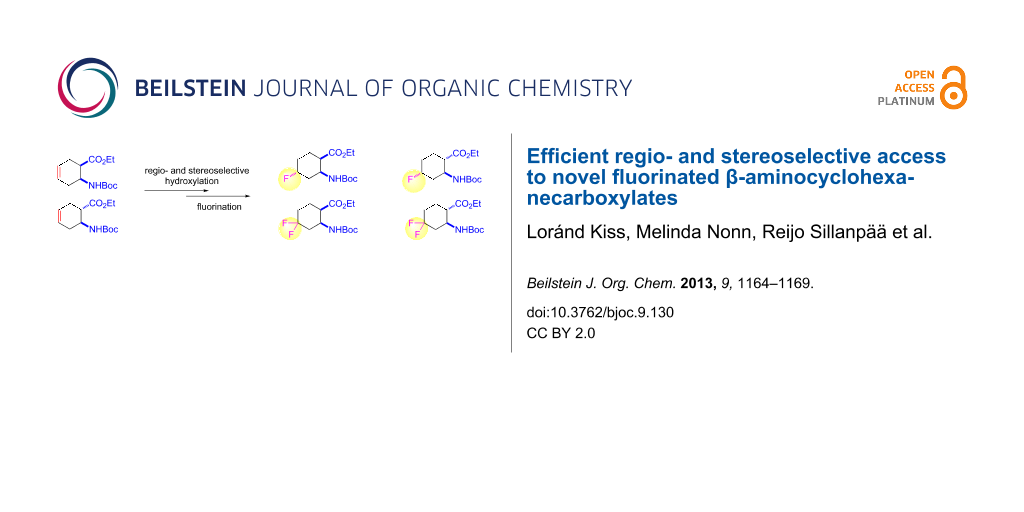
Abstract
A regio- and stereoselective method has been developed for the synthesis of novel fluorinated 2-aminocyclohexanecarboxylic acid derivatives with the fluorine attached to position 4 of the ring. The synthesis starts from either cis- or trans-β-aminocyclohex-4-enecarboxylic acids and involves regio- and stereoselective transformation of the ring C–C double bond through iodooxazine formation and hydroxylation, followed by hydroxy–fluorine or oxo–fluorine exchange.
Graphical Abstract
Introduction
Fluorine chemistry is an expanding area of research that has generated increasing interest in pharmaceutical and medicinal chemistry in recent years because of its considerable impact in drug discovery. There is currently extensive research activity in synthetic chemistry for the preparation of various biologically active fluorinated products [1-10].
Of special interest among such materials are the fluorinated amino acids, which in most cases exhibit higher bioactivities than the nonfluorinated counterparts. The fluorinated α- or acyclic β-amino acids have acquired significance as antibacterial or antifungal agents, enzyme inhibitors or as antitumoral compounds. Introduction of a fluorinated amino acid into a peptide may generate specific protein–ligand or protein–protein interactions, thereby determining thermal or metabolic stabilities, which is of great importance in peptide-based drug research [11-35]. These changes in properties may be more appreciable in the case of peptide oligomers formed from conformationally restricted fluorinated amino acids. Although cyclic β-amino acids are of major interest in pharmaceutical chemistry and in peptide research [36-60], only a relatively small number of fluorinated derivatives of this class of compounds have been synthesized so far [61-70].
Results and Discussion
We recently developed a synthetic method for the regio- and stereoselective introduction of a fluorine atom onto the skeleton of a β-aminocyclohexanecarboxylic acid. The synthesis starts from the Boc-protected 2-aminocyclohex-4-enecarboxylic acid or 2-aminocyclohex-3-enecarboxylic acid and involves ring C–C bond transformation by regio- and stereoselective hydroxylation via iodolactonization, followed by hydroxy–fluorine exchange. This protocol was applied to synthesize fluorinated β-aminocyclohexane scaffolds with the fluorine atom on either position 3 or 5 of the ring. Whereas the procedure is a convenient economical route to fluorinated cyclohexane or cyclohexene β-amino acids, it did not allow extension to the synthesis of similar derivatives with the fluorine atom on position 4.
During our work performed to fill this gap, we have developed a synthetic procedure for gaining access to fluorinated β-aminocyclohexanecarboxylic acids.
This synthesis starts from ethyl cis-2-aminocyclohex-4-enecarboxylate 1 [57] and follows two different strategies. One is based on regio- and stereoselective hydroxylation via iodooxazine formation, followed by fluorination, while the other includes stereoselective epoxidation and regioselective oxirane opening, followed by hydroxy–fluorine exchange. In the former protocol, amino ester 1 is treated with KI/I2 in H2O/CH2Cl2, which affords iodooxazinone derivative 2 stereo- and regioselectively (Scheme 1, Figure 1). Next, compound 2 is transformed to 3 by amide N-Boc protection with Boc2O and 4-dimethylaminopyridine (DMAP) in THF. Removal of the iodine from the cyclohexane skeleton in 3 is accomplished under reductive conditions. On treatment with n-Bu3SnH in the presence of a catalytic amount of azobisisobutyronitrile (AIBN) in dichloromethane under reflux, 3 undergoes deiodination to give ester 4 in 70% yield. Oxazinone 4 is then subjected to heterocycle ring opening with NaOEt in EtOH at 0 °C to furnish all-cis hydroxylated amino ester 5 with the hydroxy group on position 4 of the skeleton (Scheme 1, Figure 2).
Scheme 1: Synthesis of all-cis ethyl 4-hydroxylated β-aminocyclohexanecarboxylate 5.
