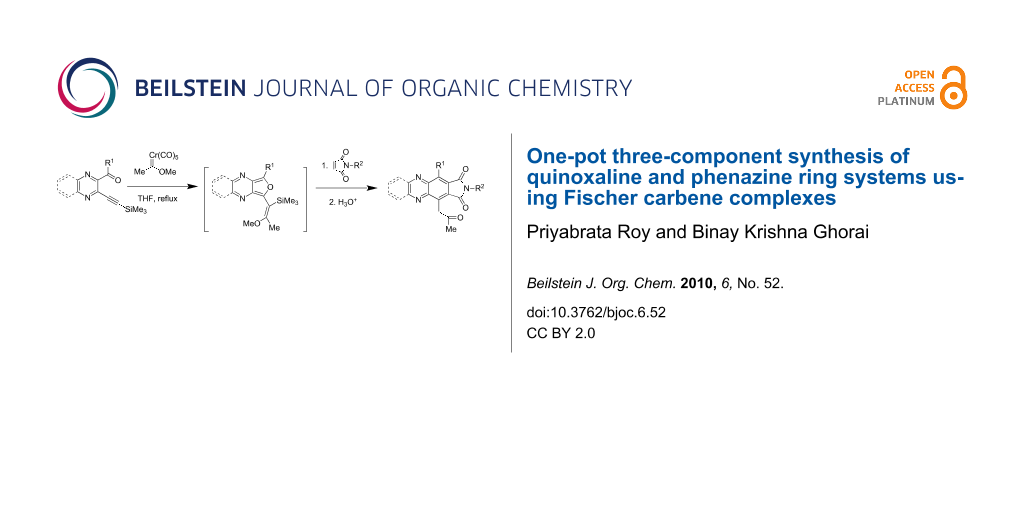
Abstract
One-pot three-component coupling of o-alkynylheteroaryl carbonyl derivatives with Fischer carbene complexes and dienophiles leading to the synthesis of quinoxaline and phenazine ring systems has been investigated. This involves the generation of furo[3,4-b]pyrazine and furo[3,4-b]quinoxaline as transient intermediates, which were trapped with Diels–Alder dienophiles. This is the first report on furo[3,4-b]pyrazine intermediates.
Graphical Abstract
Introduction
Nitrogen-containing heterocycles are abundant in nature and exhibit diverse and important biological properties [1]. Quinoxaline and phenazine derivatives are important classes of nitrogen containing heterocycles which exhibit a wide range of biological activities. Many phenazine compounds are found in nature and are produced by bacteria such as Pseudomonas spp., Streptomyces spp. and Pantoea agglomerans. These phenazine natural products have been implicated in the virulence and competitive fitness of the parent organisms [2,3]. These compounds show diverse biological activities such as antibacterial, antifungal, antiviral and antitumor properties [4-8]. While rarely found in nature, quinoxalines are well known in the pharmaceutical industry and have been shown to possess a broad spectrum of biological activity including antiviral and antibacterial properties and also act as kinase inhibitors [9-11]. These heterocyclic ring systems are most commonly assembled by the annulation of a heterocyclic ring onto a pre-existing benzene ring [12-21]. A less common approach to these ring systems is the annulation of benzene rings onto pre-existing heterocyclic rings [22]. This manuscript focuses on the successful execution of the latter transformation through a multicomponent reaction process to access these ring systems (Scheme 1). The synthetic approach involves a simultaneous one-pot construction of quinoxaline or phenazine rings which occurs in conjunction with the tandem generation and trapping of an azaisobenzofuran intermediate [23-26]. The synthesis of quinoxaline ring systems involves the coupling of Fischer carbene complexes [27-32] with 2-alkynyl-3-pyrazine carbonyl derivatives, followed by the generation of a hitherto unknown intermediate e.g. furo[3,4-b]pyrazine 4 and trapping of the latter with dienophiles. Phenazine derivatives can be synthesized using similar methodology from the coupling of 2-alkynyl-3-quinoxaline carbonyl derivative through the generation and trapping of furo[3,4-b]quinoxaline intermediates [22].
Scheme 1: Synthetic plan towards quinoxaline derivatives.
Scheme 1: Synthetic plan towards quinoxaline derivatives.
