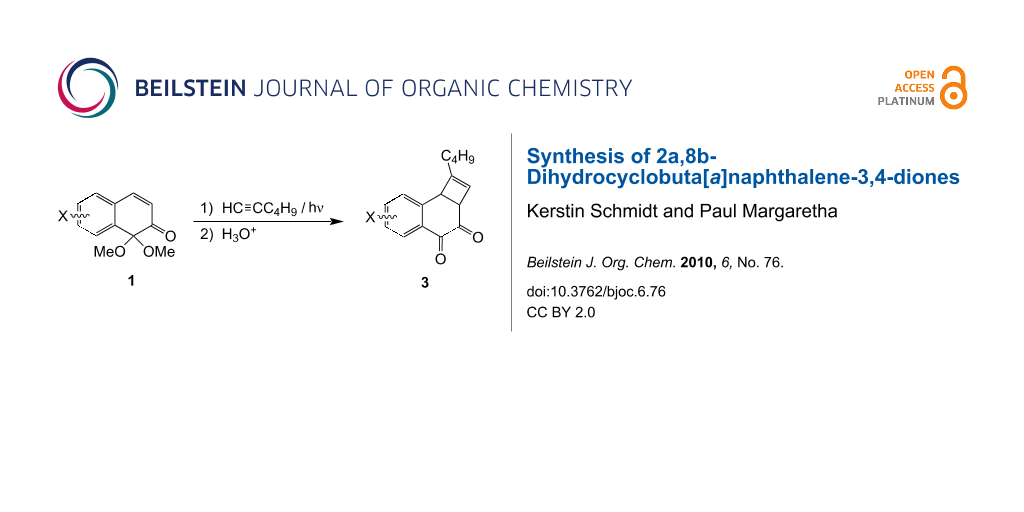
Abstract
On irradiation (λ = 350 nm) in neat hex-1-yne, naphthalene-1,2-dione monoacetals 1 afford mixtures of pentacyclic photodimers and up to 25% (isolated yield) of mixed photocycloadducts 2. Careful acidic hydrolysis of the acetal function of 2 gives the title compounds 3, the overall sequence representing a first approach to a (formal) [2 + 2] photocycloadduct of a 1,2-naphthoquinone to an alkyne.
Graphical Abstract
Introduction
The behaviour of excited 1,2- and 1,4-quinones towards ground-state molecules differs greatly. Whereas the former typically react via H-abstraction by an excited carbonyl group [1], the latter smoothly undergo [2 + 2] cycloaddition to alkenes to afford cyclobutane-type products [2]. Very recently we reported the use of 1,2-dihydro-1,1-dimethoxynaphthalen-2-ones 1 as protected precursors for the synthesis of both photocyclodimers and ketene-photocycloadducts of 1,2-naphtoquinones [3,4]. Here we report the preparation of – novel – 2a,8b-dihydrocyclobuta[a]naphthalene-3,4-diones, i.e. (formal) 1,2-naphthoquinone + alkyne [2 + 2] cycloadducts.
Results
Irradiation of 1 in the presence of alkynes affords the – known [3] – pentacyclic dimers and variable amounts (0–33%) of enone + alkyne cycloadducts as indicated by 1H NMR spectroscopy. The yields of mixed cycloadducts with many alkynes (3,3-dimethylbut-1-yne, trimethylsilylacetylene, 3-[(trimethylsilyl)oxy]prop-1-yne or hex-3-yne were invariably low (<5%). Moderately higher yields (15–25%) were obtained using hex-1-yne in either benzene or acetonitrile as solvent. Best results were obtained using hex-1-yne, both as reaction partner and as solvent. Thus, irradiation of either 1a or 1b in neat hex-1-yne affords a mixture of the corresponding dimeric dibenzophenylenediones (two regioisomers [3], 67–70%) and up to 30–33% of cycloadducts 2a or 2b, respectively. Compounds 2 can easily be isolated by chromatography (25% isolated yield) as they exhibit much higher Rf-values than the corresponding dimers. In contrast, naphthalenone 1c under the same conditions only affords <5% of 2c. Hydrolysis of cycloadducts 2 in a two phase mixture (CH2Cl2, aq HCl) at r.t. [5] leads to quantitative deprotection of the acetal function as indicated by 1H NMR spectroscopy to afford compounds 3a or 3b, respectively (Scheme 1). Compounds 3 are also easy to purify by chromatography (83–85% isolated yield) which is greatly assisted by the fact that they are easily detectable on account of their yellow colour.
