 73.95, H; 5.25%. Found: C; 73.68, H; 5.21%.
1,1′-Bis[(1,3-benzodithiol-2-ylidene)(2-thienyl)methyl]ferrocene (8) and 1-(2-thenoyl)-1′-[(1,3-benzodithiol-2-ylidene)(2-thienyl)methyl]ferrocene (9). A sample of 2-(dimethoxyphosphinyl)-1,3-benzodithiole (6, 0.786 g, 3 mmol) was stirred in dry THF (50 ml) under a stream of nitrogen at −78 °C. A solution of n-BuLi (2.3 ml, 2.6 M) was added and the mixture was stirred for 15 min. The temperature of the reaction was raised to −20 °C and for further 15 min then a solution of 1,1′-bis(2-thenoyl)ferrocene (3c; 0.888 g, 3 mmol) in dry THF (75 ml) was added portion wise. The temperature of the reaction was raised to room temperature and the reaction mixture was kept overnight with stirring. The tetrahydrofuran was removed under vacuum and the residue was washed with water and extracted with chloroform and dried over sodium sulfate. The crude oil product was chromatographed on silica gel using chloroform/hexane mixture (1:2) to give the bis(1,3-DTF)Fc 8 as a dark red oil in the first fractions, which solidified after standing in the refrigerator to be red crystals in 31% yield, mp 171–172 °C, Rf (rt, CHCl3/hexane 1:1) = 0.48. The polarity of the elutant was increased to be CHCl3/hexane (2:1) to elute a dark red solid of the corresponding Fc-DTF 9 in 12% yield.
Data for bis(1,3-DTF)Fc 8: IR (KBr) ν = 3060m, 1569s, 1542s, 1517s, 1448s, 1272m, 1216s, 1122s, 1031m, 813s, 732s, 698s cm−1. 1H NMR (CDCl3) δ 7.43 (dd, J = 1 Hz, J = 5 Hz, 2H, thiophene), 7.19 (dd, J = 2 Hz, J = 0.5 Hz, 2H, aromatic-H) 7.18–7.03 (m, 8H, 2 thiophene-H and 6 aromatic-H), 6.99–6.95 (m, 2H, thiophene-H). 4.44 (s, 4H, ferrocene-H), 4.28 (s, 4H, ferrocene-H). 13C NMR (CDCl3) δ 142.83 (thiafulvalene C=C), 136.89 (thiophene, C-2), 135.36 (aromatic-C), 127.79, 126.87, 126.04, 125.55, 125.36, 121.54, 120.62 (aromatic-CH and thiophene-CH), 113.10 (thiafulvalene C=C), 84.00 (ferrocene-C), 70.10, 68.63 (ferrocene-CH). FAB MS m/z (%) [M+ 678 (12)]. Elemental analysis for C34H22FeS6 (678.7548), Calcd: C; 60.16, H; 3.27, S; 28.34%. Found: C; 59.98, H; 3.48, S; 28.58%.
Data for Fc-DTF 9: The analytical data of this compound was in agreement with that previously reported in [28].
1,1′-Bis[(1,3-benzodithiol-2-ylidene)(2-furyl)methyl]ferrocene (10). This compound was obtained similar to the method used for 8 as an orange red oil in 62% yield, mp 98–99 °C, Rf = 0.29 (CHCl3/hexane, 25 °C). IR (KBr) ν = 3010s, 2956m, 1646m, 1569m, 1538m, 1450s, 1286m, 1214s, 1149s, 1124m, 1014s, 923m, 754s, 667s cm−1. 1H NMR (CDCl3) δ 7.44 (dd, J = 2 Hz, J = 2 Hz, 2H, furan-H), 7.19–7.18 (dd, J = 2 Hz, J = 0.5 Hz, 2H, aromatic-H), 7.15–7.14 (dd, J = 1.5 Hz, J = 0.5 Hz, 2H, aromatic-H). 7.05–7.01 (m, 4H, aromatic-H), 6.52–6.51 (dd, J = 1 Hz, J = 0.5 Hz, 2H, furan-H), 6.47–6.46 (m, 2H, furan-H), 4.48–4.47 (dd, J = 1 Hz, J = 1 Hz, 4H, ferrocene-H), 4.22 (dd, J = 1 Hz, J = 1 Hz, 4H,ferrocene-H). 13C NMR (CDCl3) δ 153.30 (furan, C-2), 140.68 (furan, C-5), 136.87, 135.70 (thiafulvalene C=C), 133.20 (aromatic-C), 125.52, 125.40, 121.26, 120.83 (aromatic-CH), 113.25 (thiafulvalene C=C), 110.55, 109.12 (furan-CH), 85.98 (ferrocene-C), 69.65, 69.48 (ferrocene-CH). FAB MS m/z (%) [M+ 646 (12)]. Elemental analysis for C34H22FeO2S4 (646.6248), Calcd: C; 63.15, H; 3.43, S; 19.83%. Found: C; 63.12, H; 3.40, S; 19.76%.