Results and Discussion
Our investigation commenced with the synthesis of o-alkynyl carbonyl derivatives 1, which were prepared in good yield from the iodoketone 6A or chloroketone 6B [33] or chloroaldehyde 6C [34] using palladium catalyzed Sonogashira coupling reactions as depicted in Scheme 2. Iodoketone 6A was prepared in 80% yield from (3-chloro-2-pyrazinyl)phenylmethanone [35] by halogen exchange with NaI in acetonitrile.
Scheme 2: Preparation of o-alkynyl carbonyl derivatives 1. A: pyrazine series; B,C: quinoxaline series.
Scheme 2: Preparation of o-alkynyl carbonyl derivatives 1. A: pyrazine series; B,C: quinoxaline series.
The three component coupling reaction of pyrazinyl ketone 1A, carbene complex 2 and N-phenylmaleimide (~ 1:1:1 ratio) in refluxing THF was initially investigated (Table 1, entry 1). This reaction led to a mixture of oxanorbornene derivative 7a and quinoxaline derivative 5a through the tandem generation and trapping of the furo[3,4-b]pyrazine intermediate 4 (R1 = Ph). Ring opening followed by extrusion of water by treatment of 7a with DBU in refluxing toluene, gave the quinoxaline derivative 5a [36]. The stereochemistry of the adduct 7a was assigned as exo based on the chemical shift of HA and HB (<4 ppm) [27]. A similar reaction process using N-methylmaleimide (entry 2) as dienophile led to the quinoxaline derivative 5b as the sole product after exposure to mild acid.
Table 1: Synthesis of quinoxaline and phenazine derivatives.
|
|
|||||
| Entry | Carbonyl compounds | R1 | R2 | Products (yielda) | |
|---|---|---|---|---|---|
| 1 | 1A | Ph | Ph | 7a (42%) | 5a (30%) |
| 2 | 1A | Ph | Me | - | 5b (55%) |
| 3 | 1C | H | Ph | 7c (10%)b | 5c (52%) |
| 4 | 1C | H | Me | - | 5d (55%) |
aIsolated yield.
bContaminated with 5c.
The three component coupling reaction of o-alkynyl quinoxaline carbonyl derivative 1C, carbene complex 2 and N-phenylmaleimide/N-methylmaleimide was also examined (Table 1, entry 3 & 4). In these cases, tandem generation and trapping of the desired furo[3,4-b]quinoxaline intermediates proceeded smoothly to give the corresponding hetero-polyaromatic phenazine derivatives 5c/5d. Although the [4 + 2] oxa-bridged adduct 7c was isolated, but it was contaminated with 5c since 7c readily converts to 5c in chloroform at room temperature (Table 1, entry 3).
The reaction was also examined with dimethyl maleate as the dienophile (Scheme 3). The reaction of pyrazinyl ketone 1A, carbene complex 2 and dimethyl maleate under the same conditions as previously described afforded the three component coupling product 9A in 40% yield via the unstable enol ether 8. No aromatized product was isolated, even under mild acidic conditions.
Scheme 3: Synthesis of quinoxaline derivative.
Scheme 3: Synthesis of quinoxaline derivative.
As part of a general effort to prepare aza-analogues of hydrophenanthrene natural products (including morphine alkaloids and abietanes) and tetracyclic triterpenes, the coupling of o-alkynyl pyrazine/quinoxaline carbonyl derivatives 1A/1B with simple γ,δ-unsaturated Fischer carbene complex 10 was investigated. This reaction proceeds via a tandem process involving the formation of azaisobenzofuran 11, followed by intramolecular Diels–Alder reaction, and ring opening of 12 to afford azahydrophenanthrone derivatives 13A/13B exclusively, in satisfactory yield (Scheme 4).
Scheme 4: Synthesis of azahydrophenanthrone derivatives.
Scheme 4: Synthesis of azahydrophenanthrone derivatives.
Conclusion
We have demonstrated a new route for the tandem generation of furo[3,4-b]pyrazine/ furo[3,4-b]quinoxaline intermediates by the coupling of o-alkynylheteroaryl carbonyl derivatives with Fischer carbene complexes. The intermediates can be trapped through Diels–Alder reaction with dienophiles leading to the synthesis of nitrogen containing heterocyclic analogues of quinoxaline and phenazine, respectively, in one-pot. This is the first report of in situ generation of furo[3,4-b]pyrazine intermediates.
