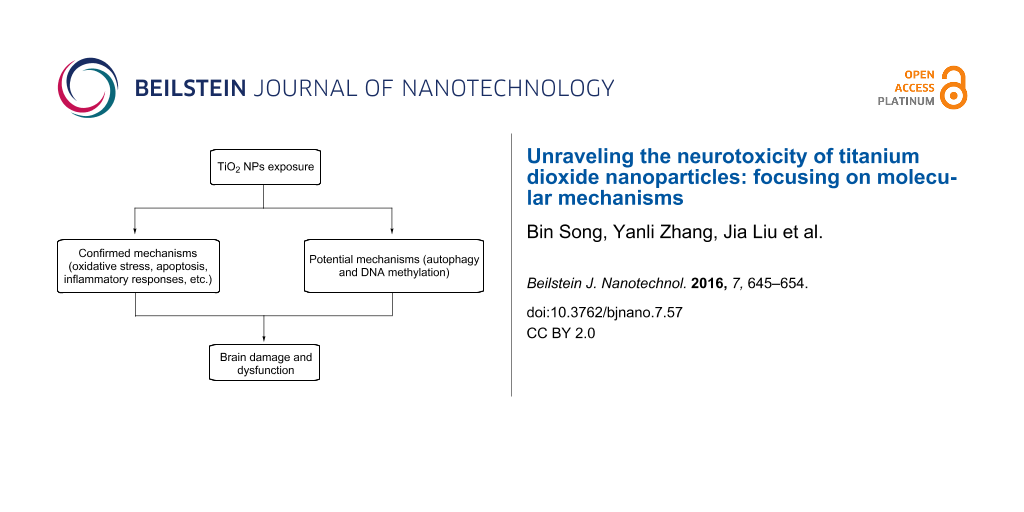
Abstract
Titanium dioxide nanoparticles (TiO2 NPs) possess unique characteristics and are widely used in many fields. Numerous in vivo studies, exposing experimental animals to these NPs through systematic administration, have suggested that TiO2 NPs can accumulate in the brain and induce brain dysfunction. Nevertheless, the exact mechanisms underlying the neurotoxicity of TiO2 NPs remain unclear. However, we have concluded from previous studies that these mechanisms mainly consist of oxidative stress (OS), apoptosis, inflammatory response, genotoxicity, and direct impairment of cell components. Meanwhile, other factors such as disturbed distributions of trace elements, disrupted signaling pathways, dysregulated neurotransmitters and synaptic plasticity have also been shown to contribute to neurotoxicity of TiO2 NPs. Recently, studies on autophagy and DNA methylation have shed some light on possible mechanisms of nanotoxicity. Therefore, we offer a new perspective that autophagy and DNA methylation could contribute to neurotoxicity of TiO2 NPs. Undoubtedly, more studies are needed to test this idea in the future. In short, to fully understand the health threats posed by TiO2 NPs and to improve the bio-safety of TiO2 NPs-based products, the neurotoxicity of TiO2 NPs must be investigated comprehensively through studying every possible molecular mechanism.
Introduction
Titanium dioxide nanoparticles, smaller than 1 μm in at least one dimension, possess specific physico-chemical characteristics [1] including antibacterial, ultraviolet-absorbing, photocatalytic, and self-cleaning properties [2]. Thus, TiO2 NPs are widely used in cosmetics, sun screens, ceramics, paints, packaging, lithium batteries, the food industry, and in medical applications [3]. However, the rapid development of nanotechnology and widespread applications of products containing TiO2 NPs have increased the risk of exposure. Therefore, numerous in vivo and in vitro studies have been performed to scrutinize the potential toxic properties of TiO2 NPs in recent years [4]. Research has demonstrated that TiO2 NPs can be detected in the main organs of experimental animals [5,6] and in exhaled breath condensate of exposed workers [7]. This accumulation can in turn damage affected organs and induce dysfunction.
