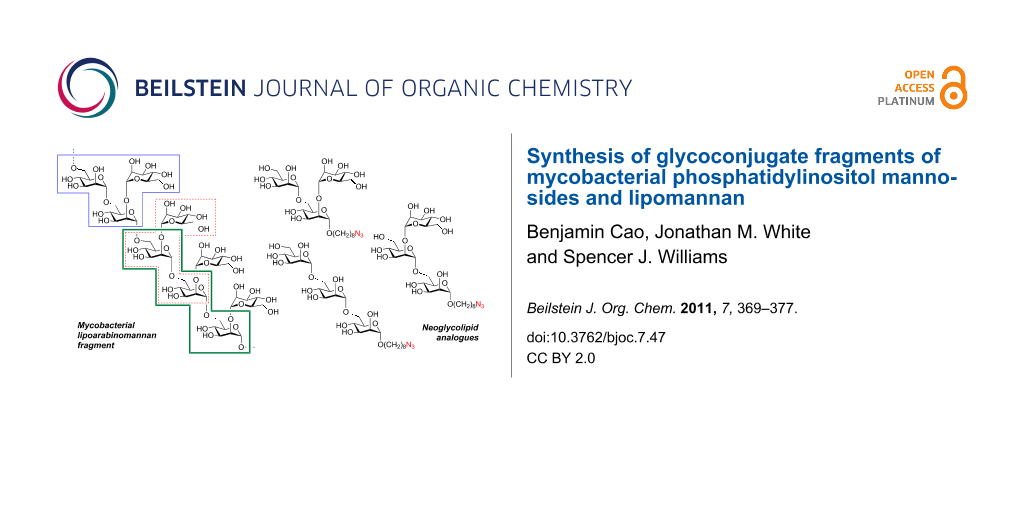
Abstract
Mycobacterium tuberculosis, the causitive agent of tuberculosis (TB), possesses a complex cell wall containing mannose-rich glycophospholids termed phosphatidylinositol mannosides (PIMs), lipomannan (LM), and lipoarabinomannan (LAM). These glycophospholipids play important roles in cell wall function and host–pathogen interactions. Synthetic PIM/LM/LAM substructures are useful biochemical tools to delineate and dissect the fine details of mannose glycophospholipid biosynthesis and their interactions with host cells. We report the efficient synthesis of a series of azidooctyl di- and trimannosides possessing the following glycan structures: α-Man-1,6-α-Man, α-Man-1,6-α-Man-1,6-α-Man, α-Man-1,2-α-Man-1,6-α-Man and 2,6-di-(α-Man)-α-Man. The synthesis includes the use of non-benzyl protecting groups compatible with the azido group and preparation of the branched trisaccharide structure 2,6-di-(α-Man)-α-Man through a double glycosylation of a 3,4-butanediacetal-protected mannoside. The azidooctyl groups of these synthetic mannans were elaborated to fluorescent glycoconjugates and squaric ester derivatives useful for further conjugation studies.
Graphical Abstract
Introduction
The incidence of TB is now at an all-time historical high with over 2 billion people infected globally [1]. TB is the leading infectious killer of people with HIV/AIDS and is second only to HIV/AIDS as an infectious cause of death for adults [2]. It is sobering that it has been more than 40 years since the last frontline TB drug (rifampicin) was deployed [3]. Drug resistance is now widespread and growing, underscoring the need for the development of new therapies to bolster the physician's armamentarium for TB control [3]. Many existing TB drugs target aspects of mycobacterial cell wall biosynthesis (e.g., thiacetazone, isoniazid, ethambutol, pyrazinamide, and ethionamide) with the cell wall of the tubercule bacillus being widely agreed as a promising target for new drugs [4,5]. The cell wall of all mycobacteria is especially rich in lipids and polysaccharides, with the major component being a macromolecule composed of mycolic acids, arabinogalactan, and peptidoglycan, termed the mycolyl–arabinogalactan–peptidoglycan complex [6,7]. One intriguing class of cell wall associated molecules are those based on a phosphatidylinositol (PI) core, which include the PIMs, LM, and LAM [8].
Through studies with gene deletion mutants of mycobacterial strains, several steps in the biosynthesis of the PIMs, LM and LAM have been shown to be essential for bacterial survival and it is now well appreciated that they are crucial cell-surface molecules that mediate host–pathogen interactions [8,9]. Biochemical studies support the general biosynthetic relationship PIMs → LM → LAM, although it is also clear that Ac2PIM2 and Ac2PIM6 represent important metabolic end products in their own right [10]. Scheme 1 summarizes the biosynthesis of the mannan core of the PIMs, LM and LAM. PIM biosynthesis commences with the stepwise transfer of two mannosyl residues onto inositol, catalyzed by the GDP-mannose dependent α-mannosyltransferases PimA [11] and PimB' [12,13], followed by acylation by the acyltransferase (Rv2611c) to give AcPIM2 [14,15]. Additional α-1,6-mannosylations of AcPIM2 give rise to AcPIM3 and AcPIM4, the last of which is hypothesized to be a key biosynthetic precursor for the synthesis of the so-called polar PIMs, AcPIM5 and Ac2PIM6, and LM and LAM [16].
Scheme 1: Indicative topology model for the biosynthesis of the glycophospholipids PIMs, LM and LAM in mycobacteria. The timing for translocation of PIM intermediates across the membrane is unclear. Hexagon = myo-inositol; closed circle = mannose; P = phosphate.
Scheme 1: Indicative topology model for the biosynthesis of the glycophospholipids PIMs, LM and LAM in mycoba...