Scheme 1: Synthesis of 2a,8b-dihydrocyclobuta[a]naphthalene-3,4-diones.
Scheme 1: Synthesis of 2a,8b-dihydrocyclobuta[a]naphthalene-3,4-diones.
Discussion
At first glance, the (relatively) low yield of mixed cycloadduct formation from excited 1 and alkynes seems disappointing. Nevertheless, one should bear in mind that a) dimer formation on irradiation of phenyl-conjugated enones, e.g., 3-phenylcyclohex-2-enone, is not suppressed even in neat alkenes as solvent [6], as these compounds tend to associate via π–π-stacking, and b) radical additions to alkynes usually proceed with significantly lower relative rates (30–50%) than those to the corresponding alkenes [7]. Taking these findings and the observed regioselectivity of the cycloaddition into consideration, the maximum relative yield (33%) of compounds 2a or 2b at total conversion of starting material is acceptable. Moreover, the fact that hydrolysis of the cycloadducts proceeds quantitatively, then the overall yields in the preparation of the – novel – 1,2-naphthoquinone + alkyne cycloadducts even becomes satisfactory. In the same experiment with 1c, the MeO-group apparently tends to increase the efficiency in photodimerization vs mixed photocycloaddition, otherwise there is no obvious explanation for this result.
Experimental
1. General. Acetals 1 were synthesized according to [8]. Both 1b, m.p. 60–62 °C, and 1c, m.p. 76–78 °C, originally described as oils, solidified on standing. Hex-1-yne was commercially available. Photolyses were conducted in a Rayonet RPR-100 photoreactor equipped with (16) 350 nm lamps. Column chromatography (CC) was carried out with silica gel 60 (Merck; 230–400 mesh). 1H and 13C NMR spectra (including 2D plots) were recorded with a Bruker WM-500 instrument at 500.13 and 125.8 MHz, resp., in CDCl3, δ in ppm, J in Hz.
2. Photolyses. Ar-Degassed solns. of 1 (1 mmol) in hex-1-yne (10 ml) were irradiated for 15 h up to total conversion (monitoring by TLC). After evaporation of the excess alkyne, the crude mixtures were analyzed by 1H NMR in order to determine the crude yield. CC (SiO2, pentane/Et2O 6:1) gave the photocycloadducts 2. 1-Butyl-3,4-dihydro-4,4-dimethoxy-2aH,8bH-cyclobuta[a]naphthalen-3-one (2a): 72 mg (25%), colourless oil, Rf = 0.65. 1H NMR: 7.70 (d, J = 8.4, 1H); 7.36 (t, J = 8.4, 1H); 7.30 (m, 2H); 5.97 (s, 1H); 4.52 (d, J = 4.6, 1H); 4.00 (bs, 1H); 3.53 & 3.00 (s, 3H); 2.16 (t, J = 7.0, 2H); 1.52 (m, 2H); 1.38 (m, 2H); 0.92 (t, J = 6.9, 3H). 13C NMR: 203.1 (s); 156.2 (s); 137.3 (s); 134.5 (s); 129.1 (d); 128.6 (d); 128.4 (d); 127.8 (d); 125.2 (d); 99.1 (s); 51.0 (q); 50.1 (d); 49.2 (q); 48.3 (d); 30.2 (t); 28.6 (t); 22.5 (t); 14.2 (q). Anal. Calcd for C18H22O3: C, 75.50; H, 7.74. Found: C, 75.43; H, 7.78. 7-Bromo-1-butyl-3,4-dihydro-4,4-dimethoxy-2aH,8bH-cyclobuta[a]naphthalen-3-one (2b): 91 mg (25%), light yellow solid, m.p. 50 – 52 °C, Rf = 0.61. 1H NMR: 7.55 (d, J = 8.4, 1H); 7.42 (s, 1H); 7.41 (d, J = 8.4, 1H); 5.96 (s, 1H); 4.46 (d, J = 4.6, 1H); 3.95 (bs, 1H); 3.52 & 2.97 (s, 3H); 2.16 (t, J = 7.0, 2H); 1.52 (m, 2H); 1.38 (m, 2H); 0.93 (t, J = 6.9, 3H). 13C NMR: 203.2 (s); 156.2 (s); 135.9 (s); 135.5 (s); 133.0 (s); 131.9 (d); 129.9 (d); 129.8 (d); 125.8 (d); 99.1 (s); 51.1 (q); 50.2 (d); 49.1 (q); 48.2 (d); 30.2 (t); 28.6 (t); 22.5 (t); 14.2 (q). Anal. Calcd for C18H21BrO3: C, 59.19; H 5.79. Found: C, 59.22; H, 5.82.