The brain is of particular interest, as it is unable to regenerate from damage. Consequently, the neurotoxicity of nanomaterials should receive considerable attention. For this reason, we discussed the application and bio-distribution of TiO2 NPs, pathways through which they are translocated into the brain, harmful effects induced by them on the brain, and factors that can regulate their neurotoxic properties in our recent review [8]. However, the molecular mechanisms underlying this neurotoxicity were not discussed in detail. Therefore, in this review, we aim to discuss all previously described molecular mechanisms underlying the neurotoxicity of TiO2 NPs by summarizing published articles. From our research, we conclude that the major mechanisms are oxidative stress (OS), inflammatory responses, apoptosis, genotoxicity, and direct impairment of cell components. However, it appears that TiO2 NPs-induced neurotoxicity results from multiple mechanisms. Furthermore, other minor mechanisms exist and include disturbed distributions of trace elements, disrupted signaling pathways, dysregulated neurotransmitters and synaptic plasticity (Table 1 and Table 2).
Table 1: Mechanisms of neurotoxicity of titanium dioxide nanoparticles in in vivo studies.
| objects | administration route | mechanisms of neurotoxicity | references |
|---|---|---|---|
| rats | intravenous injection | indirect mechanism (induced by cytokines and pro-inflammatory mediators in systemic circulation) | [6] |
| mice | nasal administration | inflammatory response (over-proliferation in glia cells) | [29] |
| rats | intravenous injection | OS and angiotensin system | [22] |
| rats | intravenous injection | multiple (OS, inflammatory response and DNA damage) | [55] |
| mice | oral administration | OS (ROS and anti-oxidant enzymes disturbed) | [23] |
| mice | inhalation | OS (H2O2 and MDA elevated) | [24] |
| mice | oral administration | other mechanisms | [57] |
| mice | intranasal administration | inflammatory response | [30] |
| pregnant rats | subcutaneous injection | OS | [26] |
| pregnant rats | oral administration | other mechanisms (cell proliferation inhibited) | [58] |
| pregnant mice | subcutaneous injection | other mechanisms (disrupted dopamine systems) | [59,60] |
| pregnant mice | subcutaneous injection | multiple mechanisms (apoptosis, OS and neurotransmitters) | [50] |
| mice | nasal instillation | OS | [21] |
| mice | nasal instillation | multiple mechanisms (OS and inflammatory response) | [49] |
| mice | delivery in abdominal cavity | OS | [25] |
| mice | intragastric administration | other mechanisms (disturbed distributions of trace elements, enzymes and neurotransmitters) | [61] |
| mice | intragastric administration | multiple mechanisms (apoptosis and OS) | [54] |
| mice | intranasal administration | P38-Nrf-2-mediated OS | [31] |
| neonatal rats | lactation exposure orally | disturbed synaptic plasticity | [62] |
| rats | trachea administration | inflammatory response | [32] |
| mice | injection in abdominal cavity | genotoxicity induced by OS | [43] |
Table 2: Main mechanisms of neurotoxicity of titanium dioxide nanoparticles in in vitro studies.
| cell types | mechanisms of neurotoxicity | references |
|---|---|---|
| BV2 | OS (ROS, H2O2 elevated) | [19,20] |
| primary hippocampal neurons | multiple mechanisms (disrupted glutamate metabolism and dysregulated levels of NMDARs) | [56] |
| primary hippocampal neurons | apoptosis mediated by mitochondria- and endoplasmic reticulum-pathways | [35] |
| primary astrocytes | direct impairment of mitochondria and ROS | [38] |
| D384 and SH-SY5Y | direct impairment of mitochondria and cell membrane | [39] |
| SH-SY5Y | direct impairment of microtubules and cell morphology | [40] |
| SH-SY5Y | multiple mechanisms (changed cell cycle, apoptosis, and DNA damage) | [53] |
| C6 and U373 | OS and impairment of mitochondria | [48] |
| C6 and U373 | multiple mechanisms (inhibited cell proliferation, morphological change and apoptosis) | [52] |
| N9 | apoptosis | [36] |
| U87 | apoptosis | [37] |
| PC12 | multiple mechanisms (OS and apoptosis) | [51] |
| PC12 | other mechanisms (signaling pathway activated and arrested cell cycle) | [63] |
Recent studies have reported that autophagy [9] and DNA methylation [10,11] are also involved in nanotoxicity (Table 3). Therefore, we hypothesized that autophagy and DNA methylation can be included as major mechanisms underlying the neurotoxicity of TiO2 NPs. Autophagy (from Greek, “auto” meaning oneself and “phagy” meaning to eat) was identified as a unique adaptation of cells to starvation and involves the cellular degradative pathway through which cytoplasmic cargo is delivered to the lysosome. Autophagy also acts as a dynamic recycling system wherein new building blocks and energy, for cellular renovation and homeostasis, are produced [12].