The biosynthesis of LM and LAM (Scheme 1) commences from AcPIM4 with the installation of a linear α-1,6-linked mannan backbone on the terminal mannose [17]. Two α-1,6-mannosyltransferases, MptB and MptA, have been identified to be involved in the elongation of the LM backbone [18,19]. The linear backbone is then elaborated with single α-1,2-linked mannose residues to give mature LM [20]. LAM is formed by addition of arabinan to the penultimate mannose residue of LM, and is subsequently capped with a variety of groups including inositol phosphate, 5-methylthioxylose and its sulfoxide, and short 1,2-mannose oligomers [7].
Studies into the biosynthesis of the PIMs, LM and LAM have been greatly facilitated by the development of glycomimetic compounds. Homogeneous synthetic substructures have been used to deconvolute aspects of substrate recognition by biosynthetic enzymes and the structural determinants of host–pathogen interactions including antibody recognition and immune pattern-recognition systems such as the dendritic cell specific intercellular adhesion molecule-grabbing non-integrin (DC-SIGN) [8]. Thus, while total syntheses of many PIM structures have now been reported, the synthesis of substructures remains a worthwhile endeavor as these are useful to clarify fine details of enzymatic substrate recognition and are substantially easier to prepare [4,8]. As a shining example, synthetic octyl α-1,6-linked oligomannoside analogues of the 1,6-mannan core are effective substrates for mycobacterial cell free systems [21-23], and were used to confirm the activity of the polymerizing α-1,6-mannosyltransferases MptB and MptA [18,19], and to demonstrate functional compartmentalization of PPM synthase activity and MptB/MptA [24]. They have also been used as glycolipid substrates supporting the development of inhibitors of PIM/LM/LAM biosynthesis [25-28]. Various 1,2-linked aminooctyl oligomannosides corresponding to the capping groups of ManLAM were prepared and conjugated to carrier proteins and used to study antibody reactivity in a serological TB assay [29,30]. A complete set of the phosphoglycan head groups of PIM1–PIM6 with a thiol linker in place of the diacylglycerol were prepared and, following immobilization on glass slides, their binding to the lectin DC-SIGN was assessed [31].
Significant questions remain in the area of PIM/LM/LAM biosynthesis that could be assisted by suitable well-defined mannan substructures. For example, the identity of the α-1,2-mannosyltransferase(s) involved in the conversion of AcPIM4 → AcPIM5 → Ac2PIM6 remain incompletely characterized [17]. Similarly, the timing of the introduction of the single α-1,2-mannose residues onto the α-1,6-linked mannan core versus the elongation of this core is unclear [32]. For these reasons, we have undertaken the synthesis of a suite of fragments of the PIMs and LM, 1–4, and report their elaboration into glycoconjugates 5–10 for use as biological reagents to study PIM/LM/LAM biosynthesis and immunogenicity (Figure 1 and Figure 2). The azidooctyl aglycon has particular utility in this regard because of its (i) lipophilicity allowing biphasic partitioning between butanol/water or purification by reversed-phase extraction, (ii) ability to be reduced to an aminooctyl chain for use in squarate conjugation chemistry, and (iii) capacity to be conjugated with fluorescent terminal alkynes using the Cu(I)-catalyzed azide–alkyne cycloaddition (CuAAC) reaction [33,34].
Figure 1: Chemical structures of (A) a representative PIM, AcPIM5 and (B) a mannan fragment of LM from mycobacteria. Boxes denote the relationship of PIMs and LM to trisaccharide fragments synthesized in this study.
Figure 1: Chemical structures of (A) a representative PIM, AcPIM5 and (B) a mannan fragment of LM from mycoba...
Figure 2: Target di- and trisaccharide glycoconjugate fragments of PIMs and LM.
Figure 2: Target di- and trisaccharide glycoconjugate fragments of PIMs and LM.
Results and Discussion
Since their introduction by Palcic and co-workers [35], hydrophobic alkyl glycosides have proven to be valuable derivatives for enzymatic assays, as their lipophilic nature allows easy product isolation by either reversed-phase chromatography or simple solvent partitioning. Incorporation of an azidooctyl group confers many of the same benefits as an octyl aglycon, with the additional advantage that the azido group may be elaborated into glycoconjugates. However, the synthesis of azidooctyl glycosides can be challenging as the use of reductively-removed protecting groups such as benzyl ethers must be avoided owing to their incompatibility with the azido group when using H2/Pd or Na/NH3. We therefore sought to develop a synthesis based on the use of esters, silyl ethers and acetals only.
Synthesis of monosaccharide building blocks
Glycosidation of 8-azidooctan-1-ol (Supporting Information File 2) using glycosyl bromide 11 [36] in the presence of AgOTf, and debenzoylation of the crude product gave 12 in 81% yield over 2 steps (Scheme 2). Regioselective silylation of the primary alcohol of 12 with TPSCl followed by benzoylation of the remaining hydroxyl groups afforded the glycoside 13, which was desilylated with HF·pyridine complex to yield 14. This chemoselective transformation uses conditions that are similar to those reported by Tam et al. [23], and result in desilylation in a significantly shorter period than that previously reported using HCl in MeOH/Et2O [22]. The diol 15 was prepared by treatment of 12 with 2,3-butanedione and trimethyl orthoformate in the presence of catalytic acid in refluxing MeOH (Scheme 2) [37]. Trace amounts of the corresponding methyl glycoside were also obtained, arising from limited methanolysis of the glycosidic linkage.
Scheme 2: Synthesis of azidooctyl alcohol 14 and diol 15.
Scheme 2: Synthesis of azidooctyl alcohol 14 and diol 15.