Scheme 1: Synthesis of all-cis ethyl 4-hydroxylated β-aminocyclohexanecarboxylate 5.
![[1860-5397-9-130-1]](/bjoc/content/figures/1860-5397-9-130-1.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 1: ORTEP diagram of iodooxazinone 2. Thermal ellipsoids have been drawn at the 20 % probability level.
Figure 1: ORTEP diagram of iodooxazinone 2. Thermal ellipsoids have been drawn at the 20 % probability level.
![[1860-5397-9-130-2]](/bjoc/content/figures/1860-5397-9-130-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: ORTEP diagram of hydroxylated amino ester 5. Thermal ellipsoids have been drawn at the 20% probability level.
Figure 2: ORTEP diagram of hydroxylated amino ester 5. Thermal ellipsoids have been drawn at the 20% probabil...
Hydroxylated amino ester 5 was also prepared via the alternative route involving stereoselective epoxidation. Cyclohexene β-amino ester 1 underwent C–C double bond oxidation with 3-chloroperbenzoic acid (MCPBA) to afford epoxy amino ester 6 cis-diastereoselectively [57] (Scheme 1). Opening of the oxirane ring in 6 with NaBH4 in EtOH at 70 °C proceeded regioselectively, providing exclusively amino ester 5 with the hydroxy function on position 4 (for analogous transformations, see reference [60]).
Hydroxylated amino ester 5 was next further used as a key compound for the synthesis of fluorinated target materials. A fluorine atom was introduced by hydroxy–fluorine exchange with bis(dimethoxyethylaminosulfur trifluoride) (Deoxo-Fluor®) reagent. The reaction was carried out under different experimental conditions, with variation of the temperature (−40 °C, 0 °C or 20 °C) and the solvent (toluene, CH2Cl2 or THF). Finally, it was found that hydroxylated amino ester 5 underwent inversion on reaction with a 50% Deoxo-Fluor toluene solution in CH2Cl2 at 0 °C [68,69] to give monofluorinated cyclohexane amino ester 7 in 32% yield (Scheme 2). This rather modest yield is attributed to the relatively large amount of elimination materials (40% overall). In continuation, the geminal difluorinated β-aminocyclohexanecarboxylic acid derivative with the fluorine atoms on position 4 was efficiently synthesized. Oxidation of the hydroxy group of amino ester 5 with pyridinium chlorochromate (PCC) in CH2Cl2 yielded the corresponding oxo-group-containing amino ester 8, which was then converted with Deoxo-Fluor in CH2Cl2 at 0 °C to the corresponding geminal difluoro amino ester 9 in good yield (Scheme 2).
Scheme 2: Syntheses of fluorinated amino esters 7 and 9.
Scheme 2: Syntheses of fluorinated amino esters 7 and 9.
The synthetic route presented above could be extended to the preparation of other 4-fluorinated cyclohexane amino acid derivatives, stereoisomers of 7 or 9. Ethyl trans-2-aminocyclohex-4-enecarboxylate 10 [57] was analogously transformed to its cis counterpart through regio- and stereoselective iodooxazine formation with KI/I2 to give compound 11 (Scheme 3). N-Protection of 11, followed by reductive deiodination, proceeded via 12 (Figure 3) to afford ester 13. Opening of the heterocyclic ring with NaOEt in EtOH at 0 °C furnished 4-hydroxylated amino ester 14, a stereoisomer of 5 (Scheme 3, Figure 4).
Scheme 3: Synthesis of ethyl 4-hydroxy-β-aminocyclohexanecarboxylate 14.
Scheme 3: Synthesis of ethyl 4-hydroxy-β-aminocyclohexanecarboxylate 14.
![[1860-5397-9-130-3]](/bjoc/content/figures/1860-5397-9-130-3.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 3: ORTEP diagram of iodooxazinone derivative 12. Thermal ellipsoids have been drawn at the 20% probability level.
Figure 3: ORTEP diagram of iodooxazinone derivative 12. Thermal ellipsoids have been drawn at the 20% probabi...
![[1860-5397-9-130-4]](/bjoc/content/figures/1860-5397-9-130-4.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 4: ORTEP diagram of hydroxylated amino ester 14. The water molecule oxygen atom O4 is situated on the twofold axis with a population parameter of 0.6. Thermal ellipsoids have been drawn at the 20% probability level.
Figure 4: ORTEP diagram of hydroxylated amino ester 14. The water molecule oxygen atom O4 is situated on the ...
It is noteworthy that in this latter case hydroxylated amino ester 14 could not be prepared by the alternative diastereoselective epoxidation and regioselective oxirane opening strategy: according to our previous results, the opening of epoxide 15 derived from 10 proceeded via a trans-diaxial chair conformation with the nucleophile attack on C4, thereby providing the 5-hydroxylated derivative [57,60].
Next, hydroxylated cyclohexane amino ester 14 was converted with Deoxo-Fluor in CH2Cl2 at 0 °C to 4-fluorinated ethyl β-aminocyclohexanecarboxylate 16, a stereoisomer of 7. Unfortunately, the formation of a substantial amount of a mixture of elimination material could again not be avoided. When subjected to oxidation with PCC, amino ester 14 gave the corresponding oxo ester 17, treatment of which with Deoxo-Fluor in CH2Cl2 at 0 °C provided geminal 4,4-difluorinated cyclohexane amino ester 18, a stereoisomer of 9 (Scheme 4).
Scheme 4: Syntheses of fluorinated amino esters 16 and 18.
Scheme 4: Syntheses of fluorinated amino esters 16 and 18.
