Abstract
Controllable wettability is important for a wide range of applications, including intelligent switching, self-cleaning and oil/water separation. In this work, rapid switching and extreme wettability changes upon ultraviolet (UV) illumination were investigated. TiO2 nanoparticles were modified in solutions of trimethoxy(alkyl)silane, and the suspensions were sprayed on glass substrates. For such samples, the water contact angle (WCA) was shown to transition from a superhydrophobic (WCA ≈ 165°) to a superhydrophilic (WCA ≈ 0°) state within 10 min upon UV illumination and subsequent recovery to superhydrophobicity occurred after heat treatment. It was found that the changes in the trimethoxy(alkyl)silane upon UV illumination can explain the rapid decrease of the WCA from more than 165° to almost 0°. To further investigate the wettability transition, trimethoxy(alkyl)silane and Al2O3 nanoparticles (which are not photocatalytic) were mixed and spray-coated onto the glass substrates as the control samples. Then the unrecoverable change of trimethoxy(alkyl)silane under UV illumination can be confirmed. It was found that the presence of trimethoxy(alkyl)silane in the TiO2–trimethoxy(alkyl)silane coating served to speed up the super-wettability transition time from superhydrophobicity to superhydrophilicity, but also limited the number of wettability recycle times. With this understanding, the effect of the trimethoxy(alkyl)silane concentration on the number of recycle cycles was investigated.
Introduction
Wettability is an important property of solid surfaces governed by surface chemistry and surface topography [1,2] and has found significant applications in various fields [3-8]. Controllable wettability that can be enabled through external stimuli, such as illumination, electric fields or heating, can be applied in chemical sensors, smart filtration and separation, and microfluidic devices [9-12]. While controlling wettability through heating is mostly limited to toxic materials, such surfaces cannot be applied in human science [13,14]. Although the application of an electric field is an efficient method to achieve switchable wettability, the range of variation in the wettability is still limited [15,16]. Among the numerous approaches to achieve switchable wettability, UV illumination is highly preferred due to its environmentally friendly, non-contact and convenient manner. TiO2 nanoparticles are a common photocatalysis material that has attracted attention since it is more sensitive to UV light [17]. The use of a photochemical method to strengthen the interaction between nanoparticles and organic materials is quite common [18,19]. TiO2 nanoparticles modified with organic materials that have a low surface energy could be used to induce the property of switchable wettability under UV illumination.
In order to be useful for many applications, the ability to rapidly switch the wettability from superhydrophobicity to superhydrophilicity is imperative. Several studies based on TiO2 have been carried out to prepare surfaces exhibiting reversible wettability [20]. Wang et al. [21] prepared a TiO2 polycrystalline film which achieved drastic changes in wettability when the water contact angle (WCA) was changed from 72° and 0° under UV illumination, followed by recovery at around 70° after storing in the dark. Jin et al. [22] modified TiO2 with 7-[4-(trifluoromethoxyphenylazo)phenoxy]pentanoic acid (CF3AZO) which could reversibly switch between hydrophobic (≈145°) and hydrophilic (≈24°) under UV illumination for about 13 h. Chagas et al. [23] fabricated surfaces that were reversibly wettable by dipping polypropylene surfaces in a suspension of TiO2 modified with trimethoxypropyl saline. In this work, the wettability transition time from superhydrophobic (WCA ≈ 158°) to superhydrophilic (WCA ≈ 0°) occurred within 120 min of UV illumination.
Although large range wettability switching can be achieved in many ways, the transition from superhydrophobic to superhydrophilic always requires a longer time, ranging from a couple of days to a couple of hours [23-27]. Some studies have been carried out to reduce the wettability transition time. Sawada et al. [28] fabricated surfaces with TiO2/fluoroalkyl end-capped vinyltrimethoxysilane sol–gel where the WCA changed from about 180° to 108° in under 50 min upon UV illumination and then further reduced to 20° after another 30 min. Thus the total transition time from superhydrophobic to superhydrophilic was about 80 min. Petroffe et al. [29] modified TiO2 with 11-(4-(phenylazo)phenoxy)undecanoic acid (AzoC11) acid to fabricate a hybrid surface which could achieve a rapid change in wettability properties within 12 min, however the wettability range was only between hydrophobic (≈146°) and hydrophilic (≈21°). Qing et al. [30] fabricated superhydrophobic TiO2 nanoparticles with (heptadecafluoro-1,1,2,2-tetradecyl)trimethoxysilane, which not only exhibited reversible wettability from 160° to 0° but also reduced the transition time to 60 min.
By modifying trimethoxy(alkyl)silane, TiO2-based surfaces can achieve a faster change from superhydrophobic to superhydrophilic. However, the reason of the faster transition process has not explained. Due to the high energy of UV illumination and the photocatalysis effect of TiO2, the –CF2−, –CF3 groups of the modified trimethoxy(alkyl)silane might become photodegraded and the end of the trimethoxy(alkyl)silane would change into –OH groups without reversibility [31,32]. It can then be assumed that the degradation of trimethoxy(alkyl)silane could reduce the transition time from superhydrophobic to superhydrophilic for surfaces modified with TiO2 nanoparticles and trimethoxy(alkyl)silane.