For α-mannosylation of primary and secondary alcohols, the mannosyl donors 16 [38] and 17 were used. Treatment of glycosyl bromide 11 with NaBH4/KI in MeCN [39] afforded the crystalline 1,2-O-benzylidene acetal 18 as a single diastereoisomer in quantitative yield (Scheme 3). The stereochemistry of the benzylidene acetal 18 formed by this method has been studied by Suzuki et al. who assigned the product as the (7S)-stereoisomer (but reported it as the (7R)-isomer) by observation of a nuclear Overhauser effect transfer between the methine proton of the benzylidene acetal and H2 [40]. Unambiguous stereochemical assignment of (7S)-18 was achieved by single crystal X-ray analysis as shown in Figure 3, and is consistent with stereoselective delivery of hydride to the exo-face of the intermediate dioxolenium ion. Acetolysis of the benzylidene acetal 18 using 2% H2SO4/Ac2O provided diacetate 19 in 60% yield. Compound 19 was converted into the trichloroacetimidate 16 following the approach of Kong and coworkers [38] or to the thioglycoside 17 by treatment with p-thiocresol and BF3·Et2O in toluene.
Scheme 3: Synthesis of mannosyl donors 16 and 17.
Scheme 3: Synthesis of mannosyl donors 16 and 17.
![[1860-5397-7-47-3]](/bjoc/content/figures/1860-5397-7-47-3.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 3: ORTEP plot of single crystal X-ray determination of (7S)-18. Thermal ellipsoids denote 20% electron probability.
Figure 3: ORTEP plot of single crystal X-ray determination of (7S)-18. Thermal ellipsoids denote 20% electron...
Assembly of mannosides 1–4
Synthesis of the protected disaccharide 20 was achieved by glycosylation of 14 with the thioglycoside donor 21 [41] using NIS/TfOH in 84% yield (Scheme 4). The protected trisaccharide 25 was prepared by an approach similar to that reported for the corresponding octyl trisaccharide [22,23]. Thus glycosylation of 14 with the silylated donor 22 using NIS/TfOH afforded the silylated disaccharide 23 (99%). Compound 23 was desilylated using HF·pyridine and the primary alcohol 24 was glycosylated using thioglycoside 21 to give the trimannoside 25. Evidence for the exclusive formation of the α-anomer in all mannosylations in this work was obtained through measurement of the 1JC,H coupling constants for the anomeric carbons of the newly formed products. Each coupling constant was >170 Hz, thereby showing that all new O-glycosidic linkages were α-configured (Supporting Information File 2) [42]. Global debenzoylation of disaccharide 20 and trisaccharide 25 with catalytic NaOMe in MeOH/CH2Cl2 provided 1 and 2 in yields of 99% and 94%, respectively.
Scheme 4: Synthesis of disaccharide 1 and trisaccharide 2.
Scheme 4: Synthesis of disaccharide 1 and trisaccharide 2.
Our strategy towards the synthesis of the trisaccharide 3 sought to utilize a glycosyl donor possessing a 2-O-acetyl group with benzoyl groups at the remaining positions, anticipating that selective deacetylation post-glycosylation could be achieved to allow the subsequent synthesis of the α-Man-1,2-Man linkage (Scheme 5). Activation of a mixture of alcohol 14 and trichloroacetimidate 16 at 0 °C with 0.1 equiv of BF3·Et2O provided disaccharide 26 in only 21% yield, with a 1,2-glycosyl orthoester as the major product (40%). Orthoesters are common by-products of glycosylation reactions and typically rearrange under acidic conditions to give trans-linked glycosides [43]. Thus, 14 and 16 were treated with 0.25 equiv of an alternative Lewis acid, TMSOTf, and allowed to react for a longer time to allow the intermediate orthoester to isomerize. Under these conditions the disaccharide 26 was isolated in an improved yield of 63%. Also isolated was a 6-O-trimethylsilyl ether (6%), resulting from the reaction of alcohol 14 with TMSOTf. Better still, treatment of 14 and 16 with 0.25 equiv of BF3·Et2O afforded 26 in 76% yield. A similar outcome was obtained using NIS/TfOH activation of thioglycoside donor 17 to furnish disaccharide 26 in 72% yield. Selective deacetylation of 26 was achieved by acidic transesterification using 3% AcCl in MeOH/CH2Cl2 to give the secondary alcohol 27 in 73% yield. Mannosylation of 27 using donor 21 under NIS/TfOH activation gave trisaccharide 28 in 61% yield, and global debenzoylation proceeded smoothly to give 3.
The trisaccharide 4 was prepared by simultaneous glycosylation of the 2 and 6 positions of acceptor 15 (Scheme 6). Treatment of the diol 15 with 4 equiv of donor 21, and NIS/TfOH afforded the protected trisaccharide 29 in 68% yield. Deprotection was achieved by sequential treatment with TFA/H2O and NaOMe/MeOH to afford the trisaccharide 4.
Synthesis of glycoconjugates 5–10
Nitrobenzodiazole (NBD) dyes are useful fluorescent labels owing to their small size, low cost, superior water solubility relative to other common alternatives, and ability to be excited using visible, rather than ultraviolet light [44]. Hindsgaul and coworkers have reported the use of glycoconjugates with NBD dyes to streamline the detection of carbohydrate-lectin interactions and report that the NBD group displayed substantially less nonspecific interaction with proteins over other fluorescent dyes [45]. Using alkynyl-NBD 30 (prepared in one step from NBD chloride and propargylamine) [34], the disaccharide 1 and trisaccharides 2 and 3 were coupled upon treatment with CuSO4, sodium ascorbate and the Cu(I)-stabilizing ligand tris(benzyltriazolylmethyl)amine (TBTA) (Scheme 7) [46,47]. The resulting dye-labelled glycoconjugates 5–7 were isolated in 90–95% yields and possessed excellent fluorescent properties with λex = 400 nm and λem = 530 nm.
Scheme 7: Synthesis of glycoconjugates 5–7 and 8–10.
Scheme 7: Synthesis of glycoconjugates 5–7 and 8–10.