Conclusion
In conclusion, a simple and convenient procedure has been developed for the introduction of one or two fluorine atoms onto the skeleton of either cis- or trans-β-aminocyclohexanecarboxylates. The synthetic concept involves regio- and stereoselective hydroxylation via iodooxazine formation, followed by hydroxy–fluorine or oxo–fluorine exchange.
Supporting Information
| Supporting Information File 1: Experimental procedures and characterization of compounds. | ||
| Format: PDF | Size: 199.2 KB | Download |
References
-
Gouverneur, V.; Müller, K., Eds. Fluorine in Pharmaceutical and Medicinal Chemistry: From Biophysical Aspects to Clinical Applications; Imperial College Press: London, 2012.
Return to citation in text: [1] -
Hagmann, W. K. J. Med. Chem. 2008, 51, 4359. doi:10.1021/jm800219f
Return to citation in text: [1] -
Isanbor, C.; O’Hagan, D. J. Fluorine Chem. 2006, 127, 303. doi:10.1016/j.jfluchem.2006.01.011
Return to citation in text: [1] -
Fustero, S.; Sanz-Cervera, J. F.; Aceña, J. L.; Sánchez-Roselló, M. Synlett 2009, 525. doi:10.1055/s-0028-1087806
Return to citation in text: [1] -
Kirk, K. L. J. Fluorine Chem. 2006, 127, 1013. doi:10.1016/j.jfluchem.2006.06.007
Return to citation in text: [1] -
O’Hagan, D. J. Fluorine Chem. 2010, 131, 1071. doi:10.1016/j.jfluchem.2010.03.003
Return to citation in text: [1] -
Bégué, J.-P.; Bonnet-Delpon, D. J. Fluorine Chem. 2006, 127, 992. doi:10.1016/j.jfluchem.2006.05.006
Return to citation in text: [1] -
Qiu, X.-L.; Xu, X.-H.; Qing, F.-L. Tetrahedron 2010, 66, 789. doi:10.1016/j.tet.2009.11.001
Return to citation in text: [1] -
Purser, S.; Moore, P. R.; Swallow, S.; Gouverneur, V. Chem. Soc. Rev. 2008, 37, 320. doi:10.1039/b610213c
Return to citation in text: [1] -
Bresciani, S.; Slawin, A. M. Z.; O’Hagan, D. J. Fluorine Chem. 2009, 130, 537. doi:10.1016/j.jfluchem.2009.03.003
Return to citation in text: [1] -
Aceña, J. L.; Sorochinsky, A. E.; Soloshonok, V. A. Synthesis 2012, 44, 1591. doi:10.1055/s-0031-1289756
Return to citation in text: [1] -
Lemonnier, G.; Lion, C.; Quirion, J.-C.; Pin, J.-P.; Goudet, C.; Jubault, P. Bioorg. Med. Chem. 2012, 20, 4716. doi:10.1016/j.bmc.2012.06.006
Return to citation in text: [1] -
Salwiczek, M.; Nyakatura, E. K.; Gerling, U. I. M.; Ye, S.; Koksch, B. Chem. Soc. Rev. 2012, 41, 2135. doi:10.1039/c1cs15241f
Return to citation in text: [1] -
Mykhailiuk, P. K.; Shishkina, S. V.; Shishkin, O. V.; Zaporozhets, O. A.; Komarov, I. V. Tetrahedron 2011, 67, 3091. doi:10.1016/j.tet.2011.02.082
Return to citation in text: [1] -
Chia, P. W.; Livesey, M. R.; Slawin, A. M. Z.; van Mourik, T.; Wyllie, D. J. A.; O’Hagan, D. Chem.–Eur. J. 2012, 18, 8813. doi:10.1002/chem.201200071
Return to citation in text: [1] -
Shibata, N.; Nishimine, T.; Shibata, N.; Tokunaga, E.; Kawada, K.; Kagawa, T.; Sorochinsky, A. E.; Soloshonok, V. A. Chem. Commun. 2012, 48, 4124. doi:10.1039/c2cc30627a
Return to citation in text: [1] -
Smits, R.; Cadicamo, C. D.; Burger, K.; Koksch, B. Chem. Soc. Rev. 2008, 37, 1727. doi:10.1039/b800310f
Return to citation in text: [1] -
Sorochinsky, A. E.; Soloshonok, V. A. J. Fluorine Chem. 2010, 131, 127. doi:10.1016/j.jfluchem.2009.09.015
Return to citation in text: [1] -
Pan, Y.; Zhao, Y.; Ma, T.; Yang, Y.; Liu, H.; Jiang, Z.; Tan, C.-H. Chem.–Eur. J. 2010, 16, 779. doi:10.1002/chem.200902830
Return to citation in text: [1] -
Acena, J. L.; Simon-Fuentes, A.; Fustero, S. Curr. Org. Chem. 2010, 14, 928. doi:10.2174/138527210791111777
Return to citation in text: [1] -
Tarui, A.; Sato, K.; Omote, M.; Kumadaki, I.; Ando, A. Adv. Synth. Catal. 2010, 352, 2733. doi:10.1002/adsc.201000506