In this study, to confirm this assumption, a series of experiments were carried out. Surfaces were coated with TiO2 nanoparticles and non-photosensitive Al2O3 nanoparticles as the control groups, respectively. The nanoparticles were modified with a short carbon chain trimethoxy(alkyl)silane, 1H,1H,2H,2H-perfluorooctyl(trimethoxy)silane (PFOS), in different concentrations. The wettability and the chemical bonding of the surfaces were tested upon UV illumination and heating treatments. The results are in agreement with the proposed assumption that the oxidization of the trimethoxy(alkyl)silane could decrease the super-wettability transition time, however the reversibility will be limited due to the irreversible oxidization process. The effect of trimethoxy(alkyl)silane concentration on the number of times the surface can be recycled was also investigated.
Results and Discussion
Characterization of the superhydrophobic coating
The hydrophilic TiO2 nanoparticles were modified in ethanol solutions of PFOS, and the suspensions were sprayed on glass substrates to make the TiO2–PFOS samples. With the same method, Al2O3–PFOS samples were prepared as control groups. As shown in Figure 1, both TiO2–PFOS and Al2O3–PFOS coated surfaces exhibited excellent superhydrophobicity, and the WCA was measured as about 165° and 171°, respectively. Based on the SEM images shown in Figure 1, two-dimensional micro–nanostructures can be obviously seen on the two kinds of surfaces due to the aggregation of nanoparticles, which can explain the good superhydrophobicity.
![[2190-4286-10-87-1]](/bjnano/content/figures/2190-4286-10-87-1.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 1: SEM images of TiO2–PFOS and Al2O3–PFOS composite surfaces.
Figure 1: SEM images of TiO2–PFOS and Al2O3–PFOS composite surfaces.
The TiO2 nanoparticles were subjected to chemical bonding as Si–O–Ti with hydrolysing PFOS. The chemical bonding is verified by the Fourier transfer infrared (FTIR) spectra of TiO2 and TiO2–PFOS coated glass surfaces, as shown in Figure 2. The asymmetric stretching vibration of the Si–O–Ti species was displayed at the absorption peak of 1065 cm−1 which further confirmed the dehydration reaction occurred between the hydrolytic PFOS and TiO2. Additionally, there were another three peaks at 1157, 1207, 1243 cm−1, which correspond to the stretching vibration of –CF2− and –CF3 groups [24,30]. Similarly, the absorption peak of –CF2− and –CF3 groups appeared in the FTIR spectra of Al2O3–PFOS but without the peak at 1065 cm−1, which indicates that there was probably no chemical bonding between Al2O3 and PFOS, suggesting the physical adhesion between Al2O3 nanoparticles and PFOS.
![[2190-4286-10-87-2]](/bjnano/content/figures/2190-4286-10-87-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: FTIR spectra of TiO2 and TiO2–PFOS, Al2O3 and Al2O3–PFOS.
Figure 2: FTIR spectra of TiO2 and TiO2–PFOS, Al2O3 and Al2O3–PFOS.
Wettability switching by UV illumination and heating
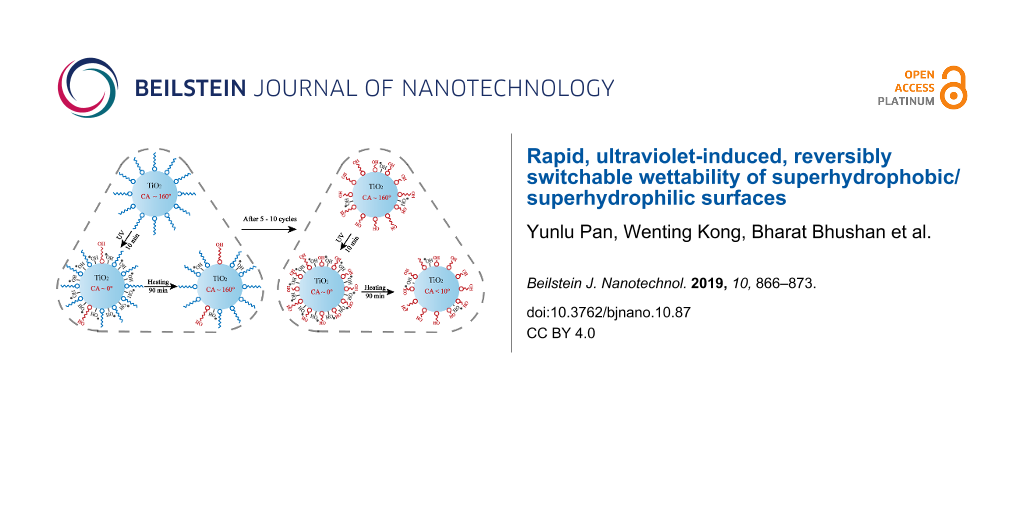
Both TiO2–PFOS coated surfaces and Al2O3–PFOS coated surfaces were directly illuminated under a UV lamp at a working distance of 10 mm, and the WCAs were recorded every 5 min. The TiO2–PFOS coated surface transitioned from superhydrophobicity to superhydrophilicity after only 10 min of UV exposure, while it took 90 min for Al2O3–PFOS surfaces to transition. Then the samples were heated at 150 °C for 90 min, the property of superhydrophobicity was recovered for the TiO2–PFOS coated surface, while the Al2O3–PFOS coated surface remained superhydrophilic. A transition time of 10 min from superhydrophobic to superhydrophilic is much faster than in previous works [22-27]. The samples in the process of wettability switching were observed by FTIR, as shown in Figure 3a. For the TiO2–PFOS surface, as the UV exposure time increases, the peak intensity at 1629 cm−1 and 3436 cm−1 (assigned to the bending vibration and stretching vibration of –OH groups, respectively) increased, while both peaks reduced after the heating treatment. At the same time, four peaks at 1207 cm−1, 1243 cm−1, 2850 cm−1 and 2919 cm−1 (attributed to the bending