The squarate diester methodology introduced by Tietze and coworkers [48] and recently refined by the Kováč group [49] for the attachment of amine-derivatized carbohydrates to carrier proteins has particular advantages over other linker methodologies as diethyl squarate is commerically available and exhibits good selectivity in each coupling step, with the intermediate squaramate ester being sufficiently stable to allow its purification and storage. It should be noted that a key limitation of the methodology is the potential immunogenicity of the squarate group [50]. The three trisaccharides 2–4 were reduced to the aminooctyl derivatives by treatment with Ph3P in THF/water, and treated with diethyl squarate according to the procedure of Kováč (Scheme 7) [49]. Purification by reversed-phase chromatography afforded the ethyl squaramyl derivatives 8–10 in 84–94% yields.
Conclusion
We report the synthesis of four di/trisaccharide fragments of mycobacterial PIMs/LM/LAM and their elaboration to fluorescently-labelled glycoconjugates and haptens for the preparation of antigens. A readily prepared and crystalline 1,2-O-benzylidene acetal 18 has been used as a central precursor for the preparation of 2-O-acetyl mannosyl donors 16 and 17. The synthetic routes are compatible with the azido group.
Supporting Information
| Supporting Information File 1: Experimental part. | ||
| Format: PDF | Size: 310.2 KB | Download |
| Supporting Information File 2:
1H and 13C NMR spectra for new compounds and fluorescence spectra for 5–7.
The crystallographic data file for the structure reported in this paper has been deposited with the Cambridge Crystallographic Data Centre as file CCDC 804936 and is available on request from http://www.ccdc.cam.ac.uk/. |
||
| Format: PDF | Size: 1.0 MB | Download |
References
-
Dye, C.; Williams, B. G. Science 2010, 328, 856–861. doi:10.1126/science.1185449
Return to citation in text: [1] -
WHO: "Tuberculosis" Fact sheet No. 104, November 2010, http://www.who.int/mediacentre/factsheets/fs104/en/index.html (accessed Jan 18, 2011).
Return to citation in text: [1] -
Ma, Z.; Lienhardt, C.; McIlleron, H.; Nunn, A. J.; Wang, X. Lancet 2010, 375, 2100–2109. doi:10.1016/S0140-6736(10)60359-9
Return to citation in text: [1] [2] -
Umesiri, F. E.; Sanki, A. K.; Boucau, J.; Ronning, D. R.; Sucheck, S. J. Med. Res. Rev. 2010, 30, 290–326. doi:10.1002/med.20190
Return to citation in text: [1] [2] -
Janin, Y. L. Bioorg. Med. Chem. 2007, 15, 2479–2513. doi:10.1016/j.bmc.2007.01.030
Return to citation in text: [1] -
Brennan, P. J.; Nikaido, H. Annu. Rev. Biochem. 1995, 64, 29–63. doi:10.1146/annurev.bi.64.070195.000333
Return to citation in text: [1] -
Kaur, D.; Guerin, M. E.; Škovierová, H.; Brennan, P. J.; Jackson, M. Adv. Appl. Microbiol. 2009, 69, 23–78. doi:10.1016/S0065-2164(09)69002-X
Return to citation in text: [1] [2] -
Cao, B.; Williams, S. J. Nat. Prod. Rep. 2010, 27, 919–947. doi:10.1039/c000604a
Return to citation in text: [1] [2] [3] [4] -
Fischer, K.; Scotet, E.; Niemeyer, M.; Koebernick, H.; Zerrahn, J.; Maillet, S.; Hurwitz, R.; Kursar, M.; Bonneville, M.; Kaufmann, S. H. E.; Schaible, U. E. Proc. Natl. Acad. Sci. U. S. A. 2004, 101, 10685–10690. doi:10.1073/pnas.0403787101
Return to citation in text: [1] -
Guerin, M. E.; Kordulakova, J.; Alzari, P. M.; Brennan, P. J.; Jackson, M. J. Biol. Chem. 2010, 285, 33577–33583. doi:10.1074/jbc.R110.168328
Return to citation in text: [1] -
Korduláková, J.; Gilleron, M.; Mikuśová, K.; Puzo, G.; Brennan, P. J.; Gicquel, B.; Jackson, M. J. Biol. Chem. 2002, 277, 31335–31344. doi:10.1074/jbc.M204060200
Return to citation in text: [1] -
Lea-Smith, D. J.; Martin, K. L.; Pyke, J. S.; Tull, D.; McConville, M. J.; Coppel, R. L.; Crellin, P. K. J. Biol. Chem. 2008, 283, 6773–6782. doi:10.1074/jbc.M707139200
Return to citation in text: [1] -
Batt, S. M.; Jabeen, T.; Mishra, A. K.; Veerapen, N.; Krumbach, K.; Eggeling, L.; Besra, G. S.; Futterer, K. J. Biol. Chem. 2010, 37741–37752. doi:10.1074/jbc.M110.165407
Return to citation in text: [1] -
Korduláková, J.; Gilleron, M.; Puzo, G.; Brennan, P. J.; Gicquel, B.; Mikuśová, K.; Jackson, M. J. Biol. Chem. 2003, 278, 36285–36295. doi:10.1074/jbc.M303639200
Return to citation in text: [1] -
Guerin, M. E.; Kaur, D.; Somashekar, B. S.; Gibbs, S.; Gest, P.; Chatterjee, D.; Brennan, P. J.; Jackson, M. J. Biol. Chem. 2009, 284, 25687–25696. doi:10.1074/jbc.M109.030593
Return to citation in text: [1] -
Morita, Y. S.; Patterson, J. H.; Billman-Jacobe, H.; McConville, M. J. Biochem. J. 2004, 378, 589–597. doi:10.1042/BJ20031372
Return to citation in text: [1] -