1,1′-Bis[(1,3-benzodithiol-2-ylidene)(m-tolyl)methyl]ferrocene (11) and 1-[(1,3-benzodithiol-2-ylidene)(m-tolyl)methyl]-1′-(m-toluoyl)ferrocene (12). A sample of the 1,3-benzodithiole-2-phosphonate (6, 0.524 g, 2 mmol) was stirred in dry THF (60 ml) under a stream of nitrogen at −78 °C. A solution of n-BuLi (1.96 ml, 2.6 M) was added portion wise and the mixture was stirred for 15 min. The reaction mixture was allowed to warm to −20 °C with continuous stirring and a solution of ferrocenyl ketone 5e (0.422 g, 2 mmol) in dry THF (50 ml) was added portion wise within 15 min. The temperature of the reaction was raised to room temperature and the reaction mixture was kept overnight with stirring. The tetrahydrofuran was removed under vacuum, the residue was washed with water and extracted with chloroform and dried over sodium sulfate. The crude oil product was chromatographed on silica gel using chloroform/hexane mixture (1:3) to give after unreacted ferrocene the corresponding 1,1′-bis(1,3-DTF)Fc 11 as dark red oils, which solidified on standing in the refrigerator in 24% yield. The polarity of the eluent was increased to be 1:2 to elute the Fc-DTF 12 as dark red semi solid material in 76% yield.
Analytical data for 11: Yield 24%, mp 80–81 °C. IR (KBr) ν = 3091w, 3052w, 3008w, 2915w, 1635s, 1600m, 1571m, 1548m, 1448s, 1373m, 1290s, 1222m, 1147m, 1122m, 1091w, 1051m, 744s, 667m cm−1. 1H NMR (CDCl3) δ 7.33 (m, 2H, aromatic-H), 7.19–7.03 (m, 4H, aromatic-H), 7.03–6.98 (m, 2H, aromatic-H), 4.35 (s, 4H, ferrocene-H), 4.24 (s, 4H, ferrocene-H), 2.37 (s, 6H, 2CH3). 13C NMR (CDCl3) δ 142.59, (aromatic-C), 137.24, 135.72 (thiafulvalene C=CS2), 128.97, 128.82, 128.42, 126.40, 125.89, 125.40, 125.17, 121.41, 120.62 (aromatic-C and CH), 86.81 (ferrocene-C), 69.79, 68.58 (ferrocene-CH), 21.53, 21.99 (2 CH3). FAB MS m/z (%) [M+, 694 (18)], EI MS m/z (%) [M+ 694 (100)]. Anal. Calcd. for C40H30FeS4 (694.06), Calcd: C; 69.15, H; 4.35, S; 18.46%. Found: C; 69.23, H; 4.21; S; 18.21%.
Analytical data for 12: Yield 76%, mp 68–69 °C. IR (KBr) ν = 3056m, 2958s, 2925s, 2869m, 1693s, 1600m, 1571s, 1548m, 1448s, 1216m, 1122s, 1089m, 1031m, 740s, 667s cm−1. 1H NMR (CDCl3) δ = 7.66 (q, J = 0.5 Hz, 2H, aromatic-H), 7.30 (m, 3H, aromatic-H), 7.23 (m, 1H, aromatic-H), 7.18 (m, 1H, aromatic-H), 7.07–7.01 (m, 5H, aromatic-H), 4.93 (t, J = 2 Hz, 2H, ferrocene-H), 4.59 (t, J = 2 Hz, 2H, ferrocene-H), 4.37 (t, J = 2 Hz, 2H, ferrocene-H), 4.24 (t, J = 2 Hz, 2H, ferrocene-H),), 2.38 (s, 6H, 2CH3). 13C NMR (CDCl3) δ 198.95 (CO), 142.17 (aromatic-C), 139.79, 138.80 (thiafulvalene C=CS2), 137.98, 136.87, 135.71, 132.25, 129.82, 129.36 (aromatic-C), 128.97, 128.78, 128.73, 128.61, 127.96, 126.25, 125.64, 125.46, 125.34, 122.39, 121.48, 120.77 (aromatic-CH), 88.18 (ferrocene-C), 78.32 (ferrocene-C), 74.18, 72.20, 70.88, 69.03 (ferrocene-CH), 21.54, 21.44 (2 CH3). FAB MS m/z (%) [M+ 558 (100)]. Anal. Calcd. for C33H26FeOS2 (558.08), Calcd: C; 70.96, H; 4.69, S; 11.48%. Found: C; 70.87, H; 4.71; S; 11.50%.