vibration and stretching vibration of –CF2- and –CF3 groups) undergo no obvious changes either under UV illumination or high temperature exposure. The change in transmittance for selected transitions are listed in Figure 3b, which shows that the change of –OH in TiO2 is much larger than the change of –CF2- or –CF3 in PFOS. The change of –CF2- or –CF3 can be observed after long time UV illumination as shown in Figure 4. Finally, PFOS was completely removed due to UV illumination and the chemical bonding between TiO2 and PFOS (Si–O–Ti) is without exception. The Al2O3–PFOS samples achieved the transition to super-wettability under UV illumination but required a much longer time of up to 90 min. The bands at 1210 cm−1 and 1240 cm−1 which represent the vibration of the –CF2- and –CF3 groups in PFOS was not recovered, while the –OH vibration in Al2O3 experienced no significant change.
![[2190-4286-10-87-3]](/bjnano/content/figures/2190-4286-10-87-3.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 3: (a) Contact angle and FTIR spectrum of the TiO2–PFOS surface under different treatment conditions. (b) The change in transmittance for selected transitions, –OH, –CF2- and –CF3.
Figure 3: (a) Contact angle and FTIR spectrum of the TiO2–PFOS surface under different treatment conditions. ...
![[2190-4286-10-87-4]](/bjnano/content/figures/2190-4286-10-87-4.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 4: (a) Contact angle and FTIR spectrum of the Al2O3–PFOS surface under different treatment conditions. (b) The change in transmittance for selected transitions, –OH, –CF2- and –CF3.
Figure 4: (a) Contact angle and FTIR spectrum of the Al2O3–PFOS surface under different treatment conditions....
The wettability transition of TiO2 under UV illumination and heat treatment has been previously reported and was explained by the formation of Ti–O–H bonds under UV light, while heating of the surface results in the decrease in the concentration of Ti–O–H bonds [33,34]. As reported, due to the low band gap energy of TiO2, the photo-induced electron–hole pairs are generated on the TiO2 surfaces under UV illumination. The holes lead to the production of oxygen vacancies to enhance the adsorption of hydroxy groups, while the hydroxy groups are replaced by oxygen atoms that have a stronger bond on the defect sites during heating process. However, the change in TiO2 cannot fully explain the fast wettability transition of the TiO2–PFOS coated surface. Based on the data shown in Figure 3 and Figure 4, the –CF2- and –CF3 groups of PFOS were degraded and oxidized due to the high energy of UV illumination. The oxygen in the air stimulates a metastable state which has a strong oxidizing property. In addition, the end of the fluorocarbon chains were changed into –OH groups which could decrease the WCA faster with increasing UV illumination time. It can thus be inferred that the oxidization of PFOS is due to UV illumination and the photocatalytic effect of TiO2. Unlike the changes occurring in TiO2, unfortunately, this change in PFOS is not reversible. The mechanism for the wettability transition of the TiO2–PFOS coated surface under UV illumination and heating treatment is shown in Figure 5. Since the change in PFOS is not reversible, the wettability transition cycles cannot continue indefinitely, and the surface will remain superhydrophilic when most of the –CF2- and –CF3 groups are replaced by the –OH groups on PFOS. PFOS has a short carbon chain which can enhance the change of the –CF2- and –CF3 groups and results in the much faster conversion time from superhydrophobicity to superhydrophilicity compared with the previous works [35,36]. The irreversible replacement of –CF2- and –CF3 groups by –OH groups on PFOS was also confirmed by the wettability transition of the Al2O3–PFOS coated surfaces.
![[2190-4286-10-87-5]](/bjnano/content/figures/2190-4286-10-87-5.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 5: The possible mechanism describing the transition of TiO2 and PFOS under UV illumination and heating.
Figure 5: The possible mechanism describing the transition of TiO2 and PFOS under UV illumination and heating....
