Search results
Search for "Negishi reaction" in Full Text gives 7 result(s) in Beilstein Journal of Organic Chemistry.
Total synthesis of asperdinones B, C, D, E and terezine D
Beilstein J. Org. Chem. 2025, 21, 2730–2738, doi:10.3762/bjoc.21.210
- gene clusters that code for a (3R) configuration. Keywords: C–H activation; indole alkaloid; Negishi reaction; prenylation; Introduction Alkaloids constitute an important family of naturally occurring compounds with a rich history in the annals of bioactive compounds [1]. Among these, the family of
Negishi-coupling-enabled synthesis of α-heteroaryl-α-amino acid building blocks for DNA-encoded chemical library applications
Beilstein J. Org. Chem. 2024, 20, 1922–1932, doi:10.3762/bjoc.20.168
- that α-heteroaryl acetates accessed through Negishi coupling can be used as key intermediates towards NCAs (Scheme 1c). Indeed, oximation of these motifs followed by reduction gave access to the desired NCAs. Results and Discussion Negishi cross-coupling step The Negishi reaction provides convenient
- access to compounds featuring C(sp2)–C(sp3) bonds. However, the general view is that this transformation is less reliable than its orthogonal counterpart, the Suzuki reaction. Recent years have seen significant developments in Negishi reaction methodologies [34][35][36][37][38][39]. In particular
- iodine (see page 11 Supporting Information File 1), affording final concentrations between 0.35 to 0.45 M in THF. The solution can be stored in the fridge under argon for one week before being used in the Negishi reaction. With concentrations above 0.4 M we observed crystallization of ethyl (bromozinc
Non-noble metal-catalyzed cross-dehydrogenation coupling (CDC) involving ether α-C(sp3)–H to construct C–C bonds
Beilstein J. Org. Chem. 2023, 19, 1259–1288, doi:10.3762/bjoc.19.94
- Negishi reaction (Zn) [1], Stille reaction (Sn) [2], Kumada reaction (Mg) [3], and Suzuki reaction (Pd) [4] (Scheme 1a). However, these coupling reactions involve a metal exchange step that generates a considerable amount of reaction waste, such as metal salts, which are not environmentally friendly. To
Synergy between supported ionic liquid-like phases and immobilized palladium N-heterocyclic carbene–phosphine complexes for the Negishi reaction under flow conditions
Beilstein J. Org. Chem. 2020, 16, 1924–1935, doi:10.3762/bjoc.16.159
- , Janssen-Cilag, S.A., C/ Jarama 75A, Toledo, Spain 10.3762/bjoc.16.159 Abstract The combination of supported ionic liquids and immobilized NHC–Pd–RuPhos led to active and more stable systems for the Negishi reaction under continuous flow conditions than those solely based on NHC–Pd–RuPhos. The fine tuning
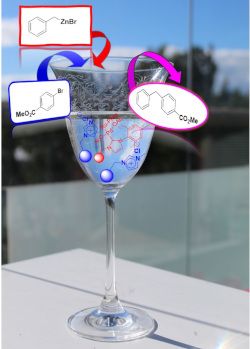
- Table 1). The slightly lower value of the Pd loading is likely to be related to the partial formation of 2:1 NHC/Pd complexes. The experimental details for the synthesis of 2a,b, 3a,b and 4a,b are given in Supporting Information File 1. The Negishi reaction of benzylzinc bromide (5) and methyl 4
- -bromobenzoate (6) to form methyl 4-benzylbenzoate (7) was used to evaluate the activity of the palladium catalysts on polymer support (Scheme 2). The Negishi reaction is a potent cross-coupling reaction in organic chemistry. Notably, it has much value for the synthesis of fine chemicals and medicinal drugs [22
Self-assembly of heteroleptic dinuclear metallosupramolecular kites from multivalent ligands via social self-sorting
Beilstein J. Org. Chem. 2015, 11, 693–700, doi:10.3762/bjoc.11.79
- (trimethylsilyl)acetylene into 7 in a yield of 85% [27]. Alkyne 7 was then subjected to a Negishi reaction with 2-bromopyridine (8) derived zinc organyl 9 to give the silyl-protected ethynylated bipyridine 10 in excellent yield of 99% which was subsequently desilylated under standard conditions to give terminal
Functionalization of heterocyclic compounds using polyfunctional magnesium and zinc reagents
Beilstein J. Org. Chem. 2011, 7, 1261–1277, doi:10.3762/bjoc.7.147
- -coupling (Negishi reaction) affords the 5-arylated furan 15 in 89% yield. Interestingly, a high chemoselectivity is observed with several heterocyclic dihalides [8][9]. Thus, the tribromopyrimidine 16 provides only the 4-zincated pyrimidine 17. After allylation, the expected allylated pyrimidine 18 is
- followed by an iodination with iodine and Ag2SO4 furnishes the tetrasubstituted aniline 125. Protection of the free amino-group followed by a Negishi-reaction provides the scaffold 126 in 80% yield (Scheme 20). Successive magnesiations at the positions 5 and 3 of the tetrasubstituted anilines 126 with
A short and efficient synthesis of valsartan via a Negishi reaction
Beilstein J. Org. Chem. 2010, 6, No. 27, doi:10.3762/bjoc.6.27




























































































