Return to citation in text: [1] -
Hook, D. F.; Gessier, F.; Noti, C.; Kast, P.; Seebach, D. ChemBioChem 2004, 5, 691. doi:10.1002/cbic.200300827
Return to citation in text: [1] -
Jäckel, C.; Koksch, B. Eur. J. Org. Chem. 2005, 4483. doi:10.1002/ejoc.200500205
Return to citation in text: [1] -
Yoder, N. C.; Kumar, K. Chem. Soc. Rev. 2002, 31, 335. doi:10.1039/b201097f
Return to citation in text: [1] -
Mathad, R. I.; Jaun, B.; Flögel, O.; Gardiner, J.; Löweneck, M.; Codée, J. D. C.; Seeberger, P. H.; Seebach, D.; Edmonds, M. K.; Graichen, F. H. M.; Abell, A. D. Helv. Chim. Acta 2007, 90, 2251. doi:10.1002/hlca.200790235
Return to citation in text: [1] -
Capone, S.; Kieltsch, I.; Flögel, O.; Lelais, G.; Togni, A.; Seebach, D. Helv. Chim. Acta 2008, 91, 2035. doi:10.1002/hlca.200890217
Return to citation in text: [1] -
Fustero, S.; Sánchez-Roselló, M.; Rodrigo, V.; Sanz-Cervera, J. F.; Piera, J.; Simón-Fuentes, A.; del Pozo, C. Chem.–Eur. J. 2008, 14, 7019. doi:10.1002/chem.200702009
Return to citation in text: [1] -
Fustero, S.; Sánchez-Roselló, M.; Rodrigo, V.; del Pozo, C.; Sanz-Cervera, J. F.; Simón, A. Org. Lett. 2006, 8, 4129. doi:10.1021/ol061733c
Return to citation in text: [1] -
Mykhailiuk, P. K.; Radchenko, D. S.; Komarov, I. V. J. Fluorine Chem. 2010, 131, 221. doi:10.1016/j.jfluchem.2009.07.017
Return to citation in text: [1] -
Pan, Y.; Calvert, K.; Silverman, R. B. Bioorg. Med. Chem. 2004, 12, 5719. doi:10.1016/j.bmc.2004.07.065
Return to citation in text: [1] -
Yasuhara, A.; Sakagami, K.; Yoshikawa, R.; Chaki, S.; Nakamura, M.; Nakazato, A. Bioorg. Med. Chem. 2006, 14, 3405. doi:10.1016/j.bmc.2005.12.061
Return to citation in text: [1] -
Wang, Z.; Silverman, R. B. Bioorg. Med. Chem. 2006, 14, 2242. doi:10.1016/j.bmc.2005.11.010
Return to citation in text: [1] -
Lu, H.; Silverman, R. B. J. Med. Chem. 2006, 49, 7404. doi:10.1021/jm0608715
Return to citation in text: [1] -
Seebach, D.; Gardiner, J. Acc. Chem. Res. 2008, 41, 1366. doi:10.1021/ar700263g
Return to citation in text: [1] -
Seebach, D. Angew. Chem., Int. Ed. 2011, 50, 96. doi:10.1002/anie.201003823
Return to citation in text: [1] -
Fülöp, F. Chem. Rev. 2001, 101, 2181. doi:10.1021/cr000456z
Return to citation in text: [1] -
Kiss, L.; Forró, E.; Fülöp, F. Synthesis of Carbocyclic β-Amino Acids. In Amino Acids, Peptides and Proteins in Organic Chemistry; Hughes, A. B., Ed.; Wiley-VCH: Weinheim, Germany, 2009; Vol. 1, pp 367 ff. doi:10.1002/9783527631766.ch8
Return to citation in text: [1] -
Hameršak, Z.; Roje, M.; Avdagić, A.; Šunjić, V. Tetrahedron: Asymmetry 2007, 18, 635. doi:10.1016/j.tetasy.2007.02.019
Return to citation in text: [1] -
Rathore, N.; Gellman, S. H.; de Pablo, J. J. Biophys. J. 2006, 91, 3425. doi:10.1529/biophysj.106.084491
Return to citation in text: [1] -
Fernandes, C.; Gauzy, C.; Yang, Y.; Roy, O.; Pereira, E.; Faure, S.; Aitken, D. J. Synthesis 2007, 2222. doi:10.1055/s-2007-983759
Return to citation in text: [1] -
Mowery, B. P.; Lee, S. E.; Kissounko, D. A.; Epand, R. F.; Epand, R. M.; Weisblum, B.; Stahl, S. S.; Gellman, S. H. J. Am. Chem. Soc. 2007, 129, 15474. doi:10.1021/ja077288d
Return to citation in text: [1] -
Gorrea, E.; Nolis, P.; Torres, E.; Da Silva, E.; Amabilino, D. B.; Branchadell, V.; Ortuño, R. M. Chem.–Eur. J. 2011, 17, 4588. doi:10.1002/chem.201002193
Return to citation in text: [1] -
Rúa, F.; Boussert, S.; Parella, T.; Díez-Pérez, I.; Branchadell, V.; Giralt, E.; Ortuño, R. M. Org. Lett. 2007, 9, 3643. doi:10.1021/ol701521k
Return to citation in text: [1] -