Limitation of switchable cycles
To further study the wettability switching of the surfaces, four samples comprised of a uniform weight of TiO2 modified by different concentrations of PFOS were sprayed onto the glass samples. All the coated surfaces were initially superhydrophobic. Then the coated samples were placed under UV illumination until they transitioned to superhydrophilic, and were thereafter heated in an air-dry oven until they recovered their superhydrophobic property – this cycle was repeated until the surfaces could no longer recover the superhydrophobic property. For each concentration of PFOS, five identical samples were prepared for statistical reliability. The experimental results are shown in Table 1, where it can be seen that the number of cycles first increases with the concentration of PFOS, then decreases, while the switching time from superhydrophobic to superhydrophilic increases constantly with increasing PFOS concentration. This phenomena indicates that more PFOS can provide more –CF2-, –CF3 and can initially increase the maximum number of conversion cycles. However, an excess of PFOS results in an increase in the time to achieve superhydrophilicity which enhances the amount of –CF2-, –CF3 changed in one cycle, and subsequently decreases the number of possible cycles. As a result, to obtain a faster wettability transition time as well as a larger number of conversion cycles, an appropriate amount of PFOS and TiO2 is needed. It should be noted that with the same concentration of PFOS, the total UV illumination time for the surface to transition to unrecovered superhydrophilicity for TiO2–PFOS and Al2O3–PFOS is 30 min and 90 min, respectively. This indicates that the oxidization time of PFOS is increased by the photocatalytic effect of TiO2.
Table 1: Effect of PFOS concentration on the wettability conversion time and number of conversion cycles.
| Amount of PFOS in 50 mL ethanol (g) | Initial contact angle (°) | Ra (μm) | Wettability conversion time (min) | Number of cycles |
|---|---|---|---|---|
| 0.5 | 169 | 2.334 | 5 | 0–1 |
| 1.0 | 171 | 2.627 | 10 | 2–3 |
| 1.5 | 172 | 2.550 | 30 | 5–6 |
| 2.0 | 168 | 2.877 | 60 | 3–4 |
For the sample with 1.5 g PFOS, the CA of the six cycles and the images of the conversion processes in the first and the last cycles are shown in Figure 6. The transition time from superhydrophobic to superhydrophilic decreased from the first cycle to the last cycle. This can be explained by the unrecoverable dissociation of the –CF2- and –CF3 groups, which is also in agreement with the proposed mechanism in Figure 5.
![[2190-4286-10-87-6]](/bjnano/content/figures/2190-4286-10-87-6.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 6: Reversible superhydrophobic/superhydrophilic switching of the composite surface under UV illumination and heating (top), and the first and last cycle wettability switching process of a sample (bottom).
Figure 6: Reversible superhydrophobic/superhydrophilic switching of the composite surface under UV illuminati...
Conclusion
TiO2 nanoparticles modified with PFOS (which has relatively short carbon chains) were applied on glass substrates to achieve fast, switchable super-wettability, demonstrated by a WCA that could be recovered from ≈165° to almost 0°. The experimental results show that the PFOS could reduce the conversion time from nearly 1 h to about 10 min, and the superhydrophobicity of the surfaces could be recovered after heating for 90 min at 120 °C. However, it was shown that certain concentrations of PFOS limit the number of recovery cycles, whereby the optimal concentration of PFOS was identified to achieve about 5 to 10 cycles. The unrecoverable change of the –CF2- and –CF3 groups on PFOS and the recoverable change of –OH groups on TiO2 were recorded by FTIR. These effects can explain both the shorter transition time and the limitation that (initially) the change of the –CF2- and –CF3 groups leads to a quicker conversion time from superhydrophobicity to superhydrophilicity. However, this is only true when most of the –CF2- and –CF3 groups are changed unrecoverably, and the whole surface cannot not transition back to a superhydrophobic state.
Experimental
Materials and chemicals
Titanium dioxide (TiO2, rutile) with a diameter of ≈25 nm and aluminium dioxide (Al2O3) with a diameter of ≈30 nm were acquired from Shanghai Aladdin Bio-Chem Technology Corporation (Shanghai, China). 1H,1H,2H,2H-Perfluorooctyl(trimethoxy)silane (PFOS) was obtained from Shanghai Macklin Biochemical Corporation (Shanghai, China). Other chemicals include 99.9% ethanol used as the solvent and deionized water was used in the CA measurements.
Preparation of coating surfaces
Rutile phase TiO2 was modified by a simple hydrolysis reaction in order to obtain superhydrophobicity. In the modification process, 1 g of PFOS was dissolved into 50 mL ethanol and stirred for 1 h at ambient temperature in order to fully hydrolyse fluoroalkylsilane. Next, 3 g of rutile TiO2 was added into the solution and stirred for another 1 h to form the suspension. The suspension could be used to spray or paint a substrate with a spray gun (20 kPa). When the surfaces were dried, the superhydrophobicity is revealed. Al2O3 was modified by the same method as TiO2.
