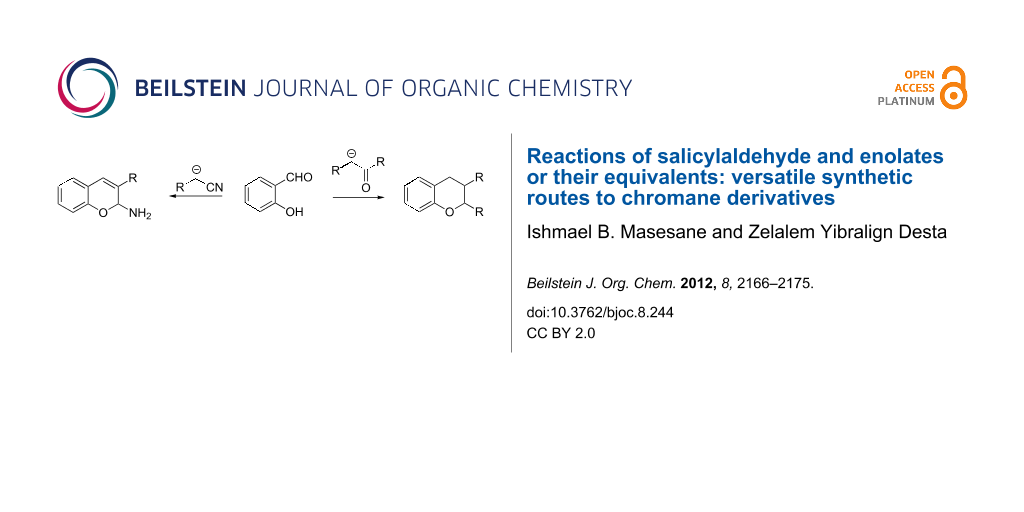
Abstract
The reported methodologies for the synthesis of chromane derivatives through the reaction of salicylaldehyde and enolates are discussed. The enolates and their equivalents involved in the reactions discussed in this article were derived from ketones, nitroalkanes, malononitrile and α,β-unsaturated compounds.
Graphical Abstract
Introduction
The chromane skeleton is found in a myriad of medicinally important compounds that have a broad range of biological activities [1-7]. Consequently, the synthesis of chromane derivatives has attracted the attention of synthetic chemists over the years [1-17]. Among the reported methodologies for the synthesis of chromane derivatives, the reaction of salicylaldehyde and enolates or their equivalents has gained a prominent position. The key features of the synthesis of chromane derivatives by the reaction of salicylaldehyde and enolates are summarized retrosynthetically in Scheme 1.
Scheme 1: Retrosynthetic analysis of chromane 1.
Scheme 1: Retrosynthetic analysis of chromane 1.
This review will summarize the reported methods for the syntheses of chromane derivatives from the reaction of salicylaldehyde and enolates or their equivalents. For the purposes of this review, chromane derivatives will include flavans, flavones, chromenes and chromones. The synthetic methods in the literature will be compared and contrasted in terms of their generality, selectivity and percentage yields.
Review
Chromane derivatives from the reaction of salicylaldehyde with enolates derived from ketones
The reaction of salicylaldehyde (5) and enolates derived from acetophenone (7) has been employed by a number of chemists in the synthesis of flavans and flavones. Flavans are chromane derivatives with a C-2 phenyl substituent while flavones are chromane derivatives with a carbonyl functional group at C-4, a carbon–carbon double bond between C-2 and C-3, and a C-2 phenyl substituent. The synthesis of flavans and flavones generally involves treatment of acetophenone (7) with a base to give enolate 8, which undergoes a Knoevenagel condensation with salicylaldehyde (5) to yield a chalcone 9. These chalcone derivatives are then cyclized by using various methodologies to give flavan 10 or flavone 11 (Scheme 2).
Scheme 2: General reaction of salicylaldehyde (5) and acetophenone (7) in the synthesis of flavan 10 and flavone 11.
Scheme 2: General reaction of salicylaldehyde (5) and acetophenone (7) in the synthesis of flavan 10 and flav...
Xue and co-workers have utilized the reaction of salicylaldehyde 12 and acetophenone 13 in the racemic synthesis of the naturally occurring flavan 16 (Scheme 3) [18]. To begin, a solution of 12 and 13 in CH3OH was stirred in the presence of KOH at room temperature to give chalcone 14. To set the stage for the cyclisation reaction, the trans carbon–carbon double bond must either be isomerized to the cis form or completely reduced. In this case, chalcone 14 was treated with H2 in the presence of a catalytic amount of Pd to give intermediate 15 in 99% yield. It is instructive to draw attention to the fact that both the carbon–carbon and carbon–oxygen double bonds of 14 were reduced by H2/Pd, a reagent usually used for the reduction of carbon–carbon double bonds. To complete the synthesis, Lewis acid mediated cyclization of intermediate 15 and acidic cleavage of the MOM protected hydroxy group delivered the desired flavan 16 in good yield.
Scheme 3: Synthesis of flavan 16 by Xue and co-workers.
Scheme 3: Synthesis of flavan 16 by Xue and co-workers.