Morita, Y. S.; Sena, C. B. C.; Waller, R. F.; Kurokawa, K.; Sernee, M. F.; Nakatani, F.; Haites, R. E.; Billman-Jacobe, H.; McConville, M. J.; Maeda, Y.; Kinoshita, T. J. Biol. Chem. 2006, 281, 25143–25155. doi:10.1074/jbc.M604214200
Return to citation in text: [1] [2] -
Mishra, A. K.; Alderwick, L. J.; Rittmann, D.; Tatituri, R. V. V.; Nigou, J.; Gilleron, M.; Eggeling, L.; Besra, G. S. Mol. Microbiol. 2007, 65, 1502–1517. doi:10.1111/j.1365-2958.2007.05884.x
Return to citation in text: [1] [2] -
Mishra, A. K.; Alderwick, L. J.; Rittman, D.; Wang, C.; Bhatt, A.; Jacobs, W. R., Jr.; Takayama, K.; Eggeling, L.; Besra, G. S. Mol. Microbiol. 2008, 68, 1595–1613. doi:10.1111/j.1365-2958.2008.06265.x
Return to citation in text: [1] [2] -
Kaur, D.; Obregon-Henao, A.; Pham, H.; Chatterjee, D.; Brennan, P. J.; Jackson, M. Proc. Natl. Acad. Sci. U. S. A. 2008, 105, 17973–17977. doi:10.1073/pnas.0807761105
Return to citation in text: [1] -
Brown, J. R.; Field, R. A.; Barker, A.; Guy, M.; Grewal, R.; Khoo, K. H.; Brennan, P. J.; Besra, G. S.; Chatterjee, D. Bioorg. Med. Chem. 2001, 9, 815–824. doi:10.1016/S0968-0896(00)00300-X
Return to citation in text: [1] -
Watt, J. A.; Williams, S. J. Org. Biomol. Chem. 2005, 3, 1982–1992. doi:10.1039/b503919c
Return to citation in text: [1] [2] [3] -
Tam, P. H.; Besra, G. S.; Lowary, T. L. ChemBioChem 2008, 9, 267–278. doi:10.1002/cbic.200700391
Return to citation in text: [1] [2] [3] -
Morita, Y. S.; Velasquez, R.; Taig, E.; Waller, R. F.; Patterson, J. H.; Tull, D.; Williams, S. J.; Billman-Jacobe, H.; McConville, M. J. J. Biol. Chem. 2005, 280, 21645–21652. doi:10.1074/jbc.M414181200
Return to citation in text: [1] -
Subramaniam, V.; Gurcha, S. S.; Besra, G. S.; Lowary, T. L. Tetrahedron: Asymmetry 2005, 16, 553–567. doi:10.1016/j.tetasy.2004.11.063
Return to citation in text: [1] -
Subramaniam, V.; Gurcha, S. S.; Besra, G. S.; Lowary, T. L. Bioorg. Med. Chem. 2005, 13, 1083–1094. doi:10.1016/j.bmc.2004.11.027
Return to citation in text: [1] -
Lo Conte, M.; Marra, A.; Chambery, A.; Gurcha, S. S.; Besra, G. S.; Dondoni, A. J. Org. Chem. 2010, 75, 6326–6336. doi:10.1021/jo100928g
Return to citation in text: [1] -
Tam, P. H.; Lowary, T. L. Org. Biomol. Chem. 2010, 8, 181–192. doi:10.1039/b916580k
Return to citation in text: [1] -
Gadikota, R. R.; Callam, C. S.; Appelmelk, B. J.; Lowary, T. L. J. Carbohydr. Chem. 2003, 22, 459–480. doi:10.1081/CAR-120025322
Return to citation in text: [1] -
Tong, M.; Jacobi, C. E.; van de Rijke, F. M.; Kujiper, S.; van de Werken, S.; Lowary, T. L.; Hokke, C. H.; Appelmelk, B. J.; Nagelkerke, N. J. D.; Tanke, H. J.; van Gijlswijk, R. P. M.; Veuskens, J.; Kolk, A. H. J.; Raap, A. K. J. Immunol. Methods 2005, 301, 154–163. doi:10.1016/j.jim.2005.04.004
Return to citation in text: [1] -
Boonyarattanakalin, S.; Liu, X.; Michieletti, M.; Lepenies, B.; Seeberger, P. H. J. Am. Chem. Soc. 2008, 130, 16791–16799. doi:10.1021/ja806283e
Return to citation in text: [1] -
Sena, C. B.; Fukuda, T.; Miyanagi, K.; Matsumoto, S.; Kobayashi, K.; Murakami, Y.; Maeda, Y.; Kinoshita, T.; Morita, Y. S. J. Biol. Chem. 2010, 285, 13326–13336. doi:10.1074/jbc.M109.077297
Return to citation in text: [1] -
Cheng, L.; Chen, Q.; Liu, J.; Du, Y. Carbohydr. Res. 2007, 342, 975. doi:10.1016/j.carres.2007.01.015
Return to citation in text: [1] -
McGill, N. W.; Williams, S. J. J. Org. Chem. 2009, 74, 9388–9398. doi:10.1021/jo902100q
Return to citation in text: [1] [2] -
Palcic, M. M.; Heerze, L. D.; Pierce, M.; Hindsgaul, O. Glycoconjugate J. 1988, 5, 49–63. doi:10.1007/BF01048331
Return to citation in text: [1] -
Ness, R. K.; Fletcher, H. G., Jr.; Hudson, C. S. J. Am. Chem. Soc. 1950, 71, 2200–2205. doi:10.1021/ja01161a091
Return to citation in text: [1] -
Hense, A.; Ley, S. V.; Osborn, H. M. I.; Owen, D. R.; Poisson, J.-F.; Warriner, S. L.; Wesson, K. E. J. Chem. Soc., Perkin Trans. 1 1997, 2023–2031. doi:10.1039/A702497E
Return to citation in text: [1] -
Heng, L.; Ning, J.; Kong, F. J. Carbohydr. Chem. 2001, 20, 285–296. doi:10.1081/CAR-100104864
Return to citation in text: [1] [2] -
Betaneli, V. I.; Ovchinnikov, M. V.; Backinowsky, L. V.; Kochetkov, N. K. Carbohydr. Res. 1982, 107, 285–291. doi:10.1016/S0008-6215(00)80547-9
Return to citation in text: [1] -
Suzuki, K.; Mizuta, T.; Yamaura, M. J. Carbohydr. Chem. 2003, 22, 143–147. doi:10.1081/CAR-120020483
Return to citation in text: [1] -
Tam, P.; Lowary, T. L. Carbohydr. Res. 2007, 342, 1741–1772. doi:10.1016/j.carres.2007.05.001
Return to citation in text: [1] -
Bock, K.; Pedersen, C. J. Chem. Soc., Perkin Trans. 2 1974, 293–299. doi:10.1039/p29740000293
Return to citation in text: [1] -
Kong, F. Carbohydr. Res. 2007, 342, 345–373. doi:10.1016/j.carres.2006.09.025
Return to citation in text: [1] -
Ghosh, P. B.; Whitehouse, M. W. Biochem. J. 1968, 108, 155.