UV illumination and heating experiments
A UV lamp (30 W) obtained from Cnlight Optical-Electrical Technology Corporation (Guangdong, China) was used to generate the UV illumination. The ultraviolet power density was set at 94 µW/cm2 and the wavelength was 254 nm. The change of the WCA under UV light illumination was measured at short intervals. For the heating process, which was employed to recover the superhydrophobicity, the surface was heated in the air-dry oven at 150 °C for 90 min after UV illumination. The switching time from superhydrophilic to superhydrophobic was found to be reduced with increasing heating temperature.
Characterization
The microstructure of the coating surfaces was studied using scanning electron microscopy (SEM, ZEISS MERLIN Compact SEM, operated at a 20 kV acceleration voltage, Carl Zeiss Jena, Germany), and the surface roughness was measured with a laser confocal microscope (LCM, OLS5000, Olympus, Japan). The chemical modification and the end group changes on the surfaces was studied by Fourier transform infrared spectroscopy (FTIR, Nicolet iS50, Thermo Scientific, USA). The contact angles were measured at room temperature with an optical contact angle meter (DropMeterTM Element A-60, Maist, Ningbo, China), where the static CAs of the droplets (6 µL) placed onto the surfaces were measured five times at different locations.
References
-
Israelachvili, J. N. Adhesion, Friction and Lubrication of Molecularly Smooth Surfaces. In Fundamentals of Friction: Macroscopic and Microscopic Processes; Singer, I. L.; Pollock, H. M., Eds.; NATO ASI Series (Series E: Applied Sciences), Vol. 220; Springer Netherlands: Dordrecht, Netherlands, 1992. doi:10.1007/978-94-011-2811-7_19
Return to citation in text: [1] -
Bhushan, B. Biomimetics; Springer International Publishing: Cham, Switzerland, 2018. doi:10.1007/978-3-642-25408-6
Return to citation in text: [1] -
Sun, Z.; Liao, T.; Liu, K.; Jiang, L.; Kim, J. H.; Dou, S. X. Small 2014, 10, 3001–3006. doi:10.1002/smll.201400516
Return to citation in text: [1] -
Jin, X.; Shi, B.; Zheng, L.; Pei, X.; Zhang, X.; Sun, Z.; Du, Y.; Kim, J. H.; Wang, X.; Dou, S.; Liu, K.; Jiang, L. Adv. Funct. Mater. 2014, 24, 2721–2726. doi:10.1002/adfm.201304184
Return to citation in text: [1] -
Wang, Y.; Ma, K.; Xin, J. H. Adv. Funct. Mater. 2018, 28, 1705128. doi:10.1002/adfm.201705128
Return to citation in text: [1] -
Jeevanandam, J.; Barhoum, A.; Chan, Y. S.; Dufresne, A.; Danquah, M. K. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. doi:10.3762/bjnano.9.98
Return to citation in text: [1] -
Li, F.; Wang, Z.; Huang, S.; Pan, Y.; Zhao, X. Adv. Funct. Mater. 2018, 28, 1706867. doi:10.1002/adfm.201706867
Return to citation in text: [1] -
Xu, Z.; Zhou, H.; Tan, S.; Jiang, X.; Wu, W.; Shi, J.; Chen, P. Beilstein J. Nanotechnol. 2018, 9, 508–519. doi:10.3762/bjnano.9.49
Return to citation in text: [1] -
Sun, Z.; Liao, T.; Liu, K.; Jiang, L.; Kim, J. H.; Dou, S. X. Nano Res. 2013, 6, 726–735. doi:10.1007/s12274-013-0350-6
Return to citation in text: [1] -
Bhushan, B., Ed. Springer Handbook of Nanotechnology; Springer Handbooks; Springer Berlin: Berlin, Germany, 2017. doi:10.1007/978-3-662-54357-3
Return to citation in text: [1] -
Liang, C.; Wen, J.; Liao, X. Beilstein J. Nanotechnol. 2018, 9, 1793–1801. doi:10.3762/bjnano.9.170
Return to citation in text: [1] -