On the basis of the above precedent by Xue and co-workers, our group accomplished the synthesis of an array of flavans of type 10 [19]. The synthesis begins with a Knoevenagel reaction of salicylaldehyde (5) and acetophenone derivatives 7 to give the corresponding chalcones of type 9 in 66–85% yields. Contrary to Xue’s reduction method where H2/Pd was used, we used NaBH4 in the reduction of both the carbon–carbon and carbon–oxygen double bonds of chalcone derivatives 9 to give the corresponding alcohols 17. It is noteworthy that the carbon–carbon double bond was also reduced by NaBH4, a reagent usually used for the reduction of carbonyl groups. Cyclization was achieved by heating intermediates 17 under reflux in acetic acid to give the corresponding flavans of type 10 in 62–87% yields (Scheme 4).
Scheme 4: Synthesis of flavans of type 10 by Mazimba and co-workers.
Scheme 4: Synthesis of flavans of type 10 by Mazimba and co-workers.
Recently, Sashidhara and co-workers achieved the synthesis of flavone 11 relying on the reaction of salicylaldehyde (5) and an enolate derived from acetophenone (7, Scheme 5) [20]. To begin, chalcone 9 was prepared in 85% yield by the Knoevenagel reaction of salicylaldehyde (5) and acetophenone (7) in the presence of KOH (aq) in ethanol as reported by Mazimba and co-workers. Chalcone 9 was then oxidatively cyclized in the presence of iodine and in a solvent-free environment to give flavone (11) in 72% yield. Methyl-, methoxy- and chloro-substituted acetophenones were also well tolerated in the reaction to give the corresponding flavones in comparable yields.
Scheme 5: Sashidhara and co-workers synthesis of flavone (11).
Scheme 5: Sashidhara and co-workers synthesis of flavone (11).
It is conceivable that enolates derived from other ketones instead of acetophenone could be reacted with salicylaldehyde to give chromane derivatives. To this end, Yu Ling and co-workers reported the efficient synthesis of chromane derivative 19 through the reaction of salicylaldehyde (5) with dimedone (18) in the presence of a catalytic amount of KF/Al2O3 (Scheme 6) [21]. The reaction is thought to proceed through a Knoevenagel condensation, a Michael addition and an intramolecular cyclization. The reaction was repeated with chloro-, bromo-, dichloro-, dibromo-, methyl- and nitro-substituted salicylaldehydes. The nitro- and 3,5-dibromo-substituted salicylaldehydes reacted with 18 to give the lowest yields of 60–70% while the other substituted salicylaldehydes reacted to give corresponding chromane derivatives in yields comparable to those achieved when 5 was used.
Scheme 6: Synthesis of chromane derivative 19 by Yu-Ling and co-workers.
Scheme 6: Synthesis of chromane derivative 19 by Yu-Ling and co-workers.
Chromane derivatives from the reactions of salicylaldehyde and enolate equivalents derived from malononitrile and its derivatives
The one-pot reaction of salicylaldehyde and malononitrile has proved to be an efficient method for the synthesis of 2-iminochromene derivatives. In general such synthetic procedures involve a Knoevenagel condensation followed by intramolecular cyclization. In a detailed study directed towards understanding the pathway of the reaction of salicylaldehyde (5) and malononitrile (20), Costa and co-workers reported the efficient synthesis of 2-iminochromene 21 in 90% yield [22]. This was achieved when salicylaldehyde (5) was reacted with 1 equivalent of malononitrile in the presence of Na2CO3 and H2O as the solvent (Scheme 7). A comparable yield was obtained when NaHCO3 was used as the base instead of Na2CO3. The use of 3-methoxy-, 3-hydroxy-, 5-bromo-, 4-N,N-diethylamino- and 5-bromo-3-methoxy-substituted salicylaldehydes gave corresponding 2-iminochromene derivatives in 86–100% yields, while the lowest yield of 62% was achieved when 3,4-dihydroxysalicylaldehyde was used.
Scheme 7: Synthesis of 2-iminochromene 21 by Costa and co-workers.
Scheme 7: Synthesis of 2-iminochromene 21 by Costa and co-workers.
Further studies by Costa and co-workers revealed that the reaction of salicyldehyde (5) with 2 equivalents of malonitrile (20) in the presence of NaHCO3 afforded 2-aminochromene 22 in 91% yield (Scheme 8) [22]. This product is thought to be the result of a Michael addition of the extra malononitrile to product 21.
Scheme 8: Synthesis of 2-aminochromene 22 by Costa and co-workers.
Scheme 8: Synthesis of 2-aminochromene 22 by Costa and co-workers.
In addition to inorganic bases such as Na2CO3 and NaHCO3, the use of amines in catalytic and quantitative amounts in the synthesis of chromane derivatives by the reaction of salicylaldehyde (5) and malononitrile (20) has been reported. Costa and co-workers used Et3N in the reaction of salicylaldehyde and 2 equivalents of malononitrile (20) in CH3OH to afford 2-aminochromene 24 in 94% yield (Scheme 9) [22].
Scheme 9: Costa and co-workers used Et3N in the synthesis of 2-aminochromene 24.
Scheme 9: Costa and co-workers used Et3N in the synthesis of 2-aminochromene 24.
In 2009, Shanthi and co-workers reported the use of the amino acid L-proline as a catalyst in a three component reaction of salicylaldehyde, malononitrile and indole for the synthesis of 2-aminochromene 27 in 90% yield (Scheme 10) [23] The synthesis proceeds through a cascade reaction of salicylaldehyde (5) and malononitrile (20) involving an aldol reaction followed by intramolecular cyclization and finally a dehydration to give intermediate 21. A subsequent Michael addition of the indole (25) to intermediate 21 gives cation 26, which loses a proton to give the product 27. Although Shanti and co-workers used a chiral catalyst, no data was provided on the stereoselectivity of this reaction.
Scheme 10: Synthesis of 2-aminochromene 27 by Shanthi and co-workers.
Scheme 10: Synthesis of 2-aminochromene 27 by Shanthi and co-workers.
In a study related to that of Shanti and co-workers, Yang and co-workers used chiral amine-thiourea catalyst 31 in a three-component enantioselective reaction of salicylaldehyde (5), acetonitrile (28) and nitromethane (30) to give 2-aminochromene 32 in 88% yield and 84% enatiomeric excess (Scheme 11) [24]. The reaction was found to be equally efficient when malononitrile (20) and cyanoacetate 29 were used instead of 28. The reaction is thought to proceed through a cascade reaction between salicylaldehyde (5) and acetonitrile (28) involving an aldol reaction, cyclization and dehydration. A subsequent Michael addition of nitromethane (30) to the product of the cascade reaction gave the desired product 32.
Scheme 11: Enantioselective synthesis of 2-aminochromenes 32–34 by Yang and co-workers.
Scheme 11: Enantioselective synthesis of 2-aminochromenes 32–34 by Yang and co-workers.
Kovalenko and co-workers used a quantitative amount of piperidine in the reaction of malononitrile derivative 35 as an enolate equivalent and salicylaldehydes 5 to give 2-iminochromenes 36 in good yields [25]. No Michael addition product was observed. 2-hydroxy-5-methoxybenzaldehyde (5a) gave product 36a in a higher yield of 81% compared to salicylaldehyde (5), which gave the corresponding product 36 in 71% yield (Scheme 12).
Scheme 12: Synthesis of 2-iminochromene derivatives of type 36 by Kovalenko and co-workers.
Scheme 12: Synthesis of 2-iminochromene derivatives of type 36 by Kovalenko and co-workers.
In another approach, Ghorbani-Vaghei and co-workers used a N,N,N’,N’-tetrabromobenzene-1,3-disulfonamide (TBBDA) mediated Knoevenagel reaction of salicylaldehyde (5) and two equivalents of malononitrile (20) or its derivative 29 to give the corresponding 2-aminochromene derivatives 22 and 37 in 92 and 82% yields respectively (Scheme 13) [26]. It is instructive to note that TBBDA is a versatile reagent in organic synthesis and has been reported to be efficient in oxidation of primary and secondary alcohols [27], in bromination of aromatic compounds [28], as catalytic reagents for silylation of alcohols, phenols, and thiols using hexamethyldisilazane [29], in conversion of urazoles to triazolinediones [30], and in oxidation of 1,3,5-trisubstituted pyrazolines [31].
Scheme 13: Synthesis of 2-aminochromenes 22 and 37 by Ghorbani-Vaghei and co-workers.
Scheme 13: Synthesis of 2-aminochromenes 22 and 37 by Ghorbani-Vaghei and co-workers.
Molecular sieves have been used as solid-phase catalysts in the preparation of 2-aminochromenes from salicylaldehyde derivatives and cyanoorganic compounds. Yu and co-workers reported the one-pot synthesis of 2-aminochromene 39 in 86% yield from the reaction of bromosalicylaldehyde 38 and cyanoacetate 29 in the presence of 3 Å molecular sieves (Scheme 14) [32]. Various derivatives of 39 were prepared in good yields by employing nitro-, methoxy-, and chloro-substituted salicylaldehydes instead of 38. Other solid catalysts such as 4 Å molecular sieves, 5 Å molecular sieves and Al2O3 were found to be effective in catalyzing the reaction but resulted in lower yields (50–63%) of product 39 [32].
Scheme 14: Synthesis of 2-aminochromene 39 by Yu and co-workers.
Scheme 14: Synthesis of 2-aminochromene 39 by Yu and co-workers.
Heravi and co-workers, on the other hand, used a mesoporous molecular sieves (MCM-41)-catalyzed Knoevenagel reaction of salicylaldehyde (5) and malononitrile (20) to give 2-iminochromene 21 in 94% yield (Scheme 15) [33]. The generality of Haravi’s method was demonstrated by the reactions of 3-hydroxy-, 4-hydroxy-, 5-hydroxy-, 4-methoxy- and 5-bromosalicylaldehyde with malononitrile (20) to give the corresponding 2-iminochromene derivatives in yields of at least 90%. MCM-41 can be reused for up to five cycles with an insignificant drop in percentage yields (80%).
Scheme 15: Synthesis of 2-iminochromene 21 by Heravi and co-workers.
Scheme 15: Synthesis of 2-iminochromene 21 by Heravi and co-workers.
At this juncture, it is instructive to draw attention to the fact that the yields of the molecular-sieve-catalyzed reactions of salicylaldehydes and enolate equivalents derived from malononitrile and its derivatives are comparable to those of reactions mediated by inorganic bases such as Na2CO3 (Scheme 7) and NaHCO3 (Scheme 8). However molecular sieves have the advantage that they are recyclable.
Chromane derivatives from the reaction of salicylaldehyde and enolates derived from α,β-unsaturated compounds
The tandem reaction of salicylaldehyde and α,β-unsaturated compounds has proved to be a reliable route to chromane derivatives. In general, this reaction involves an oxo-Michael addition of salicylaldehyde (5) to α,β-unsaturated compounds of type 40 to give enolate intermediates of type 41. Enolate intermediates 41 then undergo an intramolecular Knoevenagel condensation to give chromane derivatives 42 (Scheme 16).
Scheme 16: Tandem reaction of salicylaldehyde and α,β-unsaturated compounds.
Scheme 16: Tandem reaction of salicylaldehyde and α,β-unsaturated compounds.
Kawase and co-workers reported the K2CO3-mediated tandem reaction of salicylaldehyde derivatives of type 43 and α,β-unsaturated ester 44 in the synthesis of 2,2-dimethylchromene 45 in moderate yields (Scheme 17) [34]. The dehydration reaction in this case was accompanied by decarboxylation. The best yields were achieved when methoxy-, methyl-, chloro-, bromo- and phenyl-substituted salicylaldehydes were used as reagents. The nitro-, hydroxy-, ethoxy- and acetyl-substituted salicylaldehydes on the other hand gave poor yields or no products at all. Related reactions involving a K2CO3-mediated tandem reaction of salicylaldehyde with acrolein and alkenes with two electron withdrawing groups to give the corresponding chromane derivatives have been reported [35-37]. The percentage yields of the chromane derivatives in these reports were comparable to those reported by Kawase and co-workers.
Scheme 17: Kawase and co-workers synthesis of 2,2-dimethylchromene 45.
Scheme 17: Kawase and co-workers synthesis of 2,2-dimethylchromene 45.
In addition to K2CO3, tertiary-amine-mediated tandem reactions of salicylaldehyde and α,β-unsaturated compounds to give chromane derivatives have been reported. Stukan and co-workers, for example, used an Et3N-mediated reaction of salicylaldehyde (5) and nitropropene 46 in the synthesis of 2,3-disubstituted chromene 47 in a low yield of 28% (Scheme 18) [38]. Slightly better yields (33–40%) were achieved when 5-bromo-, 5-chloro- and 3,5-dichloro-substituted salicylaldehydes were employed in the reaction.
Scheme 18: Synthesis of 2,3-disubstituted chromene 47 by Stukan and co-workers.
Scheme 18: Synthesis of 2,3-disubstituted chromene 47 by Stukan and co-workers.
Ravichandran utilized a classical 1,4-diazabicyclo[2.2.2]octane (DABCO)-catalyzed Baylis–Hillman reaction of salicylaldehyde (5) and α,β-unsaturated compounds 48–51 in the synthesis of the corresponding chromenes 52–55 (Scheme 19) [39]. These reactions were performed in water as the solvent and the chromenes were isolated in yields of 71–79%. It is instructive to note that the Baylis–Hillman products were not detected or isolated in this work.
Scheme 19: Ravichandrans synthesis of 3-substituted chromenes 52–55.
Scheme 19: Ravichandrans synthesis of 3-substituted chromenes 52–55.
The mechanism of the DABCO-catalyzed reaction of salicylaldehyde and α,β-unsaturated compounds in the synthesis 3-substituted chromenes was proved to proceed through the Baylis–Hillman reaction by Kaye and co-workers [40,41] Their work involved the reaction of salicylaldehyde (5) with tert-butyl acrylate (56) to give the Baylis–Hillman product 57, which was subsequently cyclized in the presence of acetic acid to give chromene 58 in a low yield of 24%, together with coumarin 59 in 40% yield (Scheme 20).
Scheme 20: Synthesis of 3-substituted chromene 58 coumarin 59 by Paye and co-workers.
Scheme 20: Synthesis of 3-substituted chromene 58 coumarin 59 by Paye and co-workers.
An asymmetric amine-catalyzed reaction of salicylaldehyde (5) and α,β-unsaturated aldehyde 60 in the synthesis of 2-phenylchromene (62) was reported by Govender and co-workers [42]. The asymmetric union of salicylaldehyde (5) and aldehyde 60 was brought about by dissolving these two substances in CH2Cl2 in the presence of catalytic amounts of TMS-protected prolinol derivative 61 (Scheme 21). Methoxysalicylaldehyde 5a reacted much faster than salicylaldehyde (5) with higher isolated yield of 2-phenychromene 63 but at the expense of enantioselectivity. The best enantioselectivity (90% ee) was achieved when the aliphatic aldehyde 2-hexenal was used in the reaction instead of 60. However, the reaction suffered from very poor yields (15–21%). The reaction is thought to proceed through the condensation of aldehyde 60 and prolinol 61 to give a chiral iminium-ion intermediate. This intermediate then undergoes a domino reaction involving a Michael reaction with salicylaldehyde (5), followed by an intramolecular aldol reaction and final dehydration to give the desired chromene derivative.
Scheme 21: Govender and co-workers asymmetric synthesis of 2-phenylchromenes 62 and 63.
Scheme 21: Govender and co-workers asymmetric synthesis of 2-phenylchromenes 62 and 63.
Related work involving asymmetric reaction of salicylaldehyde derivatives and α,β-unsaturated carbonyl compounds in the synthesis of 2-phenylchromenes was reported by Li and co-workers (Scheme 22) [43]. Their strategy involved the reaction of salicylaldehyde (5) and unsaturated aldehyde 60 in the presence of catalytic amounts of TES-protected prolinol 64 and benzoic acid. High yields (87%) and excellent enantioselectivity (88%) of 2-phenylchromene 62 were achieved when the reaction was performed in 1,2-dichloroethane as the solvent. The presence of the benzoic acid additive is thought to be responsible for the increase in the enantioselectivity and higher yields of this reaction when compared to that of Govender and co-workers. It is also instructive to note that the catalyst loading for Li and co-workers was three times higher than that for Govender and co-workers.
Scheme 22: Asymmetric synthesis of 2-phenylchromene 62 by Li and co-workers.
Scheme 22: Asymmetric synthesis of 2-phenylchromene 62 by Li and co-workers.
Conclusion
This paper has demonstrated the versatility of the reactions of salicylaldehyde with enolates or their equivalents in the synthesis of chromane derivatives. These reactions can be run under quite mild conditions and are ideal for the synthesis of chromane derivatives due to their operational simplicity. The development of enantioselective reactions of salicylaldehyde and enolates to give nearly optically pure chromane derivatives is a memorable highlight of this review. Future work will undoubtedly focus on transformation of the products of the discussed reactions of salicylaldehyde with enolates to biologically active compounds and natural products.
References
-
Chang, S.; Grubbs, R. H. J. Org. Chem. 1998, 63, 864–866. doi:10.1021/jo9712198
Return to citation in text: [1] [2] -
Gowrisankar, S.; Lee, K.-Y.; Kim, J.-N. Bull. Korean Chem. Soc. 2007, 28, 624–628. doi:10.5012/bkcs.2007.28.4.624
Return to citation in text: [1] [2] -
Ibrahim, M. A.; Ali, T. E.; Alnamer, Y. A.; Gabr, Y. A. ARKIVOC 2010, (i), 98–135.
Return to citation in text: [1] [2] -
Khadem, S.; Marles, R. J. Molecules 2012, 17, 191–206. doi:10.3390/molecules17010191
Return to citation in text: [1] [2] -
Patil, R. B.; Sawant, S. D.; Thombare, P. A. Int. J. Pharm. Tech. Res. 2012, 4, 375–381.
Return to citation in text: [1] [2] -
Corradini, E.; Foglia, P.; Giansanti, P.; Gubbiotti, R.; Samperi, R.; Laganà, A. Nat. Prod. Res. 2011, 25, 469–495. doi:10.1080/14786419.2010.482054
Return to citation in text: [1] [2] -
Verma, A. K.; Pratap, R. Tetrahedron 2012, 68, 8523–8538. doi:10.1016/j.tet.2012.06.097
Return to citation in text: [1] [2] -
Cha, J.-H.; Cho, Y.-S.; Koh, H.-Y.; Lee, E.; Kim, Y.-T.; Yang, H.-H.; Kang, H.-Y. Bull. Korean Chem. Soc. 2004, 25, 1123–1124. doi:10.5012/bkcs.2004.25.8.1123
Return to citation in text: [1] -
Lee, J.-I.; Son, H.-S.; Jung, M.-G. Bull. Korean Chem. Soc. 2005, 26, 1461–1463. doi:10.5012/bkcs.2005.26.9.1461
Return to citation in text: [1] -
Miyazaki, H.; Honda, Y.; Honda, K.; Inoue, S. Tetrahedron Lett. 2000, 41, 2643–2647. doi:10.1016/S0040-4039(00)00236-7
Return to citation in text: [1] -
Petasis, N. A.; Butkevich, A. J. Organomet. Chem. 2009, 694, 1747–1753. doi:10.1016/j.jorganchem.2008.11.050
Return to citation in text: [1] -
Rodriguez, I.; Iborra, S.; Rey, F.; Corma, A. Appl. Catal., A: Gen. 2000, 194–195, 241–252. doi:10.1016/S0926-860X(99)00371-3
Return to citation in text: [1] -
Shi, Y.; Shi, M. Org. Biomol. Chem. 2007, 5, 1499–1504. doi:10.1039/b618984a
Return to citation in text: [1] -
Wang, Q.; Finn, M. G. Org. Lett. 2000, 2, 4063–4065. doi:10.1021/ol006710r
Return to citation in text: [1] -
Yadav, J. S.; Reddy, B. V. S.; Chandraiah, L.; Jagannadh, B.; Kumar, S. K.; Kunwar, A. C. Tetrahedron Lett. 2002, 43, 4527–4530. doi:10.1016/S0040-4039(02)00816-X
Return to citation in text: [1] -
Yadav, J. S.; Reddy, B. V. S.; Parisse, C.; Carvalho, P.; Rao, T. P. Tetrahedron Lett. 2002, 43, 2999–3002. doi:10.1016/S0040-4039(02)00440-9
Return to citation in text: [1] -
Yadav, J. S.; Reddy, B. V. S.; Aruna, M.; Venugopal, C.; Ramalingam, T.; Kumar, S. K.; Kunwar, A. C. J. Chem. Soc., Perkin Trans. 1 2002, 165–171. doi:10.1039/B109538M
Return to citation in text: [1] -
Xue, J. J.; Zhang, X. S.; Liang, X. Z.; Li, Y. Chin. Chem. Lett. 2003, 14, 443–444.
Return to citation in text: [1] -
Mazimba, O.; Masesane, I. B.; Majinda, R. R. Tetrahedron Lett. 2011, 51, 6716–6718. doi:10.1016/j.tetlet.2011.09.147
Return to citation in text: [1] -
Sashidhara, K. V.; Kumar, M.; Kumar, A. Tetrahedron Lett. 2012, 53, 2355–2359. doi:10.1016/j.tetlet.2012.02.108
Return to citation in text: [1] -
Li, Y.; Chen, H.; Zeng, Z.; Wang, X.; Shi, D.; Tu, S. Chin. J. Org. Chem. 2005, 25, 846–849.