Return to citation in text: [1] -
Dowlut, M.; Hall, D. G.; Hindsgaul, O. J. Org. Chem. 2005, 70, 9809–9813. doi:10.1021/jo051503w
Return to citation in text: [1] -
Donnelly, P. S.; Zanatta, S. D.; Zammit, S. C.; White, J. M.; Williams, S. J. Chem. Commun. 2008, 7, 2459–2461. doi:10.1039/b719724a
Return to citation in text: [1] -
Chan, T. R.; Hilgraf, R.; Sharpless, K. B.; Fokin, V. V. Org. Lett. 2004, 6, 2853–2855. doi:10.1021/ol0493094
Return to citation in text: [1] -
Tietze, L. F.; Schröter, C.; Gabius, S.; Brinck, U.; Goerlach-Graw, A.; Gabius, H.-J. Bioconjugate Chem. 1991, 2, 148–152. doi:10.1021/bc00009a003
Return to citation in text: [1] -
Hou, S.; Saksena, R.; Kováč, P. Carbohydr. Res. 2008, 343, 196–210. doi:10.1016/j.carres.2007.10.015
Return to citation in text: [1] [2] -
Wu, X.; Ling, C. C.; Bundle, D. R. Org. Lett. 2004, 6, 4407–4410. doi:10.1021/ol048614m
Return to citation in text: [1]
| 33. | Cheng, L.; Chen, Q.; Liu, J.; Du, Y. Carbohydr. Res. 2007, 342, 975. doi:10.1016/j.carres.2007.01.015 |
| 34. | McGill, N. W.; Williams, S. J. J. Org. Chem. 2009, 74, 9388–9398. doi:10.1021/jo902100q |
| 35. | Palcic, M. M.; Heerze, L. D.; Pierce, M.; Hindsgaul, O. Glycoconjugate J. 1988, 5, 49–63. doi:10.1007/BF01048331 |
| 36. | Ness, R. K.; Fletcher, H. G., Jr.; Hudson, C. S. J. Am. Chem. Soc. 1950, 71, 2200–2205. doi:10.1021/ja01161a091 |
| 38. | Heng, L.; Ning, J.; Kong, F. J. Carbohydr. Chem. 2001, 20, 285–296. doi:10.1081/CAR-100104864 |
| 41. | Tam, P.; Lowary, T. L. Carbohydr. Res. 2007, 342, 1741–1772. doi:10.1016/j.carres.2007.05.001 |
| 39. | Betaneli, V. I.; Ovchinnikov, M. V.; Backinowsky, L. V.; Kochetkov, N. K. Carbohydr. Res. 1982, 107, 285–291. doi:10.1016/S0008-6215(00)80547-9 |
| 40. | Suzuki, K.; Mizuta, T.; Yamaura, M. J. Carbohydr. Chem. 2003, 22, 143–147. doi:10.1081/CAR-120020483 |
| 37. | Hense, A.; Ley, S. V.; Osborn, H. M. I.; Owen, D. R.; Poisson, J.-F.; Warriner, S. L.; Wesson, K. E. J. Chem. Soc., Perkin Trans. 1 1997, 2023–2031. doi:10.1039/A702497E |
| 38. | Heng, L.; Ning, J.; Kong, F. J. Carbohydr. Chem. 2001, 20, 285–296. doi:10.1081/CAR-100104864 |
| 23. | Tam, P. H.; Besra, G. S.; Lowary, T. L. ChemBioChem 2008, 9, 267–278. doi:10.1002/cbic.200700391 |
| 22. | Watt, J. A.; Williams, S. J. Org. Biomol. Chem. 2005, 3, 1982–1992. doi:10.1039/b503919c |
| 22. | Watt, J. A.; Williams, S. J. Org. Biomol. Chem. 2005, 3, 1982–1992. doi:10.1039/b503919c |
| 23. | Tam, P. H.; Besra, G. S.; Lowary, T. L. ChemBioChem 2008, 9, 267–278. doi:10.1002/cbic.200700391 |
| 42. | Bock, K.; Pedersen, C. J. Chem. Soc., Perkin Trans. 2 1974, 293–299. doi:10.1039/p29740000293 |
| 43. | Kong, F. Carbohydr. Res. 2007, 342, 345–373. doi:10.1016/j.carres.2006.09.025 |
| 50. | Wu, X.; Ling, C. C.; Bundle, D. R. Org. Lett. 2004, 6, 4407–4410. doi:10.1021/ol048614m |
| 49. | Hou, S.; Saksena, R.; Kováč, P. Carbohydr. Res. 2008, 343, 196–210. doi:10.1016/j.carres.2007.10.015 |
| 48. | Tietze, L. F.; Schröter, C.; Gabius, S.; Brinck, U.; Goerlach-Graw, A.; Gabius, H.-J. Bioconjugate Chem. 1991, 2, 148–152. doi:10.1021/bc00009a003 |
| 49. | Hou, S.; Saksena, R.; Kováč, P. Carbohydr. Res. 2008, 343, 196–210. doi:10.1016/j.carres.2007.10.015 |
| 34. | McGill, N. W.; Williams, S. J. J. Org. Chem. 2009, 74, 9388–9398. doi:10.1021/jo902100q |
| 46. | Donnelly, P. S.; Zanatta, S. D.; Zammit, S. C.; White, J. M.; Williams, S. J. Chem. Commun. 2008, 7, 2459–2461. doi:10.1039/b719724a |