Dalei, J.; Shiyu, S.; Lei, H. Int. J. Heat Mass Transfer 2018, 128, 1344–1350. doi:10.1016/j.ijheatmasstransfer.2018.08.006
Return to citation in text: [1] -
Khongtong, S.; Ferguson, G. S. Langmuir 2004, 20, 9992–10000. doi:10.1021/la0497191
Return to citation in text: [1] -
Muthiah, P.; Boyle, T. J.; Sigmund, W. Macromol. Mater. Eng. 2013, 298, 1251–1258. doi:10.1002/mame.201200411
Return to citation in text: [1] -
Zhu, L.; Xu, J.; Xiu, Y.; Sun, Y.; Hess, D. W.; Wong, C.-P. J. Phys. Chem. B 2006, 110, 15945–15950. doi:10.1021/jp063265u
Return to citation in text: [1] -
Zhang, G.; Duan, Z.; Wang, Q.; Li, L.; Yao, W.; Liu, C. Appl. Surf. Sci. 2018, 427, 628–635. doi:10.1016/j.apsusc.2017.08.082
Return to citation in text: [1] -
Fujishima, A.; Zhang, X.; Tryk, D. A. Surf. Sci. Rep. 2008, 63, 515–582. doi:10.1016/j.surfrep.2008.10.001
Return to citation in text: [1] -
Li, W.; Guo, T.; Meng, T.; Huang, Y.; Li, X.; Yan, W.; Wang, S.; Li, X. Appl. Surf. Sci. 2013, 283, 12–18. doi:10.1016/j.apsusc.2013.05.085
Return to citation in text: [1] -
Awaja, F.; Gilbert, M.; Kelly, G.; Fox, B.; Pigram, P. J. Prog. Polym. Sci. 2009, 34, 948–968. doi:10.1016/j.progpolymsci.2009.04.007
Return to citation in text: [1] -
Lai, Y.; Huang, J.; Cui, Z.; Ge, M.; Zhang, K.-Q.; Chen, Z.; Chi, L. Small 2016, 12, 2203–2224. doi:10.1002/smll.201501837
Return to citation in text: [1] -
Wang, R.; Hashimoto, K.; Fujishima, A.; Chikuni, M.; Kojima, E.; Kitamura, A.; Shimohigoshi, M.; Watanabe, T. Nature 1997, 388, 431–432. doi:10.1038/41233
Return to citation in text: [1] -
Jin, C.; Yan, R.; Huang, J. J. Mater. Chem. 2011, 21, 17519–17525. doi:10.1039/c1jm13399c
Return to citation in text: [1] [2] -
Chagas, G. R.; Weibel, D. E. Polym. Bull. 2017, 74, 1965–1978. doi:10.1007/s00289-016-1817-x
Return to citation in text: [1] [2] [3] -
Villafiorita Monteleone, F.; Caputo, G.; Canale, C.; Cozzoli, P. D.; Cingolani, R.; Fragouli, D.; Athanassiou, A. Langmuir 2010, 26, 18557–18563. doi:10.1021/la1026398
Return to citation in text: [1] [2] [3] -
Ding, X.; Zhou, S.; Gu, G.; Wu, L. J. Mater. Chem. 2011, 21, 6161–6164. doi:10.1039/c0jm04546b
Return to citation in text: [1] [2] -
Kong, Y.; Liu, Y.; Xin, J. H. J. Mater. Chem. 2011, 21, 17978–17987. doi:10.1039/c1jm12516h
Return to citation in text: [1] [2] -
Sun, T.; Wang, G.; Feng, L.; Liu, B.; Ma, Y.; Jiang, L.; Zhu, D. Angew. Chem., Int. Ed. 2004, 43, 357–360. doi:10.1002/anie.200352565
Return to citation in text: [1] [2] -
Sawada, E.; Kakehi, H.; Chounan, Y.; Miura, M.; Sato, Y.; Isu, N.; Sawada, H. Composites, Part B 2010, 41, 498–502. doi:10.1016/j.compositesb.2010.04.002
Return to citation in text: [1] -
Petroffe, G.; Wang, C.; Sallenave, X.; Sini, G.; Goubard, F.; Péralta, S. J. Mater. Chem. A 2015, 3, 11533–11542. doi:10.1039/c5ta01710f
Return to citation in text: [1] -
Qing, Y.; Yang, C.; Yu, N.; Shang, Y.; Sun, Y.; Wang, L.; Liu, C. Chem. Eng. J. 2016, 290, 37–44. doi:10.1016/j.cej.2016.01.013
Return to citation in text: [1] [2] -
Ito, Y.; Heydari, M.; Hashimoto, A.; Konno, T.; Hirasawa, A.; Hori, S.; Kurita, K.; Nakajima, A. Langmuir 2007, 23, 1845–1850. doi:10.1021/la0624992
Return to citation in text: [1] -
Liu, C. S.; Zheng, D. M.; Zhou, J. G.; Wan, Y.; Li, Z. W. Mater. Sci. Forum 2011, 688, 102–106. doi:10.4028/www.scientific.net/msf.688.102
Return to citation in text: [1] -
Kumar, S. G.; Devi, L. G. J. Phys. Chem. A 2011, 115, 13211–13241. doi:10.1021/jp204364a