Supporting Information
| Supporting Information File 1: General procedure for the preparation of o-alkynyl carbonyl derivatives 1 and quinoxaline and phenazine derivatives and spectral data for selected compounds. | ||
| Format: PDF | Size: 141.6 KB | Download |
References
-
Porter, A. E. A. In Comprehensive Heterocyclic Chemistry: the structure, reactions, synthesis, and uses of heterocyclic compounds; Katritzky, A. R.; Rees, C. W., Eds.; Pergamon Press: Oxford, U.K., 1984; pp 157–197.
Return to citation in text: [1] -
Turner, J. M.; Messenger, A. J. Occurrence, Biochemistry and Physiology of Phenazine Pigment Production. Rose, A. H.; Tempest, D. W., Eds.; Advances in Microbial Physiology, Vol. 27; Elsevier Ltd, 1986; pp 211–275. doi:10.1016/S0065-2911(08)60306-9
Return to citation in text: [1] -
McDonald, M.; Mavrodi, D. V.; Thomashow, L. S.; Floss, H. G. J. Am. Chem. Soc. 2001, 123, 9459–9460. doi:10.1021/ja011243+
Return to citation in text: [1] -
Whistler, C. A.; Pierson, L. S., III. J. Bacteriol. 2003, 185, 3718–3725. doi:10.1128/JB.185.13.3718-3725.2003
Return to citation in text: [1] -
Muller, P. K.; Krohn, K.; Muhlradt, P. F. Infect. Immun. 1989, 57, 2591–2596.
Return to citation in text: [1] -
MacDonald, J. C. Phenazines. In Antibiotics; Gottlieb, D.; Shaw, P. D., Eds.; Springer: New York, 1967; Vol. 2, pp 52–65.
Return to citation in text: [1] -
Turner, J. M.; Messenger, A. J. Occurrence, Biochemistry and Physiology of Phenazine Pigment Production. Rose, A. H.; Tempest, D. W., Eds.; Advances in Microbial Physiology, Vol. 27; Elsevier Ltd, 1986; pp 211–275. doi:10.1016/S0065-2911(08)60306-9
Return to citation in text: [1] -
Budzikiewicz, H. FEMS Microbiol. Lett. 1993, 104, 209–228. doi:10.1111/j.1574-6968.1993.tb05868.x
Return to citation in text: [1] -
Ali, M. M.; Ismail, M. M. F.; El-Gaby, M. S. A.; Zahran, M. A.; Ammar, Y. A. Molecules 2000, 5, 864–873. doi:10.3390/50600864
Return to citation in text: [1] -
He, W.; Meyers, M. R.; Hanney, B.; Spada, A. P.; Bilder, G.; Galzcinski, H.; Amin, D.; Needle, S.; Page, K.; Jayyosi, Z.; Perrone, M. H. Bioorg. Med. Chem. Lett. 2003, 13, 3097–3100. doi:10.1016/S0960-894X(03)00655-3
Return to citation in text: [1] -
Kim, Y. B.; Kim, Y. H.; Park, J. Y.; Kim, S. K. Bioorg. Med. Chem. Lett. 2004, 14, 541–544. doi:10.1016/j.bmcl.2003.09.086
Return to citation in text: [1] -
Yadav, J. S.; Reddy, B. V. S.; Rao, Y. G.; Narsaiah, A. V. Chem. Lett. 2008, 37, 348–349. doi:10.1246/cl.2008.348
Return to citation in text: [1] -
Brown, D. J. Quinoxalines: Supplement II. In The Chemistry of Heterocyclic Compounds: A Series of Monographs; Taylor, E. C.; Wipf, P., Eds.; John Wiley & Sons: New Jersey, 2004; Vol. 61.