| 47. | Chan, T. R.; Hilgraf, R.; Sharpless, K. B.; Fokin, V. V. Org. Lett. 2004, 6, 2853–2855. doi:10.1021/ol0493094 |
| 45. | Dowlut, M.; Hall, D. G.; Hindsgaul, O. J. Org. Chem. 2005, 70, 9809–9813. doi:10.1021/jo051503w |
| 1. | Dye, C.; Williams, B. G. Science 2010, 328, 856–861. doi:10.1126/science.1185449 |
| 4. | Umesiri, F. E.; Sanki, A. K.; Boucau, J.; Ronning, D. R.; Sucheck, S. J. Med. Res. Rev. 2010, 30, 290–326. doi:10.1002/med.20190 |
| 5. | Janin, Y. L. Bioorg. Med. Chem. 2007, 15, 2479–2513. doi:10.1016/j.bmc.2007.01.030 |
| 18. | Mishra, A. K.; Alderwick, L. J.; Rittmann, D.; Tatituri, R. V. V.; Nigou, J.; Gilleron, M.; Eggeling, L.; Besra, G. S. Mol. Microbiol. 2007, 65, 1502–1517. doi:10.1111/j.1365-2958.2007.05884.x |
| 19. | Mishra, A. K.; Alderwick, L. J.; Rittman, D.; Wang, C.; Bhatt, A.; Jacobs, W. R., Jr.; Takayama, K.; Eggeling, L.; Besra, G. S. Mol. Microbiol. 2008, 68, 1595–1613. doi:10.1111/j.1365-2958.2008.06265.x |
| 3. | Ma, Z.; Lienhardt, C.; McIlleron, H.; Nunn, A. J.; Wang, X. Lancet 2010, 375, 2100–2109. doi:10.1016/S0140-6736(10)60359-9 |
| 20. | Kaur, D.; Obregon-Henao, A.; Pham, H.; Chatterjee, D.; Brennan, P. J.; Jackson, M. Proc. Natl. Acad. Sci. U. S. A. 2008, 105, 17973–17977. doi:10.1073/pnas.0807761105 |
| 3. | Ma, Z.; Lienhardt, C.; McIlleron, H.; Nunn, A. J.; Wang, X. Lancet 2010, 375, 2100–2109. doi:10.1016/S0140-6736(10)60359-9 |
| 16. | Morita, Y. S.; Patterson, J. H.; Billman-Jacobe, H.; McConville, M. J. Biochem. J. 2004, 378, 589–597. doi:10.1042/BJ20031372 |
| 2. | WHO: "Tuberculosis" Fact sheet No. 104, November 2010, http://www.who.int/mediacentre/factsheets/fs104/en/index.html (accessed Jan 18, 2011). |
| 17. | Morita, Y. S.; Sena, C. B. C.; Waller, R. F.; Kurokawa, K.; Sernee, M. F.; Nakatani, F.; Haites, R. E.; Billman-Jacobe, H.; McConville, M. J.; Maeda, Y.; Kinoshita, T. J. Biol. Chem. 2006, 281, 25143–25155. doi:10.1074/jbc.M604214200 |
| 10. | Guerin, M. E.; Kordulakova, J.; Alzari, P. M.; Brennan, P. J.; Jackson, M. J. Biol. Chem. 2010, 285, 33577–33583. doi:10.1074/jbc.R110.168328 |
| 12. | Lea-Smith, D. J.; Martin, K. L.; Pyke, J. S.; Tull, D.; McConville, M. J.; Coppel, R. L.; Crellin, P. K. J. Biol. Chem. 2008, 283, 6773–6782. doi:10.1074/jbc.M707139200 |
| 13. | Batt, S. M.; Jabeen, T.; Mishra, A. K.; Veerapen, N.; Krumbach, K.; Eggeling, L.; Besra, G. S.; Futterer, K. J. Biol. Chem. 2010, 37741–37752. doi:10.1074/jbc.M110.165407 |
| 8. | Cao, B.; Williams, S. J. Nat. Prod. Rep. 2010, 27, 919–947. doi:10.1039/c000604a |
| 9. | Fischer, K.; Scotet, E.; Niemeyer, M.; Koebernick, H.; Zerrahn, J.; Maillet, S.; Hurwitz, R.; Kursar, M.; Bonneville, M.; Kaufmann, S. H. E.; Schaible, U. E. Proc. Natl. Acad. Sci. U. S. A. 2004, 101, 10685–10690. doi:10.1073/pnas.0403787101 |
| 14. | Korduláková, J.; Gilleron, M.; Puzo, G.; Brennan, P. J.; Gicquel, B.; Mikuśová, K.; Jackson, M. J. Biol. Chem. 2003, 278, 36285–36295. doi:10.1074/jbc.M303639200 |
| 15. | Guerin, M. E.; Kaur, D.; Somashekar, B. S.; Gibbs, S.; Gest, P.; Chatterjee, D.; Brennan, P. J.; Jackson, M. J. Biol. Chem. 2009, 284, 25687–25696. doi:10.1074/jbc.M109.030593 |
| 8. | Cao, B.; Williams, S. J. Nat. Prod. Rep. 2010, 27, 919–947. doi:10.1039/c000604a |
| 6. | Brennan, P. J.; Nikaido, H. Annu. Rev. Biochem. 1995, 64, 29–63. doi:10.1146/annurev.bi.64.070195.000333 |