Return to citation in text: [1] -
Nakata, K.; Fujishima, A. J. Photochem. Photobiol., C 2012, 13, 169–189. doi:10.1016/j.jphotochemrev.2012.06.001
Return to citation in text: [1] -
Sugimura, H.; Ushiyama, K.; Hozumi, A.; Takai, O. Langmuir 2000, 16, 885–888. doi:10.1021/la990953e
Return to citation in text: [1] -
Hou, W.; Wang, Q. Langmuir 2009, 25, 6875–6879. doi:10.1021/la900151y
Return to citation in text: [1]
| 33. | Kumar, S. G.; Devi, L. G. J. Phys. Chem. A 2011, 115, 13211–13241. doi:10.1021/jp204364a |
| 34. | Nakata, K.; Fujishima, A. J. Photochem. Photobiol., C 2012, 13, 169–189. doi:10.1016/j.jphotochemrev.2012.06.001 |
| 24. | Villafiorita Monteleone, F.; Caputo, G.; Canale, C.; Cozzoli, P. D.; Cingolani, R.; Fragouli, D.; Athanassiou, A. Langmuir 2010, 26, 18557–18563. doi:10.1021/la1026398 |
| 30. | Qing, Y.; Yang, C.; Yu, N.; Shang, Y.; Sun, Y.; Wang, L.; Liu, C. Chem. Eng. J. 2016, 290, 37–44. doi:10.1016/j.cej.2016.01.013 |
| 22. | Jin, C.; Yan, R.; Huang, J. J. Mater. Chem. 2011, 21, 17519–17525. doi:10.1039/c1jm13399c |
| 23. | Chagas, G. R.; Weibel, D. E. Polym. Bull. 2017, 74, 1965–1978. doi:10.1007/s00289-016-1817-x |
| 24. | Villafiorita Monteleone, F.; Caputo, G.; Canale, C.; Cozzoli, P. D.; Cingolani, R.; Fragouli, D.; Athanassiou, A. Langmuir 2010, 26, 18557–18563. doi:10.1021/la1026398 |
| 25. | Ding, X.; Zhou, S.; Gu, G.; Wu, L. J. Mater. Chem. 2011, 21, 6161–6164. doi:10.1039/c0jm04546b |
| 26. | Kong, Y.; Liu, Y.; Xin, J. H. J. Mater. Chem. 2011, 21, 17978–17987. doi:10.1039/c1jm12516h |
| 27. | Sun, T.; Wang, G.; Feng, L.; Liu, B.; Ma, Y.; Jiang, L.; Zhu, D. Angew. Chem., Int. Ed. 2004, 43, 357–360. doi:10.1002/anie.200352565 |
| 1. | Israelachvili, J. N. Adhesion, Friction and Lubrication of Molecularly Smooth Surfaces. In Fundamentals of Friction: Macroscopic and Microscopic Processes; Singer, I. L.; Pollock, H. M., Eds.; NATO ASI Series (Series E: Applied Sciences), Vol. 220; Springer Netherlands: Dordrecht, Netherlands, 1992. doi:10.1007/978-94-011-2811-7_19 |
| 2. | Bhushan, B. Biomimetics; Springer International Publishing: Cham, Switzerland, 2018. doi:10.1007/978-3-642-25408-6 |
| 15. | Zhu, L.; Xu, J.; Xiu, Y.; Sun, Y.; Hess, D. W.; Wong, C.-P. J. Phys. Chem. B 2006, 110, 15945–15950. doi:10.1021/jp063265u |
| 16. | Zhang, G.; Duan, Z.; Wang, Q.; Li, L.; Yao, W.; Liu, C. Appl. Surf. Sci. 2018, 427, 628–635. doi:10.1016/j.apsusc.2017.08.082 |
| 30. | Qing, Y.; Yang, C.; Yu, N.; Shang, Y.; Sun, Y.; Wang, L.; Liu, C. Chem. Eng. J. 2016, 290, 37–44. doi:10.1016/j.cej.2016.01.013 |
| 13. | Khongtong, S.; Ferguson, G. S. Langmuir 2004, 20, 9992–10000. doi:10.1021/la0497191 |
| 14. | Muthiah, P.; Boyle, T. J.; Sigmund, W. Macromol. Mater. Eng. 2013, 298, 1251–1258. doi:10.1002/mame.201200411 |
| 31. | Ito, Y.; Heydari, M.; Hashimoto, A.; Konno, T.; Hirasawa, A.; Hori, S.; Kurita, K.; Nakajima, A. Langmuir 2007, 23, 1845–1850. doi:10.1021/la0624992 |
| 32. | Liu, C. S.; Zheng, D. M.; Zhou, J. G.; Wan, Y.; Li, Z. W. Mater. Sci. Forum 2011, 688, 102–106. doi:10.4028/www.scientific.net/msf.688.102 |
| 9. | Sun, Z.; Liao, T.; Liu, K.; Jiang, L.; Kim, J. H.; Dou, S. X. Nano Res. 2013, 6, 726–735. doi:10.1007/s12274-013-0350-6 |
| 10. | Bhushan, B., Ed. Springer Handbook of Nanotechnology; Springer Handbooks; Springer Berlin: Berlin, Germany, 2017. doi:10.1007/978-3-662-54357-3 |