Return to citation in text: [1] -
Huang, T. K.; Shi, L.; Wang, R.; Guo, X. Z.; Lu, X. X. Chin. Chem. Lett. 2009, 20, 161–164. doi:10.1016/j.cclet.2008.10.048
Return to citation in text: [1] -
Zhao, Z.; Wisnoski, D. D.; Wolkenberg, S. E.; Leister, W. H.; Wang, Y.; Lindsley, C. W. Tetrahedron Lett. 2004, 45, 4873–4876. doi:10.1016/j.tetlet.2004.04.144
Return to citation in text: [1] -
Bhosale, R. S.; Sarda, S. R.; Ardhapure, S. S.; Jadhav, W. N.; Bhusare, S. R.; Pawar, R. P. Tetrahedron Lett. 2005, 46, 7183–7186. doi:10.1016/j.tetlet.2005.08.080
Return to citation in text: [1] -
Boully, L.; Darabantu, M.; Turck, A.; Ple, N. J. Heterocycl. Chem. 2005, 42, 1423–1428. doi:10.1002/jhet.5570420726
Return to citation in text: [1] -
Neochoritis, C.; Stephanidou-Stephanatou, J.; Tsoleridis, C. A. Synlett 2009, 2, 302–305. doi:10.1055/s-0028-1087518
Return to citation in text: [1] -
Beifuss, U.; Tietze, M. Methanophenazine and Other Natural Biologically Active Phenazines. In Topics in Current Chemistry; Mulzer, J., Ed.; Springer: Berlin, Germany, 2005; Vol. 244, pp 77–113. doi:10.1007/b96889
Return to citation in text: [1] -
Davarani, S. S. H.; Fakhari, A. R.; Shaabani, A.; Ahmar, H.; Maleki, A.; Fumani, N. S. Tetrahedron Lett. 2008, 49, 5622–5624. doi:10.1016/j.tetlet.2008.07.063
Return to citation in text: [1] -
Pachter, J.; Kloetzel, M. C. J. Am. Chem. Soc. 1951, 73, 4958–4961. doi:10.1021/ja01154a144
Return to citation in text: [1] -
Haddadin, M. J.; Yavrouian, A.; Issidorides, C. H. Tetrahedron Lett. 1970, 11, 1409–1410. doi:10.1016/S0040-4039(01)97982-1
Return to citation in text: [1] [2] -
Basak, S.; Ghosh, S. K.; Sarkar, T. K. J. Indian Inst. Sci. 2001, 81, 431–452.
Return to citation in text: [1] -
Jana, G. P.; Ghorai, B. K. Tetrahedron 2007, 63, 12015–12025. doi:10.1016/j.tet.2007.09.007
Return to citation in text: [1] -
Jana, G. P.; Ghorai, B. K. Lett. Org. Chem. 2009, 6, 372–376.
Return to citation in text: [1] -
Mukherjee, S.; Jana, G. P.; Ghorai, B. K. J. Organomet. Chem. 2009, 694, 4100–4106. doi:10.1016/j.jorganchem.2009.08.019
Return to citation in text: [1] -
Jiang, D.; Herndon, J. W. Org. Lett. 2000, 2, 1267–1269. doi:10.1021/ol005691i
Return to citation in text: [1] [2] -
Ghorai, B. K.; Herndon, J. W.; Lam, Y.-F. Org. Lett. 2001, 3, 3535–3538. doi:10.1021/ol0166404
Return to citation in text: [1] -
Luo, Y.; Herndon, J. W.; Cervantes-Lee, F. J. Am. Chem. Soc. 2003, 125, 12720–12721. doi:10.1021/ja037500n
Return to citation in text: [1] -
Ghorai, B. K.; Herndon, J. W. Organometallics 2003, 22, 3951–3957. doi:10.1021/om030383k
Return to citation in text: [1] -
Camacho-Davila, A.; Herndon, J. W. J. Org. Chem. 2006, 71, 6682–6685. doi:10.1021/jo061053n
Return to citation in text: [1] -
Chen, Y.; Ye, S.; Jiao, L.; Liang, Y.; Sinha-Mahapatra, D. K.; Herndon, J. W.; Yu, Z.-X. J. Am. Chem. Soc. 2007, 129, 10773–10784. doi:10.1021/ja072203u
Return to citation in text: [1] -
Turck, A.; Ple, N.; Tallon, V.; Queguiner, G. J. Heterocycl. Chem. 1993, 30, 1491–1496. doi:10.1002/jhet.5570300605
Return to citation in text: [1] -
Yoshida, K.; Otomasu, H. Chem. Pharm. Bull. 1984, 32, 3361–3365.