| 7. | Kaur, D.; Guerin, M. E.; Škovierová, H.; Brennan, P. J.; Jackson, M. Adv. Appl. Microbiol. 2009, 69, 23–78. doi:10.1016/S0065-2164(09)69002-X |
| 11. | Korduláková, J.; Gilleron, M.; Mikuśová, K.; Puzo, G.; Brennan, P. J.; Gicquel, B.; Jackson, M. J. Biol. Chem. 2002, 277, 31335–31344. doi:10.1074/jbc.M204060200 |
| 4. | Umesiri, F. E.; Sanki, A. K.; Boucau, J.; Ronning, D. R.; Sucheck, S. J. Med. Res. Rev. 2010, 30, 290–326. doi:10.1002/med.20190 |
| 8. | Cao, B.; Williams, S. J. Nat. Prod. Rep. 2010, 27, 919–947. doi:10.1039/c000604a |
| 7. | Kaur, D.; Guerin, M. E.; Škovierová, H.; Brennan, P. J.; Jackson, M. Adv. Appl. Microbiol. 2009, 69, 23–78. doi:10.1016/S0065-2164(09)69002-X |
| 8. | Cao, B.; Williams, S. J. Nat. Prod. Rep. 2010, 27, 919–947. doi:10.1039/c000604a |
| 17. | Morita, Y. S.; Sena, C. B. C.; Waller, R. F.; Kurokawa, K.; Sernee, M. F.; Nakatani, F.; Haites, R. E.; Billman-Jacobe, H.; McConville, M. J.; Maeda, Y.; Kinoshita, T. J. Biol. Chem. 2006, 281, 25143–25155. doi:10.1074/jbc.M604214200 |
| 32. | Sena, C. B.; Fukuda, T.; Miyanagi, K.; Matsumoto, S.; Kobayashi, K.; Murakami, Y.; Maeda, Y.; Kinoshita, T.; Morita, Y. S. J. Biol. Chem. 2010, 285, 13326–13336. doi:10.1074/jbc.M109.077297 |
| 29. | Gadikota, R. R.; Callam, C. S.; Appelmelk, B. J.; Lowary, T. L. J. Carbohydr. Chem. 2003, 22, 459–480. doi:10.1081/CAR-120025322 |
| 30. | Tong, M.; Jacobi, C. E.; van de Rijke, F. M.; Kujiper, S.; van de Werken, S.; Lowary, T. L.; Hokke, C. H.; Appelmelk, B. J.; Nagelkerke, N. J. D.; Tanke, H. J.; van Gijlswijk, R. P. M.; Veuskens, J.; Kolk, A. H. J.; Raap, A. K. J. Immunol. Methods 2005, 301, 154–163. doi:10.1016/j.jim.2005.04.004 |
| 31. | Boonyarattanakalin, S.; Liu, X.; Michieletti, M.; Lepenies, B.; Seeberger, P. H. J. Am. Chem. Soc. 2008, 130, 16791–16799. doi:10.1021/ja806283e |
| 24. | Morita, Y. S.; Velasquez, R.; Taig, E.; Waller, R. F.; Patterson, J. H.; Tull, D.; Williams, S. J.; Billman-Jacobe, H.; McConville, M. J. J. Biol. Chem. 2005, 280, 21645–21652. doi:10.1074/jbc.M414181200 |
| 25. | Subramaniam, V.; Gurcha, S. S.; Besra, G. S.; Lowary, T. L. Tetrahedron: Asymmetry 2005, 16, 553–567. doi:10.1016/j.tetasy.2004.11.063 |
| 26. | Subramaniam, V.; Gurcha, S. S.; Besra, G. S.; Lowary, T. L. Bioorg. Med. Chem. 2005, 13, 1083–1094. doi:10.1016/j.bmc.2004.11.027 |
| 27. | Lo Conte, M.; Marra, A.; Chambery, A.; Gurcha, S. S.; Besra, G. S.; Dondoni, A. J. Org. Chem. 2010, 75, 6326–6336. doi:10.1021/jo100928g |
| 28. | Tam, P. H.; Lowary, T. L. Org. Biomol. Chem. 2010, 8, 181–192. doi:10.1039/b916580k |
| 21. | Brown, J. R.; Field, R. A.; Barker, A.; Guy, M.; Grewal, R.; Khoo, K. H.; Brennan, P. J.; Besra, G. S.; Chatterjee, D. Bioorg. Med. Chem. 2001, 9, 815–824. doi:10.1016/S0968-0896(00)00300-X |
| 22. | Watt, J. A.; Williams, S. J. Org. Biomol. Chem. 2005, 3, 1982–1992. doi:10.1039/b503919c |
| 23. | Tam, P. H.; Besra, G. S.; Lowary, T. L. ChemBioChem 2008, 9, 267–278. doi:10.1002/cbic.200700391 |
| 18. | Mishra, A. K.; Alderwick, L. J.; Rittmann, D.; Tatituri, R. V. V.; Nigou, J.; Gilleron, M.; Eggeling, L.; Besra, G. S. Mol. Microbiol. 2007, 65, 1502–1517. doi:10.1111/j.1365-2958.2007.05884.x |
| 19. | Mishra, A. K.; Alderwick, L. J.; Rittman, D.; Wang, C.; Bhatt, A.; Jacobs, W. R., Jr.; Takayama, K.; Eggeling, L.; Besra, G. S. Mol. Microbiol. 2008, 68, 1595–1613. doi:10.1111/j.1365-2958.2008.06265.x |
© 2011 Cao et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)