3. Hydrolyses. To a soln. of the acetal 2 (0.2 mmol) in CH2Cl2 (2 ml), was added 8N HCl (1.5 ml) and the mixture stirred for 5 h at room temperature. The org. phase was washed with sat. aq NaCl, dried (MgSO4) and the residue (100% conversion to product from 1H NMR) purified by CC (SiO2, pentane/Et2O 1:1) to afford the diketones 3. 1-Butyl-2a,8b-dihydrocyclobuta[a]naphthalene-3,4-dione (3a): 37 mg (85%), viscous yellow oil, Rf = 0.45. 1H NMR: 8.06 (d, J = 8.5, 1H); 7.62 (t, J = 8.5, 1H); 7.42 (t, J = 8.5, 1H); 7.37 (d, J = 8.5, 1H); 5.72 (s, 1H); 4.25 (d, J = 3.2, 1H); 4.16 (bs, 1H); 1.97 (m, 2H); 1.40 (m, 2H); 1.26 (m, 2H); 0.83 (t, J = 6.9, 3H). 13C NMR: 196.2 (s); 184.5 (s); 164.1 (s); 144.2 (s); 137.1 (s); 134.5 (d); 130.1 (d); 128.4 (d); 127.8 (d); 122.5 (d); 48.5 (d); 46.4 (d); 28.8 (t); 28.0 (t); 27.4 (t); 22.4 (q). Anal. Calcd for C16H16O2: C, 79.97; H, 6.71. Found: C, 79.92; H, 6.85. 7-Bromo-1-butyl-2a,8b-dihydrocyclobuta[a]naphthalene-3,4-dione (3b): 48 mg (83%), viscous yellow oil, Rf = 0.41. 1H NMR: 7.94 (d, J = 8.5, 1H); 7.58 (d, J = 8.5, 1H); 7.53 (s, 1H); 5.74 (s, H); 4.19 (d, J = 3.1, 1H); 4.15 (bs, 1H); 1.99 (m, 2H); 1.40 (m, 2H); 1.26 (m, 2H); 0.84 (t, J = 6.9, 3H). 13C NMR: 196.1 (s); 184.6 (s); 164.2 (s); 144.2 (s); 137.1 (s); 134.5 (d); 130.1 (s); 128.4 (d); 127.8 (d); 122.5 (d); 48.6 (d); 46.3 (d); 28.8 (t); 28.0 (t); 27.4 (t); 22.4 (q). Anal. Calcd for C16H15BrO2: C, 60.21; H, 4.71. Found: C, 60.13; H, 4.77.
References
-
Oelgemoeller, M.; Mattay, J. The “Photochemical Friedel-Crafts Acylation” of Quinones: From the Beginnings of Organic Photochemistry to Modern Solar Chemical Applications. In Handbook of Organic Photochemistry and Photobiology, 2nd ed.; Horspool, W. M.; Lenzi, F., Eds.; CRC Press: Boca Raton, USA, 2004; 88-1.