Porter, E. A.; Weisblum, B.; Gellman, S. H. J. Am. Chem. Soc. 2005, 127, 11516. doi:10.1021/ja0519785
Return to citation in text: [1] -
Roy, O.; Faure, S.; Aitken, D. J. Tetrahedron Lett. 2006, 47, 5981. doi:10.1016/j.tetlet.2006.06.027
Return to citation in text: [1] -
D’Elia, V.; Zwicknagl, H.; Reiser, O. J. Org. Chem. 2008, 73, 3262. doi:10.1021/jo800168h
Return to citation in text: [1] -
Hetényi, A.; Szakonyi, Z.; Mándity, I. M.; Szolnoki, É.; Tóth, G. K.; Martinek, T. A.; Fülöp, F. Chem. Commun. 2009, 177. doi:10.1039/b812114a
Return to citation in text: [1] -
Fülöp, F.; Martinek, T. A.; Tóth, G. K. Chem. Soc. Rev. 2006, 35, 323. doi:10.1039/b501173f
Return to citation in text: [1] -
Torres, E.; Acosta-Silva, C.; Rúa, F.; Álvarez-Larena, A.; Parella, T.; Branchadell, V.; Ortuño, R. M. Tetrahedron 2009, 65, 5669. doi:10.1016/j.tet.2009.05.039
Return to citation in text: [1] -
Farnández, D.; Torres, E.; Avilés, F. X.; Ortuño, R. M.; Vendrell, J. Bioorg. Med. Chem. 2009, 17, 3824. doi:10.1016/j.bmc.2009.04.035
Return to citation in text: [1] -
Fernandes, C.; Pereira, E.; Faure, S.; Aitken, D. J. J. Org. Chem. 2009, 74, 3217. doi:10.1021/jo900175p
Return to citation in text: [1] -
Celis, S.; Gorrea, E.; Nolis, P.; Illa, O.; Ortuño, R. M. Org. Biomol. Chem. 2012, 10, 861. doi:10.1039/c1ob06575k
Return to citation in text: [1] -
Szolnoki, É.; Hetényi, A.; Martinek, T. A.; Szakonyi, Z.; Fülöp, F. Org. Biomol. Chem. 2012, 10, 255. doi:10.1039/c1ob06627g
Return to citation in text: [1] -
Martinek, T. A.; Fülöp, F. Chem. Soc. Rev. 2012, 41, 687. doi:10.1039/c1cs15097a
Return to citation in text: [1] -
Mansawat, W.; Vilaivan, C.; Balázs, Á.; Aitken, D. J.; Vilaivan, T. Org. Lett. 2012, 14, 1440. doi:10.1021/ol300190u
Return to citation in text: [1] -
Berlicki, Ł.; Pilsl, L.; Wéber, E.; Mándity, I. M.; Cabrele, C.; Martinek, T. A.; Fülöp, F.; Reiser, O. Angew. Chem., Int. Ed. 2012, 51, 2208. doi:10.1002/anie.201107702
Return to citation in text: [1] -
Kiss, L.; Forró, E.; Fülöp, F. Tetrahedron 2012, 68, 4438. doi:10.1016/j.tet.2011.12.065
Return to citation in text: [1] [2] [3] [4] [5] -
Nonn, M.; Kiss, L.; Sillanpää, R.; Fülöp, F. Beilstein J. Org. Chem. 2012, 8, 100. doi:10.3762/bjoc.8.10
Return to citation in text: [1] -
Kiss, L.; Forró, E.; Sillanpää, R.; Fülöp, F. Tetrahedron 2010, 66, 3599. doi:10.1016/j.tet.2010.03.030
Return to citation in text: [1] -
Kiss, L.; Forró, E.; Martinek, T. A.; Bernáth, G.; De Kimpe, N.; Fülöp, F. Tetrahedron 2008, 64, 5036. doi:10.1016/j.tet.2008.03.068
Return to citation in text: [1] [2] [3] -
Qiu, X.-L.; Qing, F.-L. Eur. J. Org. Chem. 2011, 3261. doi:10.1002/ejoc.201100032
Return to citation in text: [1] -
Mikami, K.; Fustero, S.; Sánchez-Roselló, M.; Aceña, J. L.; Soloshonok, V.; Sorochinsky, A. Synthesis 2011, 3045. doi:10.1055/s-0030-1260173
Return to citation in text: [1] -
Fustero, S.; Sanz-Cervera, J. F.; Piera, J.; Sánchez-Roselló, M.; Chiva, G.; Simón-Fuentes, A. J. Fluorine Chem. 2004, 125, 621. doi:10.1016/j.jfluchem.2003.12.016
Return to citation in text: [1] -
Fustero, S.; Sánchez-Roselló, M.; Aceña, J. L.; Fernández, B.; Asensio, A.; Sanz-Cervera, J. F.; del Pozo, C. J. Org. Chem. 2009, 74, 3414. doi:10.1021/jo900296d
Return to citation in text: [1] -
Fustero, S.; Sánchez-Roselló, M.; Sanz-Cervera, J. F.; Aceña, J. L.; del Pozo, C.; Fernández, B.; Bartolomé, A.; Asensio, A. Org. Lett. 2006, 8, 4633. doi:10.1021/ol061892w
Return to citation in text: [1] -
Mittendorf, J.; Kunisch, F.; Matzke, M.; Militzer, H.-C.; Schmidt, A.; Schönfeld, W. Bioorg. Med. Chem. Lett. 2003, 13, 433. doi:10.1016/S0960-894X(02)00958-7
Return to citation in text: [1] -