Acknowledgements
We thank the Scientific Research from the Ministry of Education, Sciences, Sports and Culture (Japan) and Assiut University, (Egypt) for partial support of this work. The authors are gratefully acknowledging the Alexander von Humboldt Foundation for partial supporting this work by a Georg-Forster fellowship for A. Sarhan.
References
-
Wasielewski, M. R. Chem. Rev. 1992, 92, 435–461. doi:10.1021/cr00011a005
Return to citation in text: [1] -
Holten, D.; Bocian, D. F.; Lindsey, J. S. Acc. Chem. Res. 2002, 35, 57–69. doi:10.1021/ar970264z
Return to citation in text: [1] -
Kay, E. R.; Leigh, D. A. Nature 2006, 440, 286–287. doi:10.1038/440286a
Return to citation in text: [1] -
Bryce, M. R. Adv. Mater. 1999, 11, 11–23. doi:10.1002/(SICI)1521-4095(199901)11:1<11::AID-ADMA11>3.0.CO;2-3
Return to citation in text: [1] -
Leroy-Lhez, S.; Baffreau, J.; Perrin, L.; Levillain, E.; Allain, M.; Blesa, M.-J.; Hudhomme, P. J. Org. Chem. 2005, 70, 6313–6320. doi:10.1021/jo050766n
Return to citation in text: [1] -
Ferraris, J.; Cowan, D. O.; Walatka, V.; Perlstein, J. H. J. Am. Chem. Soc. 1973, 95, 948–949. doi:10.1021/ja00784a066
Return to citation in text: [1] -
Narita, M.; Pittman, C. U., Jr. Synthesis 1976, 489–514. doi:10.1055/s-1976-24099
Return to citation in text: [1] -
Segura, J. L.; Martin, N. Angew. Chem., Int. Ed. 2001, 40, 1372–1409. doi:10.1002/1521-3773(20010417)40:8<1372::AID-ANIE1372>3.0.CO;2-I
Return to citation in text: [1] -
Otero, M.; Herranz, M. A.; Seoane, C.; Martín, N.; Garín, J.; Orduna, J.; Alcalá, R.; Villacampa, B. Tetrahedron 2002, 58, 7463–7475. doi:10.1016/S0040-4020(02)00803-7
Return to citation in text: [1] -
Misaki, Y.; Ohta, T.; Higuchi, N.; Fujiwara, H.; Yamabe, T.; Mori, T.; Mori, H.; Tanaka, S. J. Mater. Chem. 1995, 5, 1571–1579. doi:10.1039/JM9950501571
Return to citation in text: [1] [2] -
Misaki, Y.; Higuchi, N.; Fujiwara, H.; Yamabe, T.; Mori, T.; Mori, H.; Tanaka, S. Angew. Chem., Int. Ed. Engl. 1995, 34, 1222–1225. doi:10.1002/anie.199512221
Return to citation in text: [1] [2] -
Sarhan, A. A. O. Tetrahedron 2005, 61, 3889–3932. doi:10.1016/j.tet.2005.02.028
Return to citation in text: [1] [2] -
Sarhan, A.; Izumi, T. Synth. Met. 2004, 140, 95–100. doi:10.1016/S0379-6779(03)00383-7
Return to citation in text: [1] [2] [3] -
Sarhan, A. A. O.; Murakami, M.; Izumi, T. Monatsh. Chem. 2002, 133, 1055–1066. doi:10.1007/s007060200074
Return to citation in text: [1] [2] -
Sarhan, A.; Kijima, T.; Izumi, T. J. Organomet. Chem. 2003, 682, 49–58. doi:10.1016/S0022-328X(03)00694-6
Return to citation in text: [1] [2] -
Moore, A. J.; Bryce, M. R.; Skabara, P. J.; Batsanov, A. S.; Goldenberg, L. M.; Howard, J. A. K. J. Chem. Soc., Perkin Trans. 1 1997, 3443–3449. doi:10.1039/a702554h