Return to citation in text: [1] -
Turck, A.; Mojovic, L.; Queguiner, G. Synthesis 1988, 881–884. doi:10.1055/s-1988-27736
Return to citation in text: [1] -
Sarkar, T. K.; Panda, N.; Basak, S. J. Org. Chem. 2003, 68, 6919–6927. doi:10.1021/jo0344081
Return to citation in text: [1]
| 1. | Porter, A. E. A. In Comprehensive Heterocyclic Chemistry: the structure, reactions, synthesis, and uses of heterocyclic compounds; Katritzky, A. R.; Rees, C. W., Eds.; Pergamon Press: Oxford, U.K., 1984; pp 157–197. |
| 12. | Yadav, J. S.; Reddy, B. V. S.; Rao, Y. G.; Narsaiah, A. V. Chem. Lett. 2008, 37, 348–349. doi:10.1246/cl.2008.348 |
| 13. | Brown, D. J. Quinoxalines: Supplement II. In The Chemistry of Heterocyclic Compounds: A Series of Monographs; Taylor, E. C.; Wipf, P., Eds.; John Wiley & Sons: New Jersey, 2004; Vol. 61. |
| 14. | Huang, T. K.; Shi, L.; Wang, R.; Guo, X. Z.; Lu, X. X. Chin. Chem. Lett. 2009, 20, 161–164. doi:10.1016/j.cclet.2008.10.048 |
| 15. | Zhao, Z.; Wisnoski, D. D.; Wolkenberg, S. E.; Leister, W. H.; Wang, Y.; Lindsley, C. W. Tetrahedron Lett. 2004, 45, 4873–4876. doi:10.1016/j.tetlet.2004.04.144 |
| 16. | Bhosale, R. S.; Sarda, S. R.; Ardhapure, S. S.; Jadhav, W. N.; Bhusare, S. R.; Pawar, R. P. Tetrahedron Lett. 2005, 46, 7183–7186. doi:10.1016/j.tetlet.2005.08.080 |
| 17. | Boully, L.; Darabantu, M.; Turck, A.; Ple, N. J. Heterocycl. Chem. 2005, 42, 1423–1428. doi:10.1002/jhet.5570420726 |
| 18. | Neochoritis, C.; Stephanidou-Stephanatou, J.; Tsoleridis, C. A. Synlett 2009, 2, 302–305. doi:10.1055/s-0028-1087518 |
| 19. | Beifuss, U.; Tietze, M. Methanophenazine and Other Natural Biologically Active Phenazines. In Topics in Current Chemistry; Mulzer, J., Ed.; Springer: Berlin, Germany, 2005; Vol. 244, pp 77–113. doi:10.1007/b96889 |
| 20. | Davarani, S. S. H.; Fakhari, A. R.; Shaabani, A.; Ahmar, H.; Maleki, A.; Fumani, N. S. Tetrahedron Lett. 2008, 49, 5622–5624. doi:10.1016/j.tetlet.2008.07.063 |
| 21. | Pachter, J.; Kloetzel, M. C. J. Am. Chem. Soc. 1951, 73, 4958–4961. doi:10.1021/ja01154a144 |
| 9. | Ali, M. M.; Ismail, M. M. F.; El-Gaby, M. S. A.; Zahran, M. A.; Ammar, Y. A. Molecules 2000, 5, 864–873. doi:10.3390/50600864 |
| 10. | He, W.; Meyers, M. R.; Hanney, B.; Spada, A. P.; Bilder, G.; Galzcinski, H.; Amin, D.; Needle, S.; Page, K.; Jayyosi, Z.; Perrone, M. H. Bioorg. Med. Chem. Lett. 2003, 13, 3097–3100. doi:10.1016/S0960-894X(03)00655-3 |
| 11. | Kim, Y. B.; Kim, Y. H.; Park, J. Y.; Kim, S. K. Bioorg. Med. Chem. Lett. 2004, 14, 541–544. doi:10.1016/j.bmcl.2003.09.086 |
| 4. | Whistler, C. A.; Pierson, L. S., III. J. Bacteriol. 2003, 185, 3718–3725. doi:10.1128/JB.185.13.3718-3725.2003 |
| 5. | Muller, P. K.; Krohn, K.; Muhlradt, P. F. Infect. Immun. 1989, 57, 2591–2596. |