Return to citation in text: [1] -
Gilbert, A. 1,4-Quinone Cycloaddition Reactions with Alkenes, Alkynes, and Related Compounds. In Handbook of Organic Photochemistry and Photobiology, 2nd ed.; Horspool, W. M.; Lenzi, F., Eds.; CRC Press: Boca Raton, USA, 2004; 87-1.
Return to citation in text: [1] -
Schmidt, K.; Kopf, J.; Margaretha, P. Helv. Chim. Acta 2007, 90, 1667. doi:10.1002/hlca.200790172
Return to citation in text: [1] [2] [3] -
Schmidt, K.; Margaretha, P. Helv. Chim. Acta 2008, 91, 1625. doi:10.1002/hlca.200890177
Return to citation in text: [1] -
De Kimpe, N.; Verhé, R.; De Buyck, L.; Schamp, N. J. Org. Chem. 1978, 43, 2933. doi:10.1021/jo00408a047
Return to citation in text: [1] -
McCullough, J. J.; Ramachandran, B. R.; Snyder, F. F.; Taylor, G. N. J. Am. Chem. Soc. 1975, 97, 6767. doi:10.1021/ja00856a028
Return to citation in text: [1] -
Santi, R.; Bergamini, F.; Citterio, A.; Sebastiano, R.; Nicolini, M. J. Org. Chem. 1992, 57, 4250. doi:10.1021/jo00041a034
Return to citation in text: [1] -
Mal, D.; Roy, H. N.; Hazra, N. K.; Adhikari, S. Tetrahedron 1997, 53, 2177. doi:10.1016/S0040-4020(96)01119-2
Return to citation in text: [1]
| 1. | Oelgemoeller, M.; Mattay, J. The “Photochemical Friedel-Crafts Acylation” of Quinones: From the Beginnings of Organic Photochemistry to Modern Solar Chemical Applications. In Handbook of Organic Photochemistry and Photobiology, 2nd ed.; Horspool, W. M.; Lenzi, F., Eds.; CRC Press: Boca Raton, USA, 2004; 88-1. |
| 3. | Schmidt, K.; Kopf, J.; Margaretha, P. Helv. Chim. Acta 2007, 90, 1667. doi:10.1002/hlca.200790172 |
| 3. | Schmidt, K.; Kopf, J.; Margaretha, P. Helv. Chim. Acta 2007, 90, 1667. doi:10.1002/hlca.200790172 |
| 3. | Schmidt, K.; Kopf, J.; Margaretha, P. Helv. Chim. Acta 2007, 90, 1667. doi:10.1002/hlca.200790172 |
| 4. | Schmidt, K.; Margaretha, P. Helv. Chim. Acta 2008, 91, 1625. doi:10.1002/hlca.200890177 |
| 2. | Gilbert, A. 1,4-Quinone Cycloaddition Reactions with Alkenes, Alkynes, and Related Compounds. In Handbook of Organic Photochemistry and Photobiology, 2nd ed.; Horspool, W. M.; Lenzi, F., Eds.; CRC Press: Boca Raton, USA, 2004; 87-1. |
| 8. | Mal, D.; Roy, H. N.; Hazra, N. K.; Adhikari, S. Tetrahedron 1997, 53, 2177. doi:10.1016/S0040-4020(96)01119-2 |
| 7. | Santi, R.; Bergamini, F.; Citterio, A.; Sebastiano, R.; Nicolini, M. J. Org. Chem. 1992, 57, 4250. doi:10.1021/jo00041a034 |
| 6. | McCullough, J. J.; Ramachandran, B. R.; Snyder, F. F.; Taylor, G. N. J. Am. Chem. Soc. 1975, 97, 6767. doi:10.1021/ja00856a028 |
| 5. | De Kimpe, N.; Verhé, R.; De Buyck, L.; Schamp, N. J. Org. Chem. 1978, 43, 2933. doi:10.1021/jo00408a047 |
© 2010 Schmidt and Margaretha; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)