Return to citation in text: [1] -
Costa, M.; Areias, F.; Abrunhosa, L.; Venancio, A.; Proenca, F. J. Org. Chem. 2008, 73, 1954–1962. doi:10.1021/jo702552f
Return to citation in text: [1] [2] [3] -
Shanthi, G.; Perumal, P. T.; Rao, U.; Sehgal, P. K. Indian J. Chem. 2009, 48B, 1319–1323.
Return to citation in text: [1] -
Yang, G.; Luo, C.; Mu, X.; Wang, T.; Liu, X.-Y. Chem. Commun. 2012, 48, 5880–5882. doi:10.1039/c2cc30731f
Return to citation in text: [1] -
Kovalenko, S. M.; Bylov, I. E.; Sytnik, K. M.; Chernykh, V. P.; Bilokin, Y. V. Molecules 2000, 5, 1146–1165. doi:10.3390/51001146
Return to citation in text: [1] -
Ghorbani-Vaghei, R.; Toghraei-Semiromi, Z.; Karimi-Nami, R. J. Braz. Chem. Soc. 2011, 22, 905–909.
Return to citation in text: [1] -
Ghorbani-Vaghei, R.; Veisi, H.; Amiri, M. J. Chin. Chem. Soc. 2007, 54, 1257–1260.
Return to citation in text: [1] -
Ghorbani-Vaghei, R.; Jalili, H. Synthesis 2005, 1099–1102. doi:10.1055/s-2005-861851
Return to citation in text: [1] -
Ghorbani-Vaghei, R.; Zolfigol, M.; Chegeny, M.; Veisi, H. Tetrahedron Lett. 2006, 47, 4505–4508. doi:10.1016/j.tetlet.2006.03.157
Return to citation in text: [1] -
Zolfigol, M. A.; Ghorbani-Vaghei, R.; Mallakpour, S.; Chehardoli, G.; Choghamarani, A. G.; Yazdi, A. H. Synthesis 2006, 1631–1634. doi:10.1055/s-2006-926446
Return to citation in text: [1] -
Ghorbani-Vaghei, R.; Azarifar, D.; Maleki, B. Bull. Korean Chem. Soc. 2004, 25, 953–954. doi:10.5012/bkcs.2004.25.7.953
Return to citation in text: [1] -
Yu, N.; Aramini, J. M.; Germann, M. W.; Huang, Z. Tetrahedron Lett. 2000, 41, 6993–6996. doi:10.1016/S0040-4039(00)01195-3
Return to citation in text: [1] [2] -
Heravi, M. M.; Poormohammad, N.; Yahia, Sh.; Beheshtiha, Y. S.; Baghernejad, B.; Malakooti, R. Bull. Chem. Soc. Ethiop. 2010, 24, 273–276.
Return to citation in text: [1] -
Kawase, Y.; Yamaguchi, S.; Horita, H.; Taneko, J.; Kameyama, H. Bull. Chem. Soc. Jpn. 1982, 55, 1153–1155. doi:10.1246/bcsj.55.1153
Return to citation in text: [1] -
Conti, C.; Desideri, N. Bioorg. Med. Chem. 2010, 18, 6480–6488. doi:10.1016/j.bmc.2010.06.103
Return to citation in text: [1] -
Yamaguchi, S.; Saitoh, T.; Kamiumezawa, M.; Enomoto, H.; Kawase, Y. J. Heterocycl. Chem. 1992, 29, 755–758. doi:10.1002/jhet.5570290412
Return to citation in text: [1] -
Sharma, K. K.; Krupadanam, G. L. D. Synth. Commun. 2002, 32, 1557–1562. doi:10.1081/SCC-120004146
Return to citation in text: [1] -
Stukan, E. V.; Makarenko, S. V.; Berestovitskaya, V. M. Russ. J. Gen. Chem. 2011, 81, 155–157. doi:10.1134/S1070363211010294
Return to citation in text: [1] -
Ravichandran, S. Synth. Commun. 2001, 31, 1233–1235. doi:10.1081/SCC-100104009
Return to citation in text: [1] -
Kaye, P. T.; Musa, M. A.; Xolani, W.; Nocanda, X. N.; Robinson, R. S. Org. Biomol. Chem. 2003, 1, 1133–1138. doi:10.1039/b300360d
Return to citation in text: [1] -
Musa, M. A. Applications of the Baylis-Hillman reaction in the synthesis of coumarin derivatives. Ph.D. Thesis, Rhodes University, 2002.