| 11. | Liang, C.; Wen, J.; Liao, X. Beilstein J. Nanotechnol. 2018, 9, 1793–1801. doi:10.3762/bjnano.9.170 |
| 12. | Dalei, J.; Shiyu, S.; Lei, H. Int. J. Heat Mass Transfer 2018, 128, 1344–1350. doi:10.1016/j.ijheatmasstransfer.2018.08.006 |
| 28. | Sawada, E.; Kakehi, H.; Chounan, Y.; Miura, M.; Sato, Y.; Isu, N.; Sawada, H. Composites, Part B 2010, 41, 498–502. doi:10.1016/j.compositesb.2010.04.002 |
| 3. | Sun, Z.; Liao, T.; Liu, K.; Jiang, L.; Kim, J. H.; Dou, S. X. Small 2014, 10, 3001–3006. doi:10.1002/smll.201400516 |
| 4. | Jin, X.; Shi, B.; Zheng, L.; Pei, X.; Zhang, X.; Sun, Z.; Du, Y.; Kim, J. H.; Wang, X.; Dou, S.; Liu, K.; Jiang, L. Adv. Funct. Mater. 2014, 24, 2721–2726. doi:10.1002/adfm.201304184 |
| 5. | Wang, Y.; Ma, K.; Xin, J. H. Adv. Funct. Mater. 2018, 28, 1705128. doi:10.1002/adfm.201705128 |
| 6. | Jeevanandam, J.; Barhoum, A.; Chan, Y. S.; Dufresne, A.; Danquah, M. K. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. doi:10.3762/bjnano.9.98 |
| 7. | Li, F.; Wang, Z.; Huang, S.; Pan, Y.; Zhao, X. Adv. Funct. Mater. 2018, 28, 1706867. doi:10.1002/adfm.201706867 |
| 8. | Xu, Z.; Zhou, H.; Tan, S.; Jiang, X.; Wu, W.; Shi, J.; Chen, P. Beilstein J. Nanotechnol. 2018, 9, 508–519. doi:10.3762/bjnano.9.49 |
| 29. | Petroffe, G.; Wang, C.; Sallenave, X.; Sini, G.; Goubard, F.; Péralta, S. J. Mater. Chem. A 2015, 3, 11533–11542. doi:10.1039/c5ta01710f |
| 21. | Wang, R.; Hashimoto, K.; Fujishima, A.; Chikuni, M.; Kojima, E.; Kitamura, A.; Shimohigoshi, M.; Watanabe, T. Nature 1997, 388, 431–432. doi:10.1038/41233 |
| 23. | Chagas, G. R.; Weibel, D. E. Polym. Bull. 2017, 74, 1965–1978. doi:10.1007/s00289-016-1817-x |
| 20. | Lai, Y.; Huang, J.; Cui, Z.; Ge, M.; Zhang, K.-Q.; Chen, Z.; Chi, L. Small 2016, 12, 2203–2224. doi:10.1002/smll.201501837 |
| 23. | Chagas, G. R.; Weibel, D. E. Polym. Bull. 2017, 74, 1965–1978. doi:10.1007/s00289-016-1817-x |
| 24. | Villafiorita Monteleone, F.; Caputo, G.; Canale, C.; Cozzoli, P. D.; Cingolani, R.; Fragouli, D.; Athanassiou, A. Langmuir 2010, 26, 18557–18563. doi:10.1021/la1026398 |
| 25. | Ding, X.; Zhou, S.; Gu, G.; Wu, L. J. Mater. Chem. 2011, 21, 6161–6164. doi:10.1039/c0jm04546b |
| 26. | Kong, Y.; Liu, Y.; Xin, J. H. J. Mater. Chem. 2011, 21, 17978–17987. doi:10.1039/c1jm12516h |
| 27. | Sun, T.; Wang, G.; Feng, L.; Liu, B.; Ma, Y.; Jiang, L.; Zhu, D. Angew. Chem., Int. Ed. 2004, 43, 357–360. doi:10.1002/anie.200352565 |
| 18. | Li, W.; Guo, T.; Meng, T.; Huang, Y.; Li, X.; Yan, W.; Wang, S.; Li, X. Appl. Surf. Sci. 2013, 283, 12–18. doi:10.1016/j.apsusc.2013.05.085 |
| 19. | Awaja, F.; Gilbert, M.; Kelly, G.; Fox, B.; Pigram, P. J. Prog. Polym. Sci. 2009, 34, 948–968. doi:10.1016/j.progpolymsci.2009.04.007 |
| 35. | Sugimura, H.; Ushiyama, K.; Hozumi, A.; Takai, O. Langmuir 2000, 16, 885–888. doi:10.1021/la990953e |
| 36. | Hou, W.; Wang, Q. Langmuir 2009, 25, 6875–6879. doi:10.1021/la900151y |
| 17. | Fujishima, A.; Zhang, X.; Tryk, D. A. Surf. Sci. Rep. 2008, 63, 515–582. doi:10.1016/j.surfrep.2008.10.001 |
| 22. | Jin, C.; Yan, R.; Huang, J. J. Mater. Chem. 2011, 21, 17519–17525. doi:10.1039/c1jm13399c |
© 2019 Pan et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0). Please note that the reuse, redistribution and reproduction in particular requires that the authors and source are credited.
The license is subject to the Beilstein Journal of Nanotechnology terms and conditions: (https://www.beilstein-journals.org/bjnano)