Return to citation in text: [1] -
Liu, S.-G.; Pérez, I.; Martín, N.; Echegoyen, L. J. Org. Chem. 2000, 65, 9092–9102. doi:10.1021/jo001149w
Return to citation in text: [1] -
Iyoda, M.; Takano, T.; Otani, N.; Ugawa, K.; Yoshida, M.; Matsuyama, H.; Kuwatani, Y. Chem. Lett. 2001, 1310–1311. doi:10.1246/cl.2001.1310
Return to citation in text: [1] -
Furuta, K.; Akutsu, H.; Yamada, J.-i.; Nakatsuji, S. Chem. Lett. 2004, 33, 1214–1215. doi:10.1246/cl.2004.1214
Return to citation in text: [1] -
Furuta, K.; Akutsu, H.; Yamada, J.-i.; Nakatsuji, S.; Turner, S. S. J. Mater. Chem. 2006, 16, 1504–1506. doi:10.1039/b601614f
Return to citation in text: [1] -
Green, A.; Bryce, M. R.; Batsanov, A. S.; Howard, J. A. K. J. Organomet. Chem. 1999, 590, 180–183. doi:10.1016/S0022-328X(99)00454-4
Return to citation in text: [1] -
Bryce, M. R.; Skabara, P. J.; Moore, A. J.; Batsanov, A. S.; Howard, J. A. K.; Hoy, V. J. Tetrahedron 1997, 53, 17781–17794. doi:10.1016/S0040-4020(97)10243-5
Return to citation in text: [1] -
Godbert, N.; Batsanov, A. S.; Bryce, M. R.; Howard, J. A. K. J. Org. Chem. 2001, 66, 713–719. doi:10.1021/jo001014q
Return to citation in text: [1] -
Williams, J. M.; Schultz, A. J.; Geiser, U.; Carlson, K. D.; Kini, A. M.; Wang, H. H.; Kwok, W.-K.; Whangbo, M.-H.; Schirber, J. E. Science 1991, 252, 1501–1508. doi:10.1126/science.252.5012.1501
Return to citation in text: [1] -
Yagubskii, E. B. Mol. Cryst. Liq. Cryst. 1993, 230, 139–156. doi:10.1080/10587259308032222
Return to citation in text: [1] -
Hobi, M.; Ruppert, O.; Gramlich, V.; Togni, A. Organometallics 1997, 16, 1384–1391. doi:10.1021/om9609978
Return to citation in text: [1] -
Newton, M. D.; Sutin, N. Annu. Rev. Phys. Chem. 1984, 35, 437–480. doi:10.1146/annurev.pc.35.100184.002253
Return to citation in text: [1] [2] -
Nakayama, J.; Fujiwara, K.; Hoshino, M. Chem. Lett. 1975, 1099–1102. doi:10.1246/cl.1975.1099
Return to citation in text: [1] [2] -
Nakayama, J. Synthesis 1975, 38–39. doi:10.1055/s-1975-23654
Return to citation in text: [1] -
Togni, A.; Hobi, M.; Rihs, G.; Rist, G.; Albinati, A.; Zanello, P.; Zech, D.; Keller, H. Organometallics 1994, 13, 1224–1234. doi:10.1021/om00016a027
Return to citation in text: [1] [2] [3] -
Sarhan, A.; Nouchi, Y.; Izumi, T. Tetrahedron 2003, 59, 6353–6362. doi:10.1016/S0040-4020(03)00951-7
Return to citation in text: [1] [2] -
Sarhan, A. A. O.; Izumi, T. J. Organomet. Chem. 2003, 675, 1–12. doi:10.1016/S0022-328X(03)00216-X
Return to citation in text: [1]
| 30. | Togni, A.; Hobi, M.; Rihs, G.; Rist, G.; Albinati, A.; Zanello, P.; Zech, D.; Keller, H. Organometallics 1994, 13, 1224–1234. doi:10.1021/om00016a027 |
