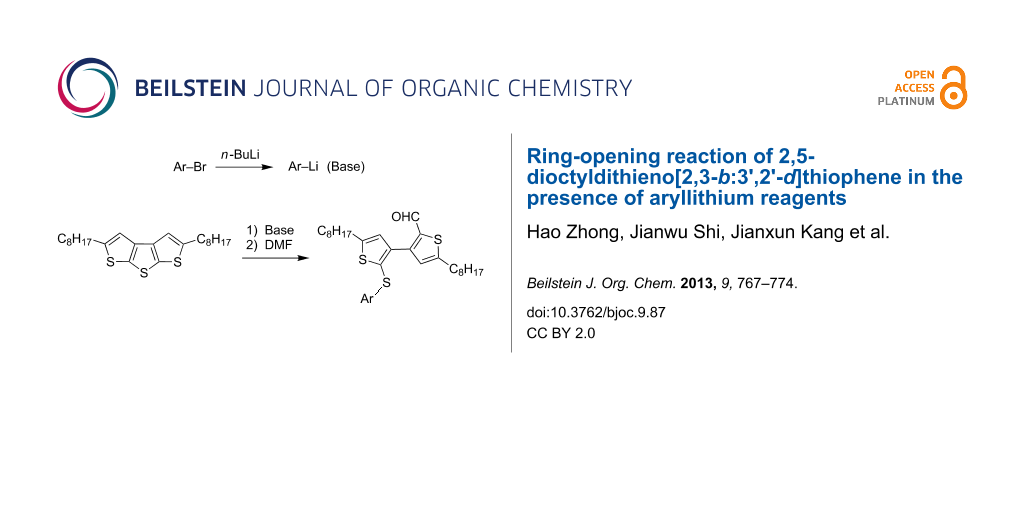
Abstract
In this paper, the ring-opening reaction of 2,5-dioctyldithieno[2,3-b:3',2'-d]thiophene with aryllithium in THF at low temperature to generate 2'-arylthio-3,3'-bithiophene-2-carbaldehydes is studied. Nine examples are explored and all the products are characterized by 1H NMR, 13C NMR and HRMS. The relative relationship between the structures of aryl groups and the efficiency of ring-opening reactions are discussed.
Graphical Abstract
Introduction
Due to the promising optical and electrical properties, the derivatives of dithieno[2,3-b:3',2'-d]thiophene (DTT), as one type of fused oligothiophene, have shown their potential applications in organic electronics [1-4]. The work on the synthesis of DTT derivatives and the chemical stability of the DTT core is of particular interest. To construct DTT functional materials, deprotonation of DTT with organolithium reagents seems to be one of the most important approaches. However, the ring-opening reaction of DTT leading to the cleavage of the center ring can be observed in the presence of n-BuLi. In our previous work, we reported the synthesis of a series of symmetric substituted dithieno[2,3-b:3',2'-d]thiophenes and their ring-opening reactions in the presence of n-BuLi. The 3,3'-bithiophene-2-carbaldehydes were generated after quenching with an electrophile, i.e., dry DMF (Scheme 1) [5].
Scheme 1: The ring-opening reaction of symmetric 2,5-disubstituted-dithieno[2,3-b:3',2'-d]thiophenes in the presence of n-BuLi in THF.
Scheme 1: The ring-opening reaction of symmetric 2,5-disubstituted-dithieno[2,3-b:3',2'-d]thiophenes in the p...
The uncommon ring opening of fused thiophene derivatives in the presence of n-BuLi, though it has been reported, does not draw as much attention as the ring opening of other heterocyclic compounds [6-9]. The limited number of reports include derivatives of benzo[b]thiophene [10-12], thieno[3,2-d]thiazole [10,11], and thieno[3,2-b]thiophene [13]. More recently, Nenajdenko et al. reported that fused thieno[2,3-b]thiophenes and some [3,2-b]-fused oligothiophenes were attacked by organo-lithium reagents resulting in the cleavage of thiophene rings [14]. They found when competitive deprotonation of the substrate was possible, high selectivity towards the ring opening was observed with n-BuLi when compared with other organolithium reagents. However, most of these ring-opening reactions mentioned above take place by using n-BuLi as the nucleophile to attack the sulfur atoms of thiophenes. Other organolithium reagents have rarely been employed for this kind of reaction. Furthermore, the relationship between the nucleophilicity of organolithium reagents and the efficiency of the ring opening of fused thiophenes has not been discussed.
In this paper, we present the ring opening of 2,5-dioctyldithieno[2,3-b:3',2'-d]thiophene (1) with nine aryllithium reagents and the characterization of the nine corresponding ring-opening products. These studies should facilitate the understanding of the chemical stability of dithieno[2,3-b:3',2'-d]thiophene, which may be of importance in both organic chemistry and materials science. Furthermore, we show a novel method for the synthesis of 2'-arylthio-3,3'-bithiophene-2-carbaldehydes based on the ring-opening reaction.
Results and Discussion
Ring-opening reaction of 2,5-dioctyldithieno[2,3-b:3',2'-d]thiophene in the presence of aryllithium reagents
The aryllithium reagents (Ar–Li) were obtained from the metal–halogen exchange of Ar–Br and n-BuLi. To avoid the possible influence from excess n-BuLi, 1.4 equiv Ar–Br was treated with 1.3 equiv n-BuLi at −78 °C for 2 h to make sure that only excess Ar–Li (1.3 equiv) was employed for the ring-opening reaction. Then a solution of 1 was added at −78 °C, at which point the reaction mixture was slowly warmed to −30 °C for 3 h. After quenching with dry DMF, the corresponding ring-opening products 2'-arylthio-3,3'-bithiophene-2-carbaldehydes were obtained.
Nine aryllithium reagents were employed for the ring-opening reaction of 1 as shown in Table 1. The molecular structures and the effects of electron-donating groups (EDG) and electron-withdrawing groups (EWG) of the aryllithium reagents were studied. Compared to the compound 2d (Table 1, entry 4) bearing EWG groups, the compounds 2b, 2c and 2e (Table 1, entries 2, 3 and 5), all of which have EDG groups, generated higher yields of products, namely 73% (3b), 74% (3c) and 83% (3e), respectively. In the case of 2b, not only was 3b obtained, but also a byproduct with similar polarity, 2'-butylsulfanyl-5,5'-dioctyl-[3,3'-bithiophene]-2-carbaldehyde [5], was generated when the metal–halogen exchange temperature was set to −78 °C. If the reaction temperature of the metal–halogen exchange was set to −78 °C first, and then warmed up to −30 °C for 3 h, only 3b could be generated in 70% yield (Table 1, entry 3). The byproduct formed in the case of 2b implies that the metal–halogen exchange cannot be completed at −78 °C.
Table 1: The ring opening of 2,5-dioctyldithieno[2,3-b:3’,2’-d]thiophene (1) with aryllithium reagents 2a–i.
|
|
|||
| Entry | Ar–Br | Yield (%)a | Product |
|---|---|---|---|
| 1 |
2a |
88 |
3a |
| 2 |
2b |
70b |
3b |
| 3 |
2c |
71b |
3c |
| 4 |
2d |
42 |
3d |
| 5 |
2e |
83 |
3e |
| 6 |
2f |
61 |
3f |
| 7 |
2g |
56 |
3g |
| 8 |
2h |
76 |
3h |
| 9 |
2i |
48c |
3i |
aYield of the isolated product. bn-BuLi was added at −78 °C then warmed up to −30 °C for 3 h. ct-BuLi was employed instead of n-BuLi.
Similar to the case of 2e bearing the EDG group of triphenylamine, 2h also gave a good yield of 3h (76%, Table 1, entry 8). These results indicate that an increase of the electron donating character of the substituted aryl groups leads to a higher nucleophilicity of the aryllithium reagents and thereby promotes the process of a ring-opening reaction. On the other hand, in the case of 2d, which has a cyano group, a poor yield of 3d (42%, Table 1, entry 4) was obtained due to the high stability of cyano-aryl anion with a low nucleophilicity.
The steric hinderance effect can be observed in the case of 2a, 2f and 2g (Table 1, entries 1, 6 and 7). When the bulkiness of aryl groups increased from benzene to anthracene, the yields of ring-opening products decreased from 3a (88%), to 3f (61%) and to 3g (56%). Therefore, the aryllithium reagents with bulky groups, such as anthryllithium, generate lower yields of ring-opening products than phenyllithium as the nucleophilic reagent.
An interesting result was observed for the metal–halogen exchange of 1-bromopyrene (2i) with n-BuLi or t-BuLi at −78 °C, which delivered different types of ring-opening products (Scheme 2). When n-BuLi was used for the metal–halogen exchange, the reaction temperature was set at 0 °C for the ring-opening reaction of 1 for 3 h. Instead of the expected product, however, an unexpected ring-opening product 2'-butyl-5,5'-dioctyl-2-(1-pyrenylthio)-3,3'-bithiophene (4, 45%) along with 1-pyrenecarboxaldehyde (5, 35%) was generated when the reaction mixture was quenched with dry DMF. If t-BuLi was employed for the metal–halogen exchange, only anticipated product 3i was obtained in 48% yield and no 4 was observed. The structure of 3i was confirmed by a single-crystal structure analysis (Figure 1).
Scheme 2: The ring-opening reaction of 1 in the presence of n-BuLi and t-BuLi employed for metal–halogen exchange.
Scheme 2: The ring-opening reaction of 1 in the presence of n-BuLi and t-BuLi employed for metal–halogen exch...
In our work, the metal–halogen exchange of 1-bromopyrene (2i) with n-BuLi or t-BuLi at −78 °C delivered different types of ring-opening products. We believe that the two metal–halogen exchange processes went to complete conversion. The formation of 4 is possibly due to the in situ generation of n-BuBr that is trapped by the carbanion generated from an attack of pyrenyllithium (PyLi) on the central sulfur atom of 1. The presence of the pyrene carbanion intermediate is responsible for the formation of 5 by dry DMF quenching. However, if t-BuLi was used for the metal–halogen exchange, t-BuBr could not be efficiently generated due to the fast elimination occurring between the formed t-BuBr and another equivalent of t-BuLi. The formed pyrene–Li attacks the sulfur center of 1 and generates the carbanion intermediate via a ring-opening mechanism, which was quenched only by dry DMF, resulting in 3i.
Crystal structure of 2'-(1-pyrenylsulfanyl)-5,5’-dioctyl-[3,3’-bithiophene]-2-carbaldehyde (3i)
The crystallographic structure of 3i is shown in Figure 1 [15]. The crystal of 3i belongs to the triclinic space group P−1. The two thiophene rings are linked together in the molecule with a torsional angle (C3–C4–C5–C6) of 55.4° and a dihedral angle of 56.1°. The pyrenylsulfanyl group is not coplanar to the neighboring thiophene ring with a dihedral angle of 79.5° and a torsion angle (C5–C6–S2–C10) of 89.7°. There are short contacts of hydrogen bonding found in the crystal packing. However, no π–π interaction was observed between two pyrene rings, as usually seen in the crystal (Figure 1, right).
![[1860-5397-9-87-1]](/bjoc/content/figures/1860-5397-9-87-1.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 1: Crystallographic structure of 3i (left, top view) and crystal packing (right). Carbon, silicon, oxygen and sulfur atoms are depicted with thermal ellipsoids set at 50% probability level, and all hydrogen atoms are omitted for clarity.
Figure 1: Crystallographic structure of 3i (left, top view) and crystal packing (right). Carbon, silicon, oxy...
Conclusion
In summary, aryllithium reagents are suitable for the ring opening of 2,5-dioctyldithieno[2,3-b:3',2'-d]thiophene. We obtained nine ring-opening products. Their yields indicated that strong nucleophilicity of the aryllithium reagents can intensify the efficiency of the ring-opening process, and the steric effect is another factor that could influence the yield. This ring opening can be applied extensively to derivatives including 2'-arylthio-3,3'-dithiophenyl-2-aldehydes, which may provide access to a broad range of compounds for pharmaceutical chemistry, organic chemistry and materials science.
Experimental
Synthesis of 2'-phenylsulfanyl-5,5'-dioctyl-[3,3'-bithiophene]-2-carbaldehyde (3a): To a solution of 2a (49.7 mg, 0.32 mmol, 1.4 equiv) in dry THF (10 mL), n-BuLi (2.38 M in hexane, 0.12 mL, 0.29 mmol, 1.3 equiv) was added dropwise at −78 °C. After stirring at −78 °C for 2 h, a solution of 1 (95.0 mg, 0.23 mmol, 1.0 equiv) in dry THF (10 mL) was added dropwise. The mixture was slowly warmed to −30 °C for 3 h, then dry DMF (0.04 mL, 0.45 mmol, 2.0 equiv) was added dropwise at −78 °C, and the reaction mixture was slowly warmed to ambient temperature overnight. After quenching with H2O (30 mL), the reaction mixture was extracted with CHCl3 (2 × 30 mL) and then washed with H2O (30 mL). After drying over MgSO4, the solvent was removed in vacuum. The residue was purified by column chromatography on silica gel with petrol ether (60–90 °C)/chloroform (1:4, v/v) as eluents to yield 3a (105.1 mg, 88.4%) as a light yellow oil. 1H NMR (400 MHz, CDCl3) δ 9.70 (s, 1H), 7.22–7.19 (m, 2H), 7.14–7.07 (m, 3H), 6.87 (s, 1H), 6.82 (s, 1H), 2.82 (t, J = 7.6 Hz, 2H), 2.79 (d, J = 7.4 Hz, 2H), 1.74–1.61 (m, 4H), 1.40–1.27 (m, 20H), 0.90–0.87 (m, 6H); 13C NMR (100 MHz, CDCl3) δ 183.03, 155.28, 150.54, 144.49, 139.86, 137.93, 136.87, 128.83, 128.30, 127.74, 127.16, 126.06, 126.02, 31.71, 31.12, 30.85, 30.56, 30.31, 29.14, 29.11, 29.06, 29.04, 28.98, 28.82, 22.54, 14.01; IR (KBr): 2955, 2926, 2855 (C-H), 1661 (C=O) cm−1; HRMS–EI m/z: [M+ + Na] calcd for C31H42OS3Na, 549.2293; found, 549.2290.
Synthesis of 2'-(4-methylphenylsulfanyl)-5,5'-dioctyl-[3,3'-bithiophene]-2-carbaldehyde (3b): The same procedure was used as for the synthesis of 3a except that after the addition of n-BuLi, the reaction temperature was raised to −30 °C for 3 h, and then cooled back to −78 °C before the addition of 1. From the reaction on the 54.2 mg scale of 2b, 90.0 mg (70.3%) of 3b was obtained as a yellow oil. 1H NMR (400 MHz, CDCl3) δ 9.69 (s, 1H), 7.02 (s , 4H), 6.84 (d, J = 1.3 Hz, 2H), 2.80 (t, J = 7.2 Hz, 2H), 2.79 (t, J = 7.2 Hz, 2H), 2.28 (s, 3H), 1.73–1.63 (m, 4H), 1.39–1.28 (m, 20H), 0.88 (t, J = 6.8 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ 183.28, 155.38, 150.07, 144.78, 139.14, 136.86, 136.41, 134.09, 129.70, 128.44, 128.13, 127.65, 127.42, 31.77, 31.18, 30.96, 30.66, 30.35, 29.20, 29.19, 29.12, 29.04, 28.92, 22.60, 20.92, 14.07; IR (KBr): 2956, 2926, 2855 (C-H), 1661 (C=O) cm−1; HRMS–EI m/z: [M+ + Na] calcd for C32H44OS3Na, 563.2430; found, 563.2437.
Synthesis of 2'-(4-methoxylphenylsulfanyl)-5,5'-dioctyl-[3,3'-bithiophene]-2-carbaldehyde (3c): The same procedure was used as for the synthesis of 3b. From the reaction on the 61.9 mg scale of 2c, 92.9 mg (70.5%) of 3c was obtained as a yellow oil. 1H NMR (400 MHz, CDCl3) δ 9.68 (s, 1H), 7.13–7.10 (m, 2H), 6.85 (s, 1H), 6.78 (s, 1H), 6.77–6.74 (m, 2H), 3.75 (s, 3H), 2.82 (t, J = 7.6 Hz, 2H), 2.76 (t, J = 7.7 Hz, 2H), 1.73–1.63 (m, 4H), 1.37–1.27 (m, 20H), 0.90–0.86 (m, 6H); 13C NMR (100 MHz, CDCl3) δ 183.34, 158.98, 155.41, 149.24, 144.91, 137.82, 136.78, 131.34, 129.42, 128.46, 127.50, 127.47, 114.57, 55.21, 31.76, 31.17, 31,02, 30.68, 30,29, 29.19, 29.11, 29.03, 28.94, 22.59, 14.06; IR (KBr): 2958, 2926, 2856 (C-H), 1661 (C=O) cm−1; HRMS–EI m/z: [M+ + Na] calcd for C32H44O2S3Na, 579.2395; found, 579.2396.
Synthesis of 2'-(4-cyanolphenylsulfanyl)-5,5'-dioctyl-[3,3'-bithiophene]-2-carbaldehyde (3d): The same procedure was used as for the synthesis of 3a. From the reaction on the 60.2 mg scale of 2d, 54.9 mg (42.1%) of 3d was obtained as a yellow oil. 1H NMR (400 MHz, CDCl3) δ 9.69 (s, 1H), 7.46 (d, J = 7.3 Hz, 2H), 7.05 (d, J = 7.3 Hz, 2H), 6.94 (s, 1H), 6.73 (s, 1H), 2.85 (t, J = 7.6 Hz, 2H), 2.77 (t, J = 7.5 Hz, 2H), 1.75–1.58 (m, 4H), 1.40–1.24 (m, 20H), 0.89–0.86 (m, 6H); 13C NMR (100 MHz, CDCl3) δ 182.81, 155.90, 152.59, 145.75, 143.64, 141.81, 137.13, 132.37, 128.18, 127.94, 125.80, 121.97, 118.53, 108.94, 31.77, 31.75, 31.17, 30.95, 30.64, 30.49, 29.19, 29.15, 29.12, 29.10, 29.06, 28.96, 28.88, 22.60, 14.07; IR (KBr): 2956, 2926, 2856 (C-H), 2228 (C≡N), 1661 (C=O) cm−1; HRMS–EI m/z: [M+ + Na] calcd for C32H41NOS3Na, 574.2244; found, 574.2243.
Synthesis of 2'-[4-(N,N-dimethylamino)phenylsulfanyl]-5,5'-dioctyl-[3,3'-bithiophene]-2-carbaldehyde (3e): The same procedure was used as for the synthesis of 3a. From the reaction on the 66.3 mg scale of 2e, 112.2 mg (83.2%) of 3e was obtained as a yellow oil. 1H NMR (400 MHz, CDCl3) δ 9.72 (s, 1H), 7.16–7.14 (m, 2H), 6.92 (s, 1H), 6.74 (s, 1H), 6.59–6.56 (m, 2H), 2.93 (s, 6H), 2.85 (t, J = 7.6 Hz, 2H), 2.73 (t, J = 7.7 Hz, 2H), 1.72 (quint, J = 6.5 Hz, 2H), 1.65 (quint, J = 7.6 Hz, 2H), 1.41–1.28 (m, 20H), 0.89 (t, J = 10.8 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ 183.48, 155.26, 150.00, 147.86, 145.27, 136.60, 135.78, 132.82, 132.29, 128.57, 127.25, 120.98, 112.59, 40.17, 31.74, 31.73, 31.16, 31.01, 30.68, 30.20, 29.18, 29.16, 29.09, 29.07, 29.01, 28.94, 22.56, 14.02; IR (KBr): 2954, 2924, 2851 (C-H), 1659 (C=O) cm−1; HRMS–EI m/z: [M+ + Na] calcd for C33H47NOS3Na, 592.2709; found, 592.2712.
Synthesis of 2'-(1-naphthylsulfanyl)-5,5'-dioctyl-[3,3'-bithiophene]-2-carbaldehyde (3f): The same procedure was used as for the synthesis of 3a. From the reaction on the 72.4 mg scale of 2f, 84.7 mg (60.5%) of 3f was obtained as a yellow oil. 1H NMR (400 MHz, CDCl3) δ 9.76 (s, 1H), 8.22–8.19 (m, 1H), 7.84–7.82 (m, 1H), 7.71 (d, J = 8.2 Hz, 1H), 7.51–7.49 (m, 2H), 7.34 (t, J = 7.56 Hz, 1H), 7.25 (d, J = 7.21 Hz, 1H), 6.87 (s, 1H), 6.84 (s, 1H), 2.81 (t, J = 7.6 Hz, 2H), 2.75 (t, J = 7.5 Hz, 2H), 1.70 (quint, J = 7.3 Hz, 2H), 1.59 (quint, J = 7.6 Hz, 2H), 1.39–1.25 (m, 22H), 0.90 (t, J = 5.8 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ 183.23, 155.54, 150.30, 144.76, 139.41, 136.91, 134.74, 133.70, 131.40, 128.35, 128.34, 127.71, 127.42, 127.12, 126.73, 126.39, 126.26, 125.56, 124.25, 31.76, 31.16, 30.85, 30.60, 30.36, 29.19, 29.12, 29.06, 29.04, 28.88, 22.60, 14.08; IR (KBr): 2956, 2926, 2855 (C-H), 1661 (C=O) cm−1; HRMS–EI m/z: [M+ + Na] calcd for C35H44OS3Na, 599.2443; found, 599.2447.
Synthesis of 2'-(9-anthrylsulfanyl)-5,5'-dioctyl-[3,3'-bithiophene]-2-carbaldehyde (3g): The same procedure was used as for the synthesis of 3a. From the reaction on the 87.4 mg scale of 2g, 85.8 mg (56.4%) of 3g was obtained as a yellow oil. 1H NMR (400 MHz, CDCl3) δ 9.60 (s, 1H), 8.62 (d, J = 9.4 Hz, 2H), 8.47 (s, 1H), 7.98 (d, J = 9.1 Hz, 2H), 7.51–7.45 (m, 4H), 6.91 (s, 1H), 6.60 ( s, 1H), 2.85 (t, J = 7.6 Hz, 2H), 2.54 (t, J = 7.7 Hz, 2H), 1.75 (quint, J = 3.0 Hz, 2H), 1.48–1.20 (m, 22H), 0.89 (dd, J = 6.4 Hz, 12.8 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ 183.32, 155.58, 147.19, 145.39, 137.02, 135.14, 134.08, 133.82, 132.41, 131.76, 130.06, 128.82, 128.76, 128.14, 127.18, 126.94, 126.57, 125.41, 31.82, 31.74, 31.09, 31.06, 30.77, 30.10, 29.68, 29.27, 29.18, 29.13, 29.09, 20.08, 28.99, 22.63, 22.58, 14.07, 14.06; IR (KBr): 2956, 2926, 2856 (C-H), 1649 (C=O) cm−1; HRMS–EI m/z: [M+ + Na] calcd for C39H46OS3Na, 649.2613; found, 649.2603.
Synthesis of 2'-[4-(N,N-diphenylamino)phenylsulfanyl]-5,5'-dioctyl-[3,3'-bithiophene]-2-carbaldehyde (3h): The same procedure was used as for the synthesis of 3a. From the reaction on the 100.1 mg scale of 2h, 127.4 mg (75.6%) of 3h was obtained as a yellow oil. 1H NMR (400 MHz, CDCl3) δ 9.65 (s, 1H), 7.26–7.22 (m, 3H), 7.06–7.02 (m, 6H), 7.00–6.99 (m, 1H), 6.98–6.97 (m, 2H), 6.91–6.88 (m, 2H) 6.88 (s, 1H), 6.81 (s, 1H), 2.85–2.77 (m, 4H), 1.73–1.65 (m, 4H), 1.39–1.26 (m, 20H), 0.90 (td, J = 6.4 Hz, 3.2 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ 183.28, 155.41, 149.67, 147.28, 146.82, 144.92, 138.63, 136.89, 129.94, 129.55, 129.25, 128.50, 128.27, 127.69, 124.47, 123.63, 123.12, 31.80, 31.78, 31.23, 31.04, 30.75, 30.36, 29.23, 29.21, 29.14, 29.09, 29.00, 22.62, 14.10. IR (KBr): 2956, 2926, 2855 (C-H), 1661 (C=O) cm−1; HRMS–EI m/z: [M+ + Na] calcd for C43H51NOS3 Na, 716.3037; found, 716.3025.
Synthesis of 2'-(1-pyrenylsulfanyl)-5,5'-dioctyl-[3,3'-bithiophene]-2-carbaldehyde (3i): The same procedure was used as for the synthesis of 3a except for two differences. One is that t-BuLi (2.6 equiv) was employed for the metal–halogen exchange, and another one is that the temperature for the ring-opening reaction of 1 was set to 0 °C for 3h. From the reaction on the 87.7 mg (1.4 equiv) scale of 2i, 69.1 mg (47.7%) of 3i and a side product, 1-pyrenecarboxaldehyde (27.8 mg, 42.0%) were generated. 3i was obtained as a light yellow solid, mp 66–68 °C; 1H NMR (400 MHz, CDCl3) δ 9.79 (s, 1H), 8.43 (d, J = 9.2 Hz, 1H), 8.18–8.16 (m, 2H), 8.08–7.95 (m, 5H), 7.80 (d, J = 8.1 Hz, 1H), 6.84 (s, 1H), 6.75 (s, 1H), 2.77 (t, J = 7.6 Hz, 2H), 2.63 (t, J = 7.6 Hz, 2H), 1.70–1.63 (m, 2H), 1.47–1.13 (m, 22H), 0.88 (t, J = 6.8 Hz, 3H), 0.85 (t, J = 7.0 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 183.27, 155.54, 149.92, 144.82, 138.90, 136.93, 131.40, 131.15, 130.71, 130.36, 129.58, 128.27, 128.12, 127.92, 127.62, 127.54, 127.00, 126.12, 125.36, 125.28, 124.94, 124.86, 124.18, 123.54, 31.75, 31.70, 31.15, 30,74, 30.52, 30.34, 29.17, 29.10, 29.03, 29.01, 28.84, 22.59, 22.55, 14.06, 14.04; IR (KBr): 2956, 2926, 2854 (C-H), 1653 (C=O) cm−1; HRMS–EI m/z: [M+ + Na] calcd for C41H46OS3Na, 673.2611; found, 673.2603.
Synthesis of 2-butyl-2'-(1-pyrenylsulfanyl)-5,5'-dioctyl-3,3'-bithiophene (4): To a solution of 2i (93.7 mg, 0.33 mmol, 1.4 equiv) in dry THF (10 mL), n-BuLi (2.39 M in hexane, 0.13 mL, 0.31 mmol, 1.3 equiv) was added dropwise at −78 °C. After slowly warming to −78 °C for 2 h, a solution of 1 (100.1 mg, 0.24 mmol, 1.0 equiv) in dry THF (10 mL) was added dropwise. The reaction mixture was slowly warmed to 0 °C for 3 h, then cooled back to −78 °C. Dry DMF (0.05 mL, 0.48 mmol, 2.0 equiv) was added dropwise, and the reaction mixture was slowly warmed to ambient temperature overnight. After quenching with H2O (30 mL), the reaction mixture was extracted with CHCl3 (2 × 30 mL) and then washed with H2O (30 mL). After drying over MgSO4, the solvent was removed in vacuum. The residue was purified by column chromatography on silica gel with petrol ether (60–90 °C)/chloroform (1:4, v/v) as eluents, 72.6 mg (45%) of 4 was obtained as a brown oil, and 5 (24.5 mg, 35%) was also obtained. For 4, 1H NMR (400 MHz, CDCl3) δ 8.49 (d, J = 9.2 Hz, 1H), 8.18–8.15 (dd, J = 3.6, 7.7 Hz, 2H), 8.07 (d, 1H), 8.04–7.95 (m, 4H), 7.80 (d, J = 8.1 Hz, 1H), 6.77 (s, 1H), 6.58 (s, 1H), 2.78 (t, J = 7.5 Hz, 2H), 2.69 (t, J = 7.5 Hz, 2H), 2.60 (t, J = 7.6 Hz, 2H), 1.67 (qt, J = 7.4 Hz, 2H), 1.51–1.42 (m, 4H), 1.32–1.10 (m, 22H), 0.89 (t, J = 6.8 Hz, 3H), 0.85 (t, J = 7.0 Hz, 3H), 0.80 (t, J = 7.4 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 149.28, 143.27, 141.10, 139.92, 133.08, 131.48, 131.34, 130.95, 129.96, 129.25, 127.62, 127.43, 127.36, 127.21, 126.07, 126.02, 125.16, 125.11, 124.95, 124.55, 124.45, 123.89, 34.04, 31.83, 31.81, 31.27, 31.22, 30.53, 29.92, 29.71, 29.28, 29.21, 29.15, 29.06, 28.52, 22.66, 22.62, 22.39, 14.11, 13.79; IR (KBr): 3435 (Ar-H), 2956, 2924, 2855 (C-H) cm−1; HRMS–EI m/z: [M]+ calcd for C44H54S3, 678.3392; found, 678.3382.
References
-
Shi, J.; Xu, L.; Li, Y.; Jia, M.; Kan, Y.; Wang, H. Org. Electron. 2013, 14, 934–941. doi:10.1016/j.orgel.2013.01.002
Return to citation in text: [1] -
Shi, J.; Li, Y.; Jia, M.; Xu, L.; Wang, H. J. Mater. Chem. 2011, 21, 17612–17614. doi:10.1039/c1jm14383b
Return to citation in text: [1] -
Zhang, L.; Tan, L.; Wang, Z.; Hu, W.; Zhu, D. Chem. Mater. 2009, 21, 1993–1999. doi:10.1021/cm900369s
Return to citation in text: [1] -
Tan, L.; Zhang, L.; Jiang, X.; Yang, X.; Wang, L.; Wang, Z.; Li, L.; Hu, W.; Shuai, Z.; Li, L.; Zhu, D. Adv. Funct. Mater. 2009, 19, 272–276. doi:10.1002/adfm.200800933
Return to citation in text: [1] -
Wang, Z.; Zhao, C.; Zhao, D.; Li, C.; Zhang, J.; Wang, H. Tetrahedron 2010, 66, 2168–2174. doi:10.1016/j.tet.2010.01.056
Return to citation in text: [1] [2] -
Gronowitz, S.; Hallberg, A.; Frejd, T. Tetrahedron 1979, 35, 2607–2610. doi:10.1016/0040-4020(79)88028-X
Return to citation in text: [1] -
Huang, H.; Li, J.; Lescop, C.; Duan, Z. Org. Lett. 2011, 13, 5252–5255. doi:10.1021/ol2021302
Return to citation in text: [1] -
Liang, Y.; Geng, W.; Wei, J.; Xi, Z. Angew. Chem., Int. Ed. 2012, 51, 1934–1937. doi:10.1002/anie.201108154
Return to citation in text: [1] -
Wang, Y.; Chi, Y.; Zhang, W.-X.; Xi, Z. J. Am. Chem. Soc. 2012, 134, 2926–2929. doi:10.1021/ja211486f
Return to citation in text: [1] -
Belley, M.; Douida, Z.; Mancuso, J.; De Vleeschauwer, M. Synlett 2005, 247–250. doi:10.1055/s-2004-837230
Return to citation in text: [1] [2] -
Hill, B.; De Vleeschauwer, M.; Houde, K.; Belley, M. Synlett 1998, 407–410. doi:10.1055/s-1998-1674
Return to citation in text: [1] [2] -
Dickinson, R. P.; Iddon, B. J. Chem. Soc. C 1971, 3447–3454. doi:10.1039/J39710003447
Return to citation in text: [1] -
Fuller, L. S.; Iddon, B.; Smith, K. A. J. Chem. Soc., Perkin Trans. 1 1999, 1273–1278. doi:10.1039/a901300h
Return to citation in text: [1] -
Chernichenko, K.; Emelyanov, N.; Gridnev, I.; Nenajdenko, V. G. Tetrahedron 2011, 67, 6812–6818. doi:10.1016/j.tet.2011.06.082
Return to citation in text: [1] -
Crystal data for 3i: M = 650.96, C41H46OS3, triclinic, space group P−1, a = 9.395(2) Å, b = 11.384(2) Å, c = 18.540(3) Å, α = 83.241(4)˚, β = 79.382(4)˚, γ = 67.287(3)˚, V = 1795.3(5) Å3, Z = 2, Dcalc = 1.204 g/cm3. A colorless crystal of dimensions 0.44 × 0.35 × 0.11 mm was used for measurement at 296(2) K. The final cycle of full-matrix least-squares refinement was based on 6253 observed reflections [I>2σ(I)] and 380 variable parameters with R1 = 0.0805, wR2 = 0.1802.
Return to citation in text: [1]
| 1. | Shi, J.; Xu, L.; Li, Y.; Jia, M.; Kan, Y.; Wang, H. Org. Electron. 2013, 14, 934–941. doi:10.1016/j.orgel.2013.01.002 |
| 2. | Shi, J.; Li, Y.; Jia, M.; Xu, L.; Wang, H. J. Mater. Chem. 2011, 21, 17612–17614. doi:10.1039/c1jm14383b |
| 3. | Zhang, L.; Tan, L.; Wang, Z.; Hu, W.; Zhu, D. Chem. Mater. 2009, 21, 1993–1999. doi:10.1021/cm900369s |
| 4. | Tan, L.; Zhang, L.; Jiang, X.; Yang, X.; Wang, L.; Wang, Z.; Li, L.; Hu, W.; Shuai, Z.; Li, L.; Zhu, D. Adv. Funct. Mater. 2009, 19, 272–276. doi:10.1002/adfm.200800933 |
| 10. | Belley, M.; Douida, Z.; Mancuso, J.; De Vleeschauwer, M. Synlett 2005, 247–250. doi:10.1055/s-2004-837230 |
| 11. | Hill, B.; De Vleeschauwer, M.; Houde, K.; Belley, M. Synlett 1998, 407–410. doi:10.1055/s-1998-1674 |
| 10. | Belley, M.; Douida, Z.; Mancuso, J.; De Vleeschauwer, M. Synlett 2005, 247–250. doi:10.1055/s-2004-837230 |
| 11. | Hill, B.; De Vleeschauwer, M.; Houde, K.; Belley, M. Synlett 1998, 407–410. doi:10.1055/s-1998-1674 |
| 12. | Dickinson, R. P.; Iddon, B. J. Chem. Soc. C 1971, 3447–3454. doi:10.1039/J39710003447 |
| 6. | Gronowitz, S.; Hallberg, A.; Frejd, T. Tetrahedron 1979, 35, 2607–2610. doi:10.1016/0040-4020(79)88028-X |
| 7. | Huang, H.; Li, J.; Lescop, C.; Duan, Z. Org. Lett. 2011, 13, 5252–5255. doi:10.1021/ol2021302 |
| 8. | Liang, Y.; Geng, W.; Wei, J.; Xi, Z. Angew. Chem., Int. Ed. 2012, 51, 1934–1937. doi:10.1002/anie.201108154 |
| 9. | Wang, Y.; Chi, Y.; Zhang, W.-X.; Xi, Z. J. Am. Chem. Soc. 2012, 134, 2926–2929. doi:10.1021/ja211486f |
| 5. | Wang, Z.; Zhao, C.; Zhao, D.; Li, C.; Zhang, J.; Wang, H. Tetrahedron 2010, 66, 2168–2174. doi:10.1016/j.tet.2010.01.056 |
| 15. | Crystal data for 3i: M = 650.96, C41H46OS3, triclinic, space group P−1, a = 9.395(2) Å, b = 11.384(2) Å, c = 18.540(3) Å, α = 83.241(4)˚, β = 79.382(4)˚, γ = 67.287(3)˚, V = 1795.3(5) Å3, Z = 2, Dcalc = 1.204 g/cm3. A colorless crystal of dimensions 0.44 × 0.35 × 0.11 mm was used for measurement at 296(2) K. The final cycle of full-matrix least-squares refinement was based on 6253 observed reflections [I>2σ(I)] and 380 variable parameters with R1 = 0.0805, wR2 = 0.1802. |
| 5. | Wang, Z.; Zhao, C.; Zhao, D.; Li, C.; Zhang, J.; Wang, H. Tetrahedron 2010, 66, 2168–2174. doi:10.1016/j.tet.2010.01.056 |
| 14. | Chernichenko, K.; Emelyanov, N.; Gridnev, I.; Nenajdenko, V. G. Tetrahedron 2011, 67, 6812–6818. doi:10.1016/j.tet.2011.06.082 |
| 13. | Fuller, L. S.; Iddon, B.; Smith, K. A. J. Chem. Soc., Perkin Trans. 1 1999, 1273–1278. doi:10.1039/a901300h |
© 2013 Zhong et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)