http://eprints.ru.ac.za/2319/
Return to citation in text: [1] -
Govender, T.; Hojabri, L.; Moghaddam, F. M.; Arvidsson, P. I. Tetrahedron: Asymmetry 2006, 17, 1763–1767. doi:10.1016/j.tetasy.2006.06.028
Return to citation in text: [1] -
Li, H.; Wang, J.; E-Nunu, T.; Zu, L.; Jiang, W.; Wei, S.; Wang, W. Chem. Commun. 2007, 507–509. doi:10.1039/b611502k
Return to citation in text: [1]
| 43. | Li, H.; Wang, J.; E-Nunu, T.; Zu, L.; Jiang, W.; Wei, S.; Wang, W. Chem. Commun. 2007, 507–509. doi:10.1039/b611502k |
| 1. | Chang, S.; Grubbs, R. H. J. Org. Chem. 1998, 63, 864–866. doi:10.1021/jo9712198 |
| 2. | Gowrisankar, S.; Lee, K.-Y.; Kim, J.-N. Bull. Korean Chem. Soc. 2007, 28, 624–628. doi:10.5012/bkcs.2007.28.4.624 |
| 3. | Ibrahim, M. A.; Ali, T. E.; Alnamer, Y. A.; Gabr, Y. A. ARKIVOC 2010, (i), 98–135. |
| 4. | Khadem, S.; Marles, R. J. Molecules 2012, 17, 191–206. doi:10.3390/molecules17010191 |
| 5. | Patil, R. B.; Sawant, S. D.; Thombare, P. A. Int. J. Pharm. Tech. Res. 2012, 4, 375–381. |
| 6. | Corradini, E.; Foglia, P.; Giansanti, P.; Gubbiotti, R.; Samperi, R.; Laganà, A. Nat. Prod. Res. 2011, 25, 469–495. doi:10.1080/14786419.2010.482054 |
| 7. | Verma, A. K.; Pratap, R. Tetrahedron 2012, 68, 8523–8538. doi:10.1016/j.tet.2012.06.097 |
| 20. | Sashidhara, K. V.; Kumar, M.; Kumar, A. Tetrahedron Lett. 2012, 53, 2355–2359. doi:10.1016/j.tetlet.2012.02.108 |
| 28. | Ghorbani-Vaghei, R.; Jalili, H. Synthesis 2005, 1099–1102. doi:10.1055/s-2005-861851 |
| 19. | Mazimba, O.; Masesane, I. B.; Majinda, R. R. Tetrahedron Lett. 2011, 51, 6716–6718. doi:10.1016/j.tetlet.2011.09.147 |
| 29. | Ghorbani-Vaghei, R.; Zolfigol, M.; Chegeny, M.; Veisi, H. Tetrahedron Lett. 2006, 47, 4505–4508. doi:10.1016/j.tetlet.2006.03.157 |
| 18. | Xue, J. J.; Zhang, X. S.; Liang, X. Z.; Li, Y. Chin. Chem. Lett. 2003, 14, 443–444. |
| 26. | Ghorbani-Vaghei, R.; Toghraei-Semiromi, Z.; Karimi-Nami, R. J. Braz. Chem. Soc. 2011, 22, 905–909. |
| 1. | Chang, S.; Grubbs, R. H. J. Org. Chem. 1998, 63, 864–866. doi:10.1021/jo9712198 |
| 2. | Gowrisankar, S.; Lee, K.-Y.; Kim, J.-N. Bull. Korean Chem. Soc. 2007, 28, 624–628. doi:10.5012/bkcs.2007.28.4.624 |
| 3. | Ibrahim, M. A.; Ali, T. E.; Alnamer, Y. A.; Gabr, Y. A. ARKIVOC 2010, (i), 98–135. |
| 4. | Khadem, S.; Marles, R. J. Molecules 2012, 17, 191–206. doi:10.3390/molecules17010191 |
| 5. | Patil, R. B.; Sawant, S. D.; Thombare, P. A. Int. J. Pharm. Tech. Res. 2012, 4, 375–381. |
| 6. | Corradini, E.; Foglia, P.; Giansanti, P.; Gubbiotti, R.; Samperi, R.; Laganà, A. Nat. Prod. Res. 2011, 25, 469–495. doi:10.1080/14786419.2010.482054 |
| 7. | Verma, A. K.; Pratap, R. Tetrahedron 2012, 68, 8523–8538. doi:10.1016/j.tet.2012.06.097 |
| 8. | Cha, J.-H.; Cho, Y.-S.; Koh, H.-Y.; Lee, E.; Kim, Y.-T.; Yang, H.-H.; Kang, H.-Y. Bull. Korean Chem. Soc. 2004, 25, 1123–1124. doi:10.5012/bkcs.2004.25.8.1123 |
| 9. | Lee, J.-I.; Son, H.-S.; Jung, M.-G. Bull. Korean Chem. Soc. 2005, 26, 1461–1463. doi:10.5012/bkcs.2005.26.9.1461 |
| 10. | Miyazaki, H.; Honda, Y.; Honda, K.; Inoue, S. Tetrahedron Lett. 2000, 41, 2643–2647. doi:10.1016/S0040-4039(00)00236-7 |
| 11. | Petasis, N. A.; Butkevich, A. J. Organomet. Chem. 2009, 694, 1747–1753. doi:10.1016/j.jorganchem.2008.11.050 |
| 12. | Rodriguez, I.; Iborra, S.; Rey, F.; Corma, A. Appl. Catal., A: Gen. 2000, 194–195, 241–252. doi:10.1016/S0926-860X(99)00371-3 |
| 13. | Shi, Y.; Shi, M. Org. Biomol. Chem. 2007, 5, 1499–1504. doi:10.1039/b618984a |