| 32. | Sarhan, A. A. O.; Izumi, T. J. Organomet. Chem. 2003, 675, 1–12. doi:10.1016/S0022-328X(03)00216-X |
| 27. | Newton, M. D.; Sutin, N. Annu. Rev. Phys. Chem. 1984, 35, 437–480. doi:10.1146/annurev.pc.35.100184.002253 |
| 30. | Togni, A.; Hobi, M.; Rihs, G.; Rist, G.; Albinati, A.; Zanello, P.; Zech, D.; Keller, H. Organometallics 1994, 13, 1224–1234. doi:10.1021/om00016a027 |
| 1. | Wasielewski, M. R. Chem. Rev. 1992, 92, 435–461. doi:10.1021/cr00011a005 |
| 2. | Holten, D.; Bocian, D. F.; Lindsey, J. S. Acc. Chem. Res. 2002, 35, 57–69. doi:10.1021/ar970264z |
| 3. | Kay, E. R.; Leigh, D. A. Nature 2006, 440, 286–287. doi:10.1038/440286a |
| 8. | Segura, J. L.; Martin, N. Angew. Chem., Int. Ed. 2001, 40, 1372–1409. doi:10.1002/1521-3773(20010417)40:8<1372::AID-ANIE1372>3.0.CO;2-I |
| 9. | Otero, M.; Herranz, M. A.; Seoane, C.; Martín, N.; Garín, J.; Orduna, J.; Alcalá, R.; Villacampa, B. Tetrahedron 2002, 58, 7463–7475. doi:10.1016/S0040-4020(02)00803-7 |
| 31. | Sarhan, A.; Nouchi, Y.; Izumi, T. Tetrahedron 2003, 59, 6353–6362. doi:10.1016/S0040-4020(03)00951-7 |
| 6. | Ferraris, J.; Cowan, D. O.; Walatka, V.; Perlstein, J. H. J. Am. Chem. Soc. 1973, 95, 948–949. doi:10.1021/ja00784a066 |
| 7. | Narita, M.; Pittman, C. U., Jr. Synthesis 1976, 489–514. doi:10.1055/s-1976-24099 |
| 13. | Sarhan, A.; Izumi, T. Synth. Met. 2004, 140, 95–100. doi:10.1016/S0379-6779(03)00383-7 |
| 5. | Leroy-Lhez, S.; Baffreau, J.; Perrin, L.; Levillain, E.; Allain, M.; Blesa, M.-J.; Hudhomme, P. J. Org. Chem. 2005, 70, 6313–6320. doi:10.1021/jo050766n |
| 14. | Sarhan, A. A. O.; Murakami, M.; Izumi, T. Monatsh. Chem. 2002, 133, 1055–1066. doi:10.1007/s007060200074 |
| 28. | Nakayama, J.; Fujiwara, K.; Hoshino, M. Chem. Lett. 1975, 1099–1102. doi:10.1246/cl.1975.1099 |
| 29. | Nakayama, J. Synthesis 1975, 38–39. doi:10.1055/s-1975-23654 |
| 4. | Bryce, M. R. Adv. Mater. 1999, 11, 11–23. doi:10.1002/(SICI)1521-4095(199901)11:1<11::AID-ADMA11>3.0.CO;2-3 |
| 30. | Togni, A.; Hobi, M.; Rihs, G.; Rist, G.; Albinati, A.; Zanello, P.; Zech, D.; Keller, H. Organometallics 1994, 13, 1224–1234. doi:10.1021/om00016a027 |
| 24. | Williams, J. M.; Schultz, A. J.; Geiser, U.; Carlson, K. D.; Kini, A. M.; Wang, H. H.; Kwok, W.-K.; Whangbo, M.-H.; Schirber, J. E. Science 1991, 252, 1501–1508. doi:10.1126/science.252.5012.1501 |
| 25. | Yagubskii, E. B. Mol. Cryst. Liq. Cryst. 1993, 230, 139–156. doi:10.1080/10587259308032222 |
| 26. | Hobi, M.; Ruppert, O.; Gramlich, V.; Togni, A. Organometallics 1997, 16, 1384–1391. doi:10.1021/om9609978 |
| 27. | Newton, M. D.; Sutin, N. Annu. Rev. Phys. Chem. 1984, 35, 437–480. doi:10.1146/annurev.pc.35.100184.002253 |
| 17. | Liu, S.-G.; Pérez, I.; Martín, N.; Echegoyen, L. J. Org. Chem. 2000, 65, 9092–9102. doi:10.1021/jo001149w |