Hamashima, Y.; Suzuki, T.; Takano, H.; Shimura, Y.; Tsuchiya, Y.; Moriya, K.; Goto, T.; Sodeoka, M. Tetrahedron 2006, 62, 7168. doi:10.1016/j.tet.2005.12.070
Return to citation in text: [1] -
Kiss, L.; Forró, E.; Fustero, S.; Fülöp, F. Eur. J. Org. Chem. 2011, 4993. doi:10.1002/ejoc.201100583
Return to citation in text: [1] [2] -
Kiss, L.; Forró, E.; Fustero, S.; Fülöp, F. Org. Biomol. Chem. 2011, 9, 6528. doi:10.1039/c1ob05648d
Return to citation in text: [1] [2] -
Nonn, M.; Kiss, L.; Hänninen, M. M.; Sillanpää, R.; Fülöp, F. Chem. Biodiversity 2012, 9, 2571. doi:10.1002/cbdv.201200323
Return to citation in text: [1]
| 1. | Gouverneur, V.; Müller, K., Eds. Fluorine in Pharmaceutical and Medicinal Chemistry: From Biophysical Aspects to Clinical Applications; Imperial College Press: London, 2012. |
| 2. | Hagmann, W. K. J. Med. Chem. 2008, 51, 4359. doi:10.1021/jm800219f |
| 3. | Isanbor, C.; O’Hagan, D. J. Fluorine Chem. 2006, 127, 303. doi:10.1016/j.jfluchem.2006.01.011 |
| 4. | Fustero, S.; Sanz-Cervera, J. F.; Aceña, J. L.; Sánchez-Roselló, M. Synlett 2009, 525. doi:10.1055/s-0028-1087806 |
| 5. | Kirk, K. L. J. Fluorine Chem. 2006, 127, 1013. doi:10.1016/j.jfluchem.2006.06.007 |
| 6. | O’Hagan, D. J. Fluorine Chem. 2010, 131, 1071. doi:10.1016/j.jfluchem.2010.03.003 |
| 7. | Bégué, J.-P.; Bonnet-Delpon, D. J. Fluorine Chem. 2006, 127, 992. doi:10.1016/j.jfluchem.2006.05.006 |
| 8. | Qiu, X.-L.; Xu, X.-H.; Qing, F.-L. Tetrahedron 2010, 66, 789. doi:10.1016/j.tet.2009.11.001 |
| 9. | Purser, S.; Moore, P. R.; Swallow, S.; Gouverneur, V. Chem. Soc. Rev. 2008, 37, 320. doi:10.1039/b610213c |
| 10. | Bresciani, S.; Slawin, A. M. Z.; O’Hagan, D. J. Fluorine Chem. 2009, 130, 537. doi:10.1016/j.jfluchem.2009.03.003 |
| 57. | Kiss, L.; Forró, E.; Fülöp, F. Tetrahedron 2012, 68, 4438. doi:10.1016/j.tet.2011.12.065 |
| 61. | Qiu, X.-L.; Qing, F.-L. Eur. J. Org. Chem. 2011, 3261. doi:10.1002/ejoc.201100032 |
| 62. | Mikami, K.; Fustero, S.; Sánchez-Roselló, M.; Aceña, J. L.; Soloshonok, V.; Sorochinsky, A. Synthesis 2011, 3045. doi:10.1055/s-0030-1260173 |
| 63. | Fustero, S.; Sanz-Cervera, J. F.; Piera, J.; Sánchez-Roselló, M.; Chiva, G.; Simón-Fuentes, A. J. Fluorine Chem. 2004, 125, 621. doi:10.1016/j.jfluchem.2003.12.016 |
| 64. | Fustero, S.; Sánchez-Roselló, M.; Aceña, J. L.; Fernández, B.; Asensio, A.; Sanz-Cervera, J. F.; del Pozo, C. J. Org. Chem. 2009, 74, 3414. doi:10.1021/jo900296d |
| 65. | Fustero, S.; Sánchez-Roselló, M.; Sanz-Cervera, J. F.; Aceña, J. L.; del Pozo, C.; Fernández, B.; Bartolomé, A.; Asensio, A. Org. Lett. 2006, 8, 4633. doi:10.1021/ol061892w |
| 66. | Mittendorf, J.; Kunisch, F.; Matzke, M.; Militzer, H.-C.; Schmidt, A.; Schönfeld, W. Bioorg. Med. Chem. Lett. 2003, 13, 433. doi:10.1016/S0960-894X(02)00958-7 |
| 67. | Hamashima, Y.; Suzuki, T.; Takano, H.; Shimura, Y.; Tsuchiya, Y.; Moriya, K.; Goto, T.; Sodeoka, M. Tetrahedron 2006, 62, 7168. doi:10.1016/j.tet.2005.12.070 |
| 68. | Kiss, L.; Forró, E.; Fustero, S.; Fülöp, F. Eur. J. Org. Chem. 2011, 4993. doi:10.1002/ejoc.201100583 |
| 69. | Kiss, L.; Forró, E.; Fustero, S.; Fülöp, F. Org. Biomol. Chem. 2011, 9, 6528. doi:10.1039/c1ob05648d |
| 70. | Nonn, M.; Kiss, L.; Hänninen, M. M.; Sillanpää, R.; Fülöp, F. Chem. Biodiversity 2012, 9, 2571. doi:10.1002/cbdv.201200323 |
| 36. | Fülöp, F. Chem. Rev. 2001, 101, 2181. doi:10.1021/cr000456z |
| 37. | Kiss, L.; Forró, E.; Fülöp, F. Synthesis of Carbocyclic β-Amino Acids. In Amino Acids, Peptides and Proteins in Organic Chemistry; Hughes, A. B., Ed.; Wiley-VCH: Weinheim, Germany, 2009; Vol. 1, pp 367 ff. doi:10.1002/9783527631766.ch8 |