| 6. | MacDonald, J. C. Phenazines. In Antibiotics; Gottlieb, D.; Shaw, P. D., Eds.; Springer: New York, 1967; Vol. 2, pp 52–65. |
| 7. | Turner, J. M.; Messenger, A. J. Occurrence, Biochemistry and Physiology of Phenazine Pigment Production. Rose, A. H.; Tempest, D. W., Eds.; Advances in Microbial Physiology, Vol. 27; Elsevier Ltd, 1986; pp 211–275. doi:10.1016/S0065-2911(08)60306-9 |
| 8. | Budzikiewicz, H. FEMS Microbiol. Lett. 1993, 104, 209–228. doi:10.1111/j.1574-6968.1993.tb05868.x |
| 36. | Sarkar, T. K.; Panda, N.; Basak, S. J. Org. Chem. 2003, 68, 6919–6927. doi:10.1021/jo0344081 |
| 2. | Turner, J. M.; Messenger, A. J. Occurrence, Biochemistry and Physiology of Phenazine Pigment Production. Rose, A. H.; Tempest, D. W., Eds.; Advances in Microbial Physiology, Vol. 27; Elsevier Ltd, 1986; pp 211–275. doi:10.1016/S0065-2911(08)60306-9 |
| 3. | McDonald, M.; Mavrodi, D. V.; Thomashow, L. S.; Floss, H. G. J. Am. Chem. Soc. 2001, 123, 9459–9460. doi:10.1021/ja011243+ |
| 27. | Jiang, D.; Herndon, J. W. Org. Lett. 2000, 2, 1267–1269. doi:10.1021/ol005691i |
| 22. | Haddadin, M. J.; Yavrouian, A.; Issidorides, C. H. Tetrahedron Lett. 1970, 11, 1409–1410. doi:10.1016/S0040-4039(01)97982-1 |
| 27. | Jiang, D.; Herndon, J. W. Org. Lett. 2000, 2, 1267–1269. doi:10.1021/ol005691i |
| 28. | Ghorai, B. K.; Herndon, J. W.; Lam, Y.-F. Org. Lett. 2001, 3, 3535–3538. doi:10.1021/ol0166404 |
| 29. | Luo, Y.; Herndon, J. W.; Cervantes-Lee, F. J. Am. Chem. Soc. 2003, 125, 12720–12721. doi:10.1021/ja037500n |
| 30. | Ghorai, B. K.; Herndon, J. W. Organometallics 2003, 22, 3951–3957. doi:10.1021/om030383k |
| 31. | Camacho-Davila, A.; Herndon, J. W. J. Org. Chem. 2006, 71, 6682–6685. doi:10.1021/jo061053n |
| 32. | Chen, Y.; Ye, S.; Jiao, L.; Liang, Y.; Sinha-Mahapatra, D. K.; Herndon, J. W.; Yu, Z.-X. J. Am. Chem. Soc. 2007, 129, 10773–10784. doi:10.1021/ja072203u |
| 35. | Turck, A.; Mojovic, L.; Queguiner, G. Synthesis 1988, 881–884. doi:10.1055/s-1988-27736 |
| 23. | Basak, S.; Ghosh, S. K.; Sarkar, T. K. J. Indian Inst. Sci. 2001, 81, 431–452. |
| 24. | Jana, G. P.; Ghorai, B. K. Tetrahedron 2007, 63, 12015–12025. doi:10.1016/j.tet.2007.09.007 |
| 25. | Jana, G. P.; Ghorai, B. K. Lett. Org. Chem. 2009, 6, 372–376. |
| 26. | Mukherjee, S.; Jana, G. P.; Ghorai, B. K. J. Organomet. Chem. 2009, 694, 4100–4106. doi:10.1016/j.jorganchem.2009.08.019 |
| 22. | Haddadin, M. J.; Yavrouian, A.; Issidorides, C. H. Tetrahedron Lett. 1970, 11, 1409–1410. doi:10.1016/S0040-4039(01)97982-1 |
| 33. | Turck, A.; Ple, N.; Tallon, V.; Queguiner, G. J. Heterocycl. Chem. 1993, 30, 1491–1496. doi:10.1002/jhet.5570300605 |
© 2010 Roy and Ghorai; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)