| 14. | Wang, Q.; Finn, M. G. Org. Lett. 2000, 2, 4063–4065. doi:10.1021/ol006710r |
| 15. | Yadav, J. S.; Reddy, B. V. S.; Chandraiah, L.; Jagannadh, B.; Kumar, S. K.; Kunwar, A. C. Tetrahedron Lett. 2002, 43, 4527–4530. doi:10.1016/S0040-4039(02)00816-X |
| 16. | Yadav, J. S.; Reddy, B. V. S.; Parisse, C.; Carvalho, P.; Rao, T. P. Tetrahedron Lett. 2002, 43, 2999–3002. doi:10.1016/S0040-4039(02)00440-9 |
| 17. | Yadav, J. S.; Reddy, B. V. S.; Aruna, M.; Venugopal, C.; Ramalingam, T.; Kumar, S. K.; Kunwar, A. C. J. Chem. Soc., Perkin Trans. 1 2002, 165–171. doi:10.1039/B109538M |
| 27. | Ghorbani-Vaghei, R.; Veisi, H.; Amiri, M. J. Chin. Chem. Soc. 2007, 54, 1257–1260. |
| 22. | Costa, M.; Areias, F.; Abrunhosa, L.; Venancio, A.; Proenca, F. J. Org. Chem. 2008, 73, 1954–1962. doi:10.1021/jo702552f |
| 24. | Yang, G.; Luo, C.; Mu, X.; Wang, T.; Liu, X.-Y. Chem. Commun. 2012, 48, 5880–5882. doi:10.1039/c2cc30731f |
| 22. | Costa, M.; Areias, F.; Abrunhosa, L.; Venancio, A.; Proenca, F. J. Org. Chem. 2008, 73, 1954–1962. doi:10.1021/jo702552f |
| 25. | Kovalenko, S. M.; Bylov, I. E.; Sytnik, K. M.; Chernykh, V. P.; Bilokin, Y. V. Molecules 2000, 5, 1146–1165. doi:10.3390/51001146 |
| 22. | Costa, M.; Areias, F.; Abrunhosa, L.; Venancio, A.; Proenca, F. J. Org. Chem. 2008, 73, 1954–1962. doi:10.1021/jo702552f |
| 21. | Li, Y.; Chen, H.; Zeng, Z.; Wang, X.; Shi, D.; Tu, S. Chin. J. Org. Chem. 2005, 25, 846–849. |
| 23. | Shanthi, G.; Perumal, P. T.; Rao, U.; Sehgal, P. K. Indian J. Chem. 2009, 48B, 1319–1323. |
| 32. | Yu, N.; Aramini, J. M.; Germann, M. W.; Huang, Z. Tetrahedron Lett. 2000, 41, 6993–6996. doi:10.1016/S0040-4039(00)01195-3 |
| 30. | Zolfigol, M. A.; Ghorbani-Vaghei, R.; Mallakpour, S.; Chehardoli, G.; Choghamarani, A. G.; Yazdi, A. H. Synthesis 2006, 1631–1634. doi:10.1055/s-2006-926446 |
| 31. | Ghorbani-Vaghei, R.; Azarifar, D.; Maleki, B. Bull. Korean Chem. Soc. 2004, 25, 953–954. doi:10.5012/bkcs.2004.25.7.953 |
| 40. | Kaye, P. T.; Musa, M. A.; Xolani, W.; Nocanda, X. N.; Robinson, R. S. Org. Biomol. Chem. 2003, 1, 1133–1138. doi:10.1039/b300360d |
| 41. |
Musa, M. A. Applications of the Baylis-Hillman reaction in the synthesis of coumarin derivatives. Ph.D. Thesis, Rhodes University, 2002.
http://eprints.ru.ac.za/2319/ |
| 42. | Govender, T.; Hojabri, L.; Moghaddam, F. M.; Arvidsson, P. I. Tetrahedron: Asymmetry 2006, 17, 1763–1767. doi:10.1016/j.tetasy.2006.06.028 |
| 38. | Stukan, E. V.; Makarenko, S. V.; Berestovitskaya, V. M. Russ. J. Gen. Chem. 2011, 81, 155–157. doi:10.1134/S1070363211010294 |
| 39. | Ravichandran, S. Synth. Commun. 2001, 31, 1233–1235. doi:10.1081/SCC-100104009 |
| 34. | Kawase, Y.; Yamaguchi, S.; Horita, H.; Taneko, J.; Kameyama, H. Bull. Chem. Soc. Jpn. 1982, 55, 1153–1155. doi:10.1246/bcsj.55.1153 |
| 35. | Conti, C.; Desideri, N. Bioorg. Med. Chem. 2010, 18, 6480–6488. doi:10.1016/j.bmc.2010.06.103 |
| 36. | Yamaguchi, S.; Saitoh, T.; Kamiumezawa, M.; Enomoto, H.; Kawase, Y. J. Heterocycl. Chem. 1992, 29, 755–758. doi:10.1002/jhet.5570290412 |
| 37. | Sharma, K. K.; Krupadanam, G. L. D. Synth. Commun. 2002, 32, 1557–1562. doi:10.1081/SCC-120004146 |
| 32. | Yu, N.; Aramini, J. M.; Germann, M. W.; Huang, Z. Tetrahedron Lett. 2000, 41, 6993–6996. doi:10.1016/S0040-4039(00)01195-3 |
| 33. | Heravi, M. M.; Poormohammad, N.; Yahia, Sh.; Beheshtiha, Y. S.; Baghernejad, B.; Malakooti, R. Bull. Chem. Soc. Ethiop. 2010, 24, 273–276. |
© 2012 Masesane and Desta; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)