| 38. | Hameršak, Z.; Roje, M.; Avdagić, A.; Šunjić, V. Tetrahedron: Asymmetry 2007, 18, 635. doi:10.1016/j.tetasy.2007.02.019 |
| 39. | Rathore, N.; Gellman, S. H.; de Pablo, J. J. Biophys. J. 2006, 91, 3425. doi:10.1529/biophysj.106.084491 |
| 40. | Fernandes, C.; Gauzy, C.; Yang, Y.; Roy, O.; Pereira, E.; Faure, S.; Aitken, D. J. Synthesis 2007, 2222. doi:10.1055/s-2007-983759 |
| 41. | Mowery, B. P.; Lee, S. E.; Kissounko, D. A.; Epand, R. F.; Epand, R. M.; Weisblum, B.; Stahl, S. S.; Gellman, S. H. J. Am. Chem. Soc. 2007, 129, 15474. doi:10.1021/ja077288d |
| 42. | Gorrea, E.; Nolis, P.; Torres, E.; Da Silva, E.; Amabilino, D. B.; Branchadell, V.; Ortuño, R. M. Chem.–Eur. J. 2011, 17, 4588. doi:10.1002/chem.201002193 |
| 43. | Rúa, F.; Boussert, S.; Parella, T.; Díez-Pérez, I.; Branchadell, V.; Giralt, E.; Ortuño, R. M. Org. Lett. 2007, 9, 3643. doi:10.1021/ol701521k |
| 44. | Porter, E. A.; Weisblum, B.; Gellman, S. H. J. Am. Chem. Soc. 2005, 127, 11516. doi:10.1021/ja0519785 |
| 45. | Roy, O.; Faure, S.; Aitken, D. J. Tetrahedron Lett. 2006, 47, 5981. doi:10.1016/j.tetlet.2006.06.027 |
| 46. | D’Elia, V.; Zwicknagl, H.; Reiser, O. J. Org. Chem. 2008, 73, 3262. doi:10.1021/jo800168h |
| 47. | Hetényi, A.; Szakonyi, Z.; Mándity, I. M.; Szolnoki, É.; Tóth, G. K.; Martinek, T. A.; Fülöp, F. Chem. Commun. 2009, 177. doi:10.1039/b812114a |
| 48. | Fülöp, F.; Martinek, T. A.; Tóth, G. K. Chem. Soc. Rev. 2006, 35, 323. doi:10.1039/b501173f |
| 49. | Torres, E.; Acosta-Silva, C.; Rúa, F.; Álvarez-Larena, A.; Parella, T.; Branchadell, V.; Ortuño, R. M. Tetrahedron 2009, 65, 5669. doi:10.1016/j.tet.2009.05.039 |
| 50. | Farnández, D.; Torres, E.; Avilés, F. X.; Ortuño, R. M.; Vendrell, J. Bioorg. Med. Chem. 2009, 17, 3824. doi:10.1016/j.bmc.2009.04.035 |
| 51. | Fernandes, C.; Pereira, E.; Faure, S.; Aitken, D. J. J. Org. Chem. 2009, 74, 3217. doi:10.1021/jo900175p |
| 52. | Celis, S.; Gorrea, E.; Nolis, P.; Illa, O.; Ortuño, R. M. Org. Biomol. Chem. 2012, 10, 861. doi:10.1039/c1ob06575k |
| 53. | Szolnoki, É.; Hetényi, A.; Martinek, T. A.; Szakonyi, Z.; Fülöp, F. Org. Biomol. Chem. 2012, 10, 255. doi:10.1039/c1ob06627g |
| 54. | Martinek, T. A.; Fülöp, F. Chem. Soc. Rev. 2012, 41, 687. doi:10.1039/c1cs15097a |
| 55. | Mansawat, W.; Vilaivan, C.; Balázs, Á.; Aitken, D. J.; Vilaivan, T. Org. Lett. 2012, 14, 1440. doi:10.1021/ol300190u |
| 56. | Berlicki, Ł.; Pilsl, L.; Wéber, E.; Mándity, I. M.; Cabrele, C.; Martinek, T. A.; Fülöp, F.; Reiser, O. Angew. Chem., Int. Ed. 2012, 51, 2208. doi:10.1002/anie.201107702 |
| 57. | Kiss, L.; Forró, E.; Fülöp, F. Tetrahedron 2012, 68, 4438. doi:10.1016/j.tet.2011.12.065 |
| 58. | Nonn, M.; Kiss, L.; Sillanpää, R.; Fülöp, F. Beilstein J. Org. Chem. 2012, 8, 100. doi:10.3762/bjoc.8.10 |
| 59. | Kiss, L.; Forró, E.; Sillanpää, R.; Fülöp, F. Tetrahedron 2010, 66, 3599. doi:10.1016/j.tet.2010.03.030 |
| 60. | Kiss, L.; Forró, E.; Martinek, T. A.; Bernáth, G.; De Kimpe, N.; Fülöp, F. Tetrahedron 2008, 64, 5036. doi:10.1016/j.tet.2008.03.068 |
| 11. | Aceña, J. L.; Sorochinsky, A. E.; Soloshonok, V. A. Synthesis 2012, 44, 1591. doi:10.1055/s-0031-1289756 |
| 12. | Lemonnier, G.; Lion, C.; Quirion, J.-C.; Pin, J.-P.; Goudet, C.; Jubault, P. Bioorg. Med. Chem. 2012, 20, 4716. doi:10.1016/j.bmc.2012.06.006 |
| 13. | Salwiczek, M.; Nyakatura, E. K.; Gerling, U. I. M.; Ye, S.; Koksch, B. Chem. Soc. Rev. 2012, 41, 2135. doi:10.1039/c1cs15241f |
| 14. | Mykhailiuk, P. K.; Shishkina, S. V.; Shishkin, O. V.; Zaporozhets, O. A.; Komarov, I. V. Tetrahedron 2011, 67, 3091. doi:10.1016/j.tet.2011.02.082 |
| 15. | Chia, P. W.; Livesey, M. R.; Slawin, A. M. Z.; van Mourik, T.; Wyllie, D. J. A.; O’Hagan, D. Chem.–Eur. J. 2012, 18, 8813. doi:10.1002/chem.201200071 |
| 16. | Shibata, N.; Nishimine, T.; Shibata, N.; Tokunaga, E.; Kawada, K.; Kagawa, T.; Sorochinsky, A. E.; Soloshonok, V. A. Chem. Commun. 2012, 48, 4124. doi:10.1039/c2cc30627a |
| 17. | Smits, R.; Cadicamo, C. D.; Burger, K.; Koksch, B. Chem. Soc. Rev. 2008, 37, 1727. doi:10.1039/b800310f |
| 18. | Sorochinsky, A. E.; Soloshonok, V. A. J. Fluorine Chem. 2010, 131, 127. doi:10.1016/j.jfluchem.2009.09.015 |
| 19. | Pan, Y.; Zhao, Y.; Ma, T.; Yang, Y.; Liu, H.; Jiang, Z.; Tan, C.-H. Chem.–Eur. J. 2010, 16, 779. doi:10.1002/chem.200902830 |
| 20. | Acena, J. L.; Simon-Fuentes, A.; Fustero, S. Curr. Org. Chem. 2010, 14, 928. doi:10.2174/138527210791111777 |
| 21. | Tarui, A.; Sato, K.; Omote, M.; Kumadaki, I.; Ando, A. Adv. Synth. Catal. 2010, 352, 2733. doi:10.1002/adsc.201000506 |
| 22. | Hook, D. F.; Gessier, F.; Noti, C.; Kast, P.; Seebach, D. ChemBioChem 2004, 5, 691. doi:10.1002/cbic.200300827 |
| 23. | Jäckel, C.; Koksch, B. Eur. J. Org. Chem. 2005, 4483. doi:10.1002/ejoc.200500205 |
| 24. | Yoder, N. C.; Kumar, K. Chem. Soc. Rev. 2002, 31, 335. doi:10.1039/b201097f |
| 25. | Mathad, R. I.; Jaun, B.; Flögel, O.; Gardiner, J.; Löweneck, M.; Codée, J. D. C.; Seeberger, P. H.; Seebach, D.; Edmonds, M. K.; Graichen, F. H. M.; Abell, A. D. Helv. Chim. Acta 2007, 90, 2251. doi:10.1002/hlca.200790235 |
| 26. | Capone, S.; Kieltsch, I.; Flögel, O.; Lelais, G.; Togni, A.; Seebach, D. Helv. Chim. Acta 2008, 91, 2035. doi:10.1002/hlca.200890217 |
| 27. | Fustero, S.; Sánchez-Roselló, M.; Rodrigo, V.; Sanz-Cervera, J. F.; Piera, J.; Simón-Fuentes, A.; del Pozo, C. Chem.–Eur. J. 2008, 14, 7019. doi:10.1002/chem.200702009 |
| 28. | Fustero, S.; Sánchez-Roselló, M.; Rodrigo, V.; del Pozo, C.; Sanz-Cervera, J. F.; Simón, A. Org. Lett. 2006, 8, 4129. doi:10.1021/ol061733c |
| 29. | Mykhailiuk, P. K.; Radchenko, D. S.; Komarov, I. V. J. Fluorine Chem. 2010, 131, 221. doi:10.1016/j.jfluchem.2009.07.017 |
| 30. | Pan, Y.; Calvert, K.; Silverman, R. B. Bioorg. Med. Chem. 2004, 12, 5719. doi:10.1016/j.bmc.2004.07.065 |
| 31. | Yasuhara, A.; Sakagami, K.; Yoshikawa, R.; Chaki, S.; Nakamura, M.; Nakazato, A. Bioorg. Med. Chem. 2006, 14, 3405. doi:10.1016/j.bmc.2005.12.061 |
| 32. | Wang, Z.; Silverman, R. B. Bioorg. Med. Chem. 2006, 14, 2242. doi:10.1016/j.bmc.2005.11.010 |
| 33. | Lu, H.; Silverman, R. B. J. Med. Chem. 2006, 49, 7404. doi:10.1021/jm0608715 |
| 34. | Seebach, D.; Gardiner, J. Acc. Chem. Res. 2008, 41, 1366. doi:10.1021/ar700263g |
| 35. | Seebach, D. Angew. Chem., Int. Ed. 2011, 50, 96. doi:10.1002/anie.201003823 |
| 57. | Kiss, L.; Forró, E.; Fülöp, F. Tetrahedron 2012, 68, 4438. doi:10.1016/j.tet.2011.12.065 |
| 68. | Kiss, L.; Forró, E.; Fustero, S.; Fülöp, F. Eur. J. Org. Chem. 2011, 4993. doi:10.1002/ejoc.201100583 |
| 69. | Kiss, L.; Forró, E.; Fustero, S.; Fülöp, F. Org. Biomol. Chem. 2011, 9, 6528. doi:10.1039/c1ob05648d |
| 60. | Kiss, L.; Forró, E.; Martinek, T. A.; Bernáth, G.; De Kimpe, N.; Fülöp, F. Tetrahedron 2008, 64, 5036. doi:10.1016/j.tet.2008.03.068 |
| 57. | Kiss, L.; Forró, E.; Fülöp, F. Tetrahedron 2012, 68, 4438. doi:10.1016/j.tet.2011.12.065 |
| 57. | Kiss, L.; Forró, E.; Fülöp, F. Tetrahedron 2012, 68, 4438. doi:10.1016/j.tet.2011.12.065 |
| 60. | Kiss, L.; Forró, E.; Martinek, T. A.; Bernáth, G.; De Kimpe, N.; Fülöp, F. Tetrahedron 2008, 64, 5036. doi:10.1016/j.tet.2008.03.068 |
© 2013 Kiss et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)