The literal meaning of “epigenetics” is “outside conventional genetics”, which is defined as all heritable alternations in gene expression. These stable alternations are not caused by changes to DNA sequence itself, but instead arise during development and cell proliferation [13,14]. DNA methylation is the one of the most extensively studied epigenetic mechanisms. Whether TiO2 NPs are able to induce neurotoxicity through altering autophagy or DNA methylation status in brain remains uncertain. Further studies are required to further verify their role in neurotoxicity induced by TiO2 NPs. To fully understand threats to the brain posed by TiO2 NPs, and improve the bio-safety of TiO2 NPs-based products, neurotoxicity of these compounds must be investigated comprehensively.
Review
Established mechanisms underlying the neurotoxicity of TiO2 NPs
To fully understand potential health threats posed by TiO2 NPs, we summarized recent articles about the neurotoxicity of TiO2 NPs in our recently published review. We found that after rats or mice were exposed to TiO2 NPs via several administration routes (e.g., nasal instillation, subcutaneous injection and oral exposure), NPs can be absorbed and translocated into the brain mainly through the blood–brain barrier (BBB) or the nose-to-brain pathway, which bypasses the BBB. Given that TiO2 NPs were able to pass the placental barrier and accumulate in the fetal brain by penetrating the undeveloped BBB, TiO2 NPs exposure during gestation can impair fetal brain development. Based on the limited excretion rate from brain, even low-dose exposure to TiO2 NPs can lead to gradual accumulation over a long period of time. This accumulation can in turn affect brain development, impair brain function, and can even result in disabilities in learning and memory assessed by poor performance in behavioral tests.
As stated previously, the major mechanisms of TiO2 NPs-induced neurotoxicity are oxidative stress (OS), inflammatory responses, apoptosis, genotoxicity, and direct impairment of cell components. However, in most situations, neurotoxicity occurs through multiple mechanisms. Furthermore, other minor mechanisms include disturbed distributions of trace elements, disrupted signaling pathways, dysregulated neurotransmitters, and synaptic plasticity.
Oxidative stress mechanism
Oxidative stress (OS) is defined as the production of reactive oxygen species (ROS) or/and reactive nitrogen species (RNS) at a rate much high than the elimination rate after the organism encounters harmful stimulus. OS can injure tissues and organs, and is often associated with diseases and aging. Meanwhile, oxidative stress, caused by NPs, is the most important and widely accepted mechanism of nano-neurotoxicity. ROS, such as superoxide, hydrogen peroxide, and hydroxyl radicals, are natural products of the regular oxygen metabolism [15,16]. However, these free radicals can interact within biological systems, resulting in oxidative damage to the organism. These harmful effects can be counteracted by biological antioxidants, including superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH-Px), the expression of which needs to be coordinately regulated with the onset of OS [17,18]. If this balance is interrupted, levels of NPs-activated OS surpass the capacity of the biological antioxidants, potentially resulting in toxic oxidative stress. As a result, central nervous system (CNS) dysfunctions might ultimately be induced.
Long et al. [19,20] first revealed in their in vitro studies that TiO2 NPs can induce dose- and time-dependent elevations in H2O2 levels in BV2 cells (an immortalized brain microglia cell line). BV2 internalized TiO2 NPs and subsequently swollen mitochondria were detected by transmission electron microscopy (TEM), indicating that the function of the mitochondria was disrupted. Mitochondria are the sites of aerobic respiration, and generally are the major energy production center in eukaryotes. Dysfunction of mitochondria would thus influence energy metabolism.
Many in vivo studies have also verified the role of OS in TiO2 NPs-induced neurotoxicity. Wang et al. [21] found that nasal instillation of TiO2 NPs can lead to histopathological changes in the mouse brain. At the same time, the activity of superoxide dismutase (SOD) was inhibited, methane dicarboxylic aldehyde (MDA) levels were increased, and acetylