Abstract
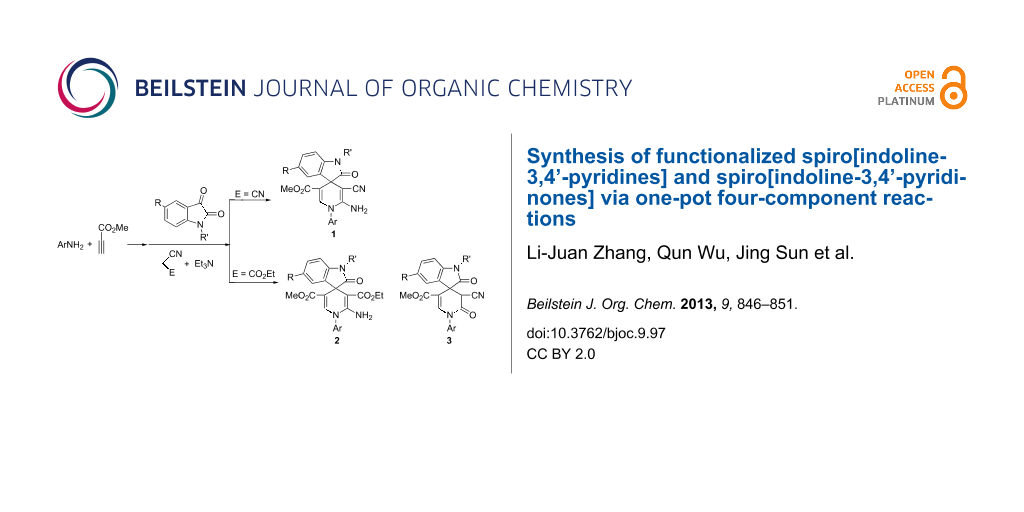
In the presence of triethylamine as catalyst, the one-pot four-component reactions of arylamines, methyl propiolate, isatin and malononitrile afforded the functionalized spiro[indoline-3,4’-pyridine] derivatives in good yields. Similar reactions with ethyl cyanoacetate successfully afforded the functionalized spiro[indoline-3,4’-pyridines] and spiro[indoline-3,4’-pyridinones] as the main products according to the structures of the arylamines and other primary amines.
Graphical Abstract
Introduction
β-Enaminones and β-enamino esters represent important synthetic building blocks for the development of versatile carbon–carbon bond-formation reactions and heterocyclic constructions [1-5]. In recent years, the in situ generated β-enamino esters, which could be easily obtained from the addition of aliphatic or aromatic primary amines to the activated alkynes, have been widely recognized as practical synthons for the synthesis of a wide variety of heterocycles and pharmaceutical compounds [6-11]. Many domino reactions have been developed by trapping this kind of β-enamino ester with sequential adding of nucleophilic or electrophilic reagents to give versatile nitrogen-containing compounds and N,O-heterocycles [12-23]. Recently, Perumal and we have both developed an efficient synthetic procedure for functionalized spiro[indoline-3,4’-pyridines] by domino reactions of in situ generated β-enamino esters, isatin and malononitrile with triethylamine as the base catalyst [24,25]. A literature survey indicated that there has been an explosion of activity around the synthesis of spirooxindoles in the past years [26-31]. Encouraged by these results, and hunting for new synthetic methods for functionalized spirooxindoles, we investigated the domino reactions of arylamines, methyl propiolate, aromatic aldehydes and malononitrile (ethyl cyanoacetate) and successfully developed a facile synthetic procedure for functionalized spiro[indoline-3,4’-pyridines] and spiro[indoline-3,4’-pyridinones].
Results and Discussion
The efficient formation of functionalized spiro[indole-3,4’-pyridines] via the four-component reaction prompted us to study the reaction scope further [25]. Another widely used electron-deficient alkyne reagent, methyl propiolate, was utilized to replace dimethyl acetylenedicarboxylate as one component. The addition reaction of aniline to methyl propiolate to give the active adducts, 3-arylaminoacrylates, usually needs more than twelve hours. Thus, we decided firstly to let arylamine and methyl propiolate react in ethanol at room temperature for 24 hours. TLC analysis indicated that the addition reaction had finished, and TLC analysis indicated that almost exclusively the desired β-arylaminoacrylates existed in the solution. Then isatin and malononitrile as well as triethylamine were added to the system and the mixture was heated under reflux for an additional 24 hours. The expected spiro[indoline-3,4’-pyridines] 1a–1p were obtained in good yields by using this one-pot domino reaction procedure (Table 1). It can be seen that aniline bearing methoxy, methyl or chloro groups all reacted smoothly to give the expected spiro[indoline-3,4’-pyridines] with marginal effect. Benzylamine also afforded good yields of the spiro product. The structures of the prepared spiro[indoline-3,4’-pyridines] 1a–1p were fully characterized by spectroscopic methods and were further confirmed by the determination of the single crystal structures of the spiro compounds 1c (Figure 1) and 1h (Figure 2).
Table 1: Synthesis of spiro[indoline-3,4’-pyridine] 1a–1p by domino reaction.
|
|
|||||
| Entry | Compd | Ar | R | R’ | Yield (%) |
|---|---|---|---|---|---|
| 1 | 1a | C6H5 | H | H | 79 |
| 2 | 1b | p-CH3C6H4 | H | H | 76 |
| 3 | 1c | p-CH3OC6H4 | H | H | 74 |
| 4 | 1d | p-ClC6H4 | H | H | 81 |
| 5 | 1e | p-BrC6H4 | H | H | 80 |
| 6 | 1f | p-ClC6H4 | Me | H | 75 |
| 7 | 1g | p-CH3C6H4 | Me | H | 73 |
| 8 | 1h | p-ClC6H4 | Cl | H | 84 |
| 9 | 1i | p-CH3C6H4 | Cl | H | 72 |
| 10 | 1j | p-ClC6H4 | H | CH2C6H5 | 64 |
| 11 | 1k | p-CH3C6H4 | H | CH2C6H5 | 75 |
| 12 | 1l | p-CH3OC6H4 | H | CH2C6H5 | 72 |
| 13 | 1m | C6H5 | H | CH2C6H5 | 66 |
| 14 | 1n | m-ClC6H4 | H | CH2C6H5 | 61 |
| 15 | 1o | m-CH3C6H4 | H | CH2C6H5 | 62 |
| 16 | 1p | C6H5CH2 | H | CH2C6H5 | 50 |
![[1860-5397-9-97-1]](/bjoc/content/figures/1860-5397-9-97-1.png?scale=1.3&max-width=1024&background=FFFFFF)
Figure 1: Molecular structure of the spiro compound 1c.
Figure 1: Molecular structure of the spiro compound 1c.
![[1860-5397-9-97-2]](/bjoc/content/figures/1860-5397-9-97-2.png?scale=1.3&max-width=1024&background=FFFFFF)
Figure 2: Molecular structure of the spiro compound 1h.
Figure 2: Molecular structure of the spiro compound 1h.
When ethyl cyanoacetate was utilized in the domino reaction under similar conditions, the reaction usually resulted in a complicated mixture of spiro[indoline-3,4’-pyridines] 2 and spiro[indoline-3,4’-pyridinones] 3 depending on whether the cyano group or the ester group was taking part in the cyclization process (Scheme 1). In order to simplify the separation process, only the main product was separated from the reaction mixtures by column chromatography. The other product was not separated or structurally characterized. The results are listed in Table 2. It is interesting to find that anilines bearing p-chloro, m-chloro or p-bromo groups afforded spiro[indoline-3,4’-pyridines] 2a–2h as the main products (Table 2, entries 1–8). Due to fact that m-nitroaniline and p-nitroaniline could not react with methyl propiolate to give the desired intermediate β-enamino ester, they were not utilized in this reaction. On the other hand p-methoxyaniline, p-methylaniline and aniline itself gave spiro[indoline-3,4’-pyridinones] 3a–3j as the main products (Table 2, entries 9–18). Thus, it seems that anilines with electron-withdrawing groups preferably give spiro[indoline-3,4’-pyridines] 2, while anilines with electron-donating groups preferably produce spiro[indoline-3,4’-pyridinones] 3.
Table 2: Synthesis of spiro[indoline-3,4’-pyridines] 2a–2h and spiro[indoline-3,4’-pyridinone] 3a–3n via domino reaction.
|
|
|||||
| Entry | Compd | Ar | R | R’ | Yield (%) |
|---|---|---|---|---|---|
| 1 | 2a | p-ClC6H4 | H | H | 49 |
| 2 | 2b | p-ClC6H4 | H | CH2C6H5 | 52 |
| 3 | 2c | p-ClC6H4 | Me | C4H9 | 47 |
| 4 | 2d | p-ClC6H4 | Me | CH2C6H5 | 35 |
| 5 | 2e | m-ClC6H4 | Me | CH2C6H5 | 36 |
| 6 | 2f | p-BrC6H4 | H | H | 52 |
| 7 | 2g | p-BrC6H4 | H | C4H9 | 36 |
| 8 | 2h | p-BrC6H4 | Me | CH2C6H5 | 56 |
| 9 | 3a | p-CH3OC6H4 | H | H | 39 |
| 10 | 3b | p-CH3OC6H4 | H | CH2C6H5 | 53 |
| 11 | 3c | p-CH3OC6H4 | H | C4H9 | 54 |
| 12 | 3d | p-CH3OC6H4 | Me | CH2C6H5 | 51 |
| 13 | 3e | p-CH3C6H4 | H | CH2C6H5 | 56 |
| 14 | 3f | p-CH3C6H4 | H | C4H9 | 47 |
| 15 | 3g | p-CH3C6H4 | Me | CH2C6H5 | 49 |
| 16 | 3h | C6H5 | Me | C4H9 | 56 |
| 17 | 3i | C6H5 | H | CH2C6H5 | 47 |
| 18 | 3j | C6H5 | Me | CH2C6H5 | 52 |
| 19 | 3k | C6H5CH2 | H | H | 68 |
| 20 | 3l | C6H5CH2 | Me | CH2C6H5 | 45 |
| 21 | 3m | C6H5CH2 | H | C4H9 | 63 |
| 22 | 3n | C6H5CH2CH2 | H | CH2C6H5 | 48 |
Benzylamine and 2-phenylethylamine could also be used in the domino reactions to give the spiro[indoline-3,4’-pyridinones] 3k–3n as the main products (Table 2, entries 19–22). The structures of the prepared spiro compounds 2a–2h and 3a–3n were fully established by spectroscopic methods. The single-crystal structures of spiro compounds 2b (Figure 3) and 3b (Figure 4) were successfully determined by X-ray diffraction methods. It should be pointed out that the 1H NMR spectra of compounds 2a–2h showed some distinguishing features. The characteristic resonance of the NH2 group usually displays one broad peak at about 7.40 ppm, which is overlapped with the peaks of aromatic protons. Comparing with the one singlet at 5.80 ppm of the NH2 group in compounds 1a–1p, the peak of the NH2 group in compounds 2a–2h is shifted to a much lower field. Secondly, the characteristic peaks of the CH2 unit of the ethoxy group in most spiro[indoline-3,4’-pyridines] 2a–2h split into two mixed peaks at about 3.75–3.72 (m, 1H), 3.37–3.34 (m, 1H), which indicated that these two protons existed in different circumstance. The CH3 unit of the ethoxy goup showed a normal peak.
![[1860-5397-9-97-3]](/bjoc/content/figures/1860-5397-9-97-3.png?scale=1.3&max-width=1024&background=FFFFFF)
Figure 3: Molecular structure of spiro compound 2b.
Figure 3: Molecular structure of spiro compound 2b.
![[1860-5397-9-97-4]](/bjoc/content/figures/1860-5397-9-97-4.png?scale=1.3&max-width=1024&background=FFFFFF)
Figure 4: Molecular structure of spiro compound 3b.
Figure 4: Molecular structure of spiro compound 3b.
From the molecular structure of the spiro compound 3b shown in Figure 4, we could clearly see that the cyano group and the phenyl group of oxindole moiety in the newly formed dihydropyridinone ring exist in cis-configuration. 1H NMR spectra of 3a–3n all display one singlet at about 5.40 ppm for the one proton in the dihydropyridinone ring, which indicated that only one isomer exists in each product. Based on the single crystal structure and 1H NMR data we could tentatively conclude that the spiro[indoline-3,4’-pyridinones] 3a–3n have cis-configuration of the cyano group and the phenyl group of the oxindole moiety.
To explain the mechanism of this domino reaction, a plausible reaction course was proposed to account for the different products based on the published reactions containing methyl propiolate [20-22], which are illustrated in Scheme 1. The first reaction is the formation of the key intermediate β-enamino ester A from the addition of arylamine to methyl propiolate. The second reaction is a Knoevenagel condensation of isatin with malononitrile or ethyl cyanoacetate under the catalysis of triethylamine to give the isatylidene deriatives B. The third reaction is a Michael addition of β-enamino ester intermediate A with isatinylidene derivative B to yield intermediate C. In the case of the reaction containing malononitrile, the nucleophilic addition of the amino group to the C–N triple bond in intermediate C resulted in spiro compound 1 with the tautomerization of the imino group to an amino group. In the case of the reaction containing ethyl cyanoacetate, the amino group could react with both the cyano group and the ester group in the adduct C. The nucleophilic addition of the amino group to the C–N triple bond finally afforded spiro[indoline-3,4’-pyridines] 2. On the other hand the amino group attacked the ester group to give spiro[indoline-3,4’-pyridinones] 3 with the elimination of ethanol. In this reaction process, the reasons that anilines with electron-withdrawing groups preferably attack the cyano group and anilines with electron-donating groups preferably attack the ester group are not very clear.
Scheme 1: The proposed mechanism for the domino reaction.
Scheme 1: The proposed mechanism for the domino reaction.
Conclusion
In summary, we have successfully developed a one-pot four-component reaction of arylamines, methyl propiolate, isatin and malononitrile or ethyl cyanoacetate with triethylamine as base catalyst. This reaction can proceed smoothly under mild conditions to afford the functionalized spiro[indoline-3,4’-pyridines] and spiro[indoline-3,4’-pyridinones] in moderate to good yields. The advantages of this reaction included readily available starting materials, mild reaction conditions, operational simplicity, a widely variety of substrates, and molecular diversity of the products. The potential uses of this reaction in synthetic and medicinal chemistry may be quite significant.
Experimental
Reagents and apparatus: Aromatic aldehydes, arylamines, methyl propiolate and other reagents are commercial reagents and were used as received. Solvents were purified by standard techniques. All reactions were monitored by TLC. Melting points were taken on a hot-plate microscope apparatus. IR spectra were obtained on a Bruker Tensor 27 spectrometer (KBr disc). NMR spectra were recorded with a Bruker AV-600 spectrometer. HPLC–MS were measured with a Fennigan LCQ Deca XP MAX instrument. High-resolution mass spectra (ESI) were obtained with a Bruker UHR–TOF maXis spectrometer. X-ray data were collected on a Bruker Smart APEX-2 CCD diffractometer. Single-crystal data for compounds 1c (CCDC 843674), 1h (CCDC 843676), 2b (CCDC 904924) and 3b (CCDC 904923) have been deposited in the Cambridge Crystallographic Data Centre.
General procedure for the synthesis of spiro[indoline-3,4’-pyridine] derivatives 1a–1p: In an analogous manner to our procedure published in [25], a solution of arylamine (2.0 mmol), methyl propiolate (2.0 mmol) in 5 mL ethanol was stirred at room temperature overnight. Then isatin (2.0 mmol), malononitrile (2.0 mmol) and triethylamine (0.4 mmol) were added. The mixture was heated under reflux for about 24 hours. Then the solution was concentrated to approximately half the volume. The resulting precipitates were collected and washed with ethanol to give the pure product for analysis.
General procedure for the synthesis of spiro[indoline-3,4’-pyridines] 2a–2h and spiro[indoline-3,4’-pyridinone] derivatives 3a–3n: A similar procedure to that above was used. A solution of arylamine (2.0 mmol), methyl propiolate (2.0 mmol, 0.168 g) in 5 mL ethanol was stirred at room temperature overnight. Then isatin (2.0 mmol), ethyl cyanoacetate (2.0 mmol, 0.226 g) and triethylamine (0.4 mmol) were added to it, and the whole mixture was heated under reflux for about 24 hours. Then the solution was concentrated to approximately half the volume, which was subjected to column chromatography with ethyl acetate and light petroleum (v/v = 1:3) as eluent to give the pure product for analysis.
Supporting Information
| Supporting Information File 1: Experimental details and detailed spectroscopic data. | ||
| Format: PDF | Size: 2.0 MB | Download |
References
-
Bagley, M. C.; Dale, J. W.; Bower, J. Chem. Commun. 2002, 1682–1683. doi:10.1039/b203900a
Return to citation in text: [1] -
Valla, A.; Valla, B.; Cartier, D.; Le Guillou, R.; Labia, R.; Potier, P. Tetrahedron Lett. 2005, 46, 6671–6674. doi:10.1016/j.tetlet.2005.07.141
Return to citation in text: [1] -
Katritzky, A. R.; Hayden, A. E.; Kirichenko, K.; Pelphrey, P.; Ji, Y. J. Org. Chem. 2004, 69, 5108–5111. doi:10.1021/jo0496594
Return to citation in text: [1] -
Vohra, R. K.; Bruneau, C.; Renaud, J.-L. Adv. Synth. Catal. 2006, 348, 2571–2574. doi:10.1002/adsc.200600343
Return to citation in text: [1] -
Sirijindalert, H.; Hansuthirakul, K.; Rashatasakhon, P.; Sukwattanasinitt, M.; Ajavakom, A. Tetrahedron 2010, 66, 5161–5167. doi:10.1016/j.tet.2010.04.102
Return to citation in text: [1] -
Shibata, Y.; Noguchi, K.; Hirano, M.; Tanaka, K. Org. Lett. 2008, 10, 2825–2828. doi:10.1021/ol800966f
Return to citation in text: [1] -
Harju, K.; Vesterinen, J.; Yli-Kauhaluoma, J. Org. Lett. 2009, 11, 2219–2221. doi:10.1021/ol900704b
Return to citation in text: [1] -
Yadav, J. S.; Reddy, B. V. S.; Yadav, N. N.; Gupta, M. K.; Sridhar, B. J. Org. Chem. 2008, 73, 6857–6859. doi:10.1021/jo8007034
Return to citation in text: [1] -
Ding, H.; Zhang, Y.; Bian, M.; Yao, W.; Ma, C. J. Org. Chem. 2008, 73, 578–584. doi:10.1021/jo702299m
Return to citation in text: [1] -
Glotova, T.; Dvorko, M. Yu.; Ushakov, I. A.; Chipanina, N. N.; Kazheva, O. N.; Chekhlov, A. N.; Dyachenko, O. A.; Gusarova, N. K.; Trofimov, B. A. Tetrahedron 2009, 65, 9814–9818. doi:10.1016/j.tet.2009.09.069
Return to citation in text: [1] -
Yavari, I.; Bayat, M. J.; Sirouspour, M.; Souri, S. Tetrahedron 2010, 66, 7995–7999. doi:10.1016/j.tet.2010.08.016
Return to citation in text: [1] -
Jiang, H.-F.; Li, J.-H.; Chen, Z.-W. Tetrahedron 2010, 66, 9721–9728. doi:10.1016/j.tet.2010.10.041
Return to citation in text: [1] -
Nguyen, T. B.; Martel, A.; Dhal, R.; Dujardin, G. Org. Lett. 2008, 10, 4493–4496. doi:10.1021/ol8017243
Return to citation in text: [1] -
Fan, M.; Yan, Z.; Liu, W.; Liang, Y. J. Org. Chem. 2005, 70, 8204–8207. doi:10.1021/jo050903g
Return to citation in text: [1] -
Naidu, B. N.; Sorenson, M. E. Org. Lett. 2005, 7, 1391–1393. doi:10.1021/ol0502073
Return to citation in text: [1] -
Alizadeh, A.; Rostamnia, S.; Zohreh, N.; Hosseinpour, R. Tetrahedron Lett. 2009, 50, 1533–1535. doi:10.1016/j.tetlet.2008.12.107
Return to citation in text: [1] -
Teimouri, M. B.; Abbasi, T. Tetrahedron 2010, 66, 3795–3800. doi:10.1016/j.tet.2010.03.058
Return to citation in text: [1] -
Sun, J.; Xia, E.-Y.; Wu, Q.; Yan, C.-G. Org. Lett. 2010, 12, 3678–3681. doi:10.1021/ol101475b
Return to citation in text: [1] -
Sun, J.; Wu, Q.; Xia, E.-Y.; Yan, C.-G. Eur. J. Org. Chem. 2011, 2981–2986. doi:10.1002/ejoc.201100008
Return to citation in text: [1] -
Sun, J.; Sun, Y.; Xia, E.-Y.; Yan, C.-G. ACS Comb. Sci. 2011, 13, 436–441. doi:10.1021/co200071v
Return to citation in text: [1] [2] -
Sun, J.; Sun, Y.; Gao, H.; Yan, C.-G. Synthesis 2012, 1069–1073. doi:10.1055/s-0031-1289718
Return to citation in text: [1] [2] -
Sun, Y.; Sun, J.; Yan, C.-G. Mol. Diversity 2012, 16, 163–171. doi:10.1007/s11030-011-9344-z
Return to citation in text: [1] [2] -
Zhang, L.-L.; Sun, J.; Yan, C.-G. Tetrahedron Lett. 2012, 53, 6965–6967. doi:10.1016/j.tetlet.2012.10.043
Return to citation in text: [1] -
Kiruthika, S. E.; Lakshmi, N. V.; Banu, B. R.; Perumal, P. T. Tetrahedron Lett. 2011, 52, 6508–6511. doi:10.1016/j.tetlet.2011.09.119
Return to citation in text: [1] -
Sun, J.; Wu, Q.; Zhang, L.; Yan, C. Chin. J. Chem. 2012, 30, 1548–1554. doi:10.1002/cjoc.201100657
Return to citation in text: [1] [2] [3] -
Trost, B. M.; Brennan, M. K. Synthesis 2009, 3003–3025. doi:10.1055/s-0029-1216975
Return to citation in text: [1] -
Singh, G. S.; Desta, Z. Y. Chem. Rev. 2012, 112, 6104–6155. doi:10.1021/cr300135y
Return to citation in text: [1] -
Ball-Jones, N. R.; Badillo, J. J.; Franz, A. K. Org. Biomol. Chem. 2012, 10, 5165–5181. doi:10.1039/c2ob25184a
Return to citation in text: [1] -
Guo, S.; Wang, R.; Li, J.; Li, C.; Deng, H.; Jia, X. Synlett 2011, 2256–2258. doi:10.1055/s-0030-1261188
Return to citation in text: [1] -
Jie, H.; Li, J.; Li, C.; Jia, X. Synlett 2012, 2274–2278. doi:10.1055/s-0032-1317030
Return to citation in text: [1] -
Sun, J.; Sun, Y.; Gong, H.; Xie, Y.-J.; Yan, C.-G. Org. Lett. 2012, 14, 5172–5175. doi:10.1021/ol302530m
Return to citation in text: [1]
| 1. | Bagley, M. C.; Dale, J. W.; Bower, J. Chem. Commun. 2002, 1682–1683. doi:10.1039/b203900a |
| 2. | Valla, A.; Valla, B.; Cartier, D.; Le Guillou, R.; Labia, R.; Potier, P. Tetrahedron Lett. 2005, 46, 6671–6674. doi:10.1016/j.tetlet.2005.07.141 |
| 3. | Katritzky, A. R.; Hayden, A. E.; Kirichenko, K.; Pelphrey, P.; Ji, Y. J. Org. Chem. 2004, 69, 5108–5111. doi:10.1021/jo0496594 |
| 4. | Vohra, R. K.; Bruneau, C.; Renaud, J.-L. Adv. Synth. Catal. 2006, 348, 2571–2574. doi:10.1002/adsc.200600343 |
| 5. | Sirijindalert, H.; Hansuthirakul, K.; Rashatasakhon, P.; Sukwattanasinitt, M.; Ajavakom, A. Tetrahedron 2010, 66, 5161–5167. doi:10.1016/j.tet.2010.04.102 |
| 26. | Trost, B. M.; Brennan, M. K. Synthesis 2009, 3003–3025. doi:10.1055/s-0029-1216975 |
| 27. | Singh, G. S.; Desta, Z. Y. Chem. Rev. 2012, 112, 6104–6155. doi:10.1021/cr300135y |
| 28. | Ball-Jones, N. R.; Badillo, J. J.; Franz, A. K. Org. Biomol. Chem. 2012, 10, 5165–5181. doi:10.1039/c2ob25184a |
| 29. | Guo, S.; Wang, R.; Li, J.; Li, C.; Deng, H.; Jia, X. Synlett 2011, 2256–2258. doi:10.1055/s-0030-1261188 |
| 30. | Jie, H.; Li, J.; Li, C.; Jia, X. Synlett 2012, 2274–2278. doi:10.1055/s-0032-1317030 |
| 31. | Sun, J.; Sun, Y.; Gong, H.; Xie, Y.-J.; Yan, C.-G. Org. Lett. 2012, 14, 5172–5175. doi:10.1021/ol302530m |
| 24. | Kiruthika, S. E.; Lakshmi, N. V.; Banu, B. R.; Perumal, P. T. Tetrahedron Lett. 2011, 52, 6508–6511. doi:10.1016/j.tetlet.2011.09.119 |
| 25. | Sun, J.; Wu, Q.; Zhang, L.; Yan, C. Chin. J. Chem. 2012, 30, 1548–1554. doi:10.1002/cjoc.201100657 |
| 12. | Jiang, H.-F.; Li, J.-H.; Chen, Z.-W. Tetrahedron 2010, 66, 9721–9728. doi:10.1016/j.tet.2010.10.041 |
| 13. | Nguyen, T. B.; Martel, A.; Dhal, R.; Dujardin, G. Org. Lett. 2008, 10, 4493–4496. doi:10.1021/ol8017243 |
| 14. | Fan, M.; Yan, Z.; Liu, W.; Liang, Y. J. Org. Chem. 2005, 70, 8204–8207. doi:10.1021/jo050903g |
| 15. | Naidu, B. N.; Sorenson, M. E. Org. Lett. 2005, 7, 1391–1393. doi:10.1021/ol0502073 |
| 16. | Alizadeh, A.; Rostamnia, S.; Zohreh, N.; Hosseinpour, R. Tetrahedron Lett. 2009, 50, 1533–1535. doi:10.1016/j.tetlet.2008.12.107 |
| 17. | Teimouri, M. B.; Abbasi, T. Tetrahedron 2010, 66, 3795–3800. doi:10.1016/j.tet.2010.03.058 |
| 18. | Sun, J.; Xia, E.-Y.; Wu, Q.; Yan, C.-G. Org. Lett. 2010, 12, 3678–3681. doi:10.1021/ol101475b |
| 19. | Sun, J.; Wu, Q.; Xia, E.-Y.; Yan, C.-G. Eur. J. Org. Chem. 2011, 2981–2986. doi:10.1002/ejoc.201100008 |
| 20. | Sun, J.; Sun, Y.; Xia, E.-Y.; Yan, C.-G. ACS Comb. Sci. 2011, 13, 436–441. doi:10.1021/co200071v |
| 21. | Sun, J.; Sun, Y.; Gao, H.; Yan, C.-G. Synthesis 2012, 1069–1073. doi:10.1055/s-0031-1289718 |
| 22. | Sun, Y.; Sun, J.; Yan, C.-G. Mol. Diversity 2012, 16, 163–171. doi:10.1007/s11030-011-9344-z |
| 23. | Zhang, L.-L.; Sun, J.; Yan, C.-G. Tetrahedron Lett. 2012, 53, 6965–6967. doi:10.1016/j.tetlet.2012.10.043 |
| 6. | Shibata, Y.; Noguchi, K.; Hirano, M.; Tanaka, K. Org. Lett. 2008, 10, 2825–2828. doi:10.1021/ol800966f |
| 7. | Harju, K.; Vesterinen, J.; Yli-Kauhaluoma, J. Org. Lett. 2009, 11, 2219–2221. doi:10.1021/ol900704b |
| 8. | Yadav, J. S.; Reddy, B. V. S.; Yadav, N. N.; Gupta, M. K.; Sridhar, B. J. Org. Chem. 2008, 73, 6857–6859. doi:10.1021/jo8007034 |
| 9. | Ding, H.; Zhang, Y.; Bian, M.; Yao, W.; Ma, C. J. Org. Chem. 2008, 73, 578–584. doi:10.1021/jo702299m |
| 10. | Glotova, T.; Dvorko, M. Yu.; Ushakov, I. A.; Chipanina, N. N.; Kazheva, O. N.; Chekhlov, A. N.; Dyachenko, O. A.; Gusarova, N. K.; Trofimov, B. A. Tetrahedron 2009, 65, 9814–9818. doi:10.1016/j.tet.2009.09.069 |
| 11. | Yavari, I.; Bayat, M. J.; Sirouspour, M.; Souri, S. Tetrahedron 2010, 66, 7995–7999. doi:10.1016/j.tet.2010.08.016 |
| 25. | Sun, J.; Wu, Q.; Zhang, L.; Yan, C. Chin. J. Chem. 2012, 30, 1548–1554. doi:10.1002/cjoc.201100657 |
| 20. | Sun, J.; Sun, Y.; Xia, E.-Y.; Yan, C.-G. ACS Comb. Sci. 2011, 13, 436–441. doi:10.1021/co200071v |
| 21. | Sun, J.; Sun, Y.; Gao, H.; Yan, C.-G. Synthesis 2012, 1069–1073. doi:10.1055/s-0031-1289718 |
| 22. | Sun, Y.; Sun, J.; Yan, C.-G. Mol. Diversity 2012, 16, 163–171. doi:10.1007/s11030-011-9344-z |
| 25. | Sun, J.; Wu, Q.; Zhang, L.; Yan, C. Chin. J. Chem. 2012, 30, 1548–1554. doi:10.1002/cjoc.201100657 |
© 2013 Zhang et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)