| 18. | Iyoda, M.; Takano, T.; Otani, N.; Ugawa, K.; Yoshida, M.; Matsuyama, H.; Kuwatani, Y. Chem. Lett. 2001, 1310–1311. doi:10.1246/cl.2001.1310 |
| 19. | Furuta, K.; Akutsu, H.; Yamada, J.-i.; Nakatsuji, S. Chem. Lett. 2004, 33, 1214–1215. doi:10.1246/cl.2004.1214 |
| 20. | Furuta, K.; Akutsu, H.; Yamada, J.-i.; Nakatsuji, S.; Turner, S. S. J. Mater. Chem. 2006, 16, 1504–1506. doi:10.1039/b601614f |
| 21. | Green, A.; Bryce, M. R.; Batsanov, A. S.; Howard, J. A. K. J. Organomet. Chem. 1999, 590, 180–183. doi:10.1016/S0022-328X(99)00454-4 |
| 22. | Bryce, M. R.; Skabara, P. J.; Moore, A. J.; Batsanov, A. S.; Howard, J. A. K.; Hoy, V. J. Tetrahedron 1997, 53, 17781–17794. doi:10.1016/S0040-4020(97)10243-5 |
| 23. | Godbert, N.; Batsanov, A. S.; Bryce, M. R.; Howard, J. A. K. J. Org. Chem. 2001, 66, 713–719. doi:10.1021/jo001014q |
| 12. | Sarhan, A. A. O. Tetrahedron 2005, 61, 3889–3932. doi:10.1016/j.tet.2005.02.028 |
| 13. | Sarhan, A.; Izumi, T. Synth. Met. 2004, 140, 95–100. doi:10.1016/S0379-6779(03)00383-7 |
| 15. | Sarhan, A.; Kijima, T.; Izumi, T. J. Organomet. Chem. 2003, 682, 49–58. doi:10.1016/S0022-328X(03)00694-6 |
| 12. | Sarhan, A. A. O. Tetrahedron 2005, 61, 3889–3932. doi:10.1016/j.tet.2005.02.028 |
| 13. | Sarhan, A.; Izumi, T. Synth. Met. 2004, 140, 95–100. doi:10.1016/S0379-6779(03)00383-7 |
| 14. | Sarhan, A. A. O.; Murakami, M.; Izumi, T. Monatsh. Chem. 2002, 133, 1055–1066. doi:10.1007/s007060200074 |
| 15. | Sarhan, A.; Kijima, T.; Izumi, T. J. Organomet. Chem. 2003, 682, 49–58. doi:10.1016/S0022-328X(03)00694-6 |
| 16. | Moore, A. J.; Bryce, M. R.; Skabara, P. J.; Batsanov, A. S.; Goldenberg, L. M.; Howard, J. A. K. J. Chem. Soc., Perkin Trans. 1 1997, 3443–3449. doi:10.1039/a702554h |
| 31. | Sarhan, A.; Nouchi, Y.; Izumi, T. Tetrahedron 2003, 59, 6353–6362. doi:10.1016/S0040-4020(03)00951-7 |
| 10. | Misaki, Y.; Ohta, T.; Higuchi, N.; Fujiwara, H.; Yamabe, T.; Mori, T.; Mori, H.; Tanaka, S. J. Mater. Chem. 1995, 5, 1571–1579. doi:10.1039/JM9950501571 |
| 11. | Misaki, Y.; Higuchi, N.; Fujiwara, H.; Yamabe, T.; Mori, T.; Mori, H.; Tanaka, S. Angew. Chem., Int. Ed. Engl. 1995, 34, 1222–1225. doi:10.1002/anie.199512221 |
| 10. | Misaki, Y.; Ohta, T.; Higuchi, N.; Fujiwara, H.; Yamabe, T.; Mori, T.; Mori, H.; Tanaka, S. J. Mater. Chem. 1995, 5, 1571–1579. doi:10.1039/JM9950501571 |
| 11. | Misaki, Y.; Higuchi, N.; Fujiwara, H.; Yamabe, T.; Mori, T.; Mori, H.; Tanaka, S. Angew. Chem., Int. Ed. Engl. 1995, 34, 1222–1225. doi:10.1002/anie.199512221 |
| 28. | Nakayama, J.; Fujiwara, K.; Hoshino, M. Chem. Lett. 1975, 1099–1102. doi:10.1246/cl.1975.1099 |
© 2009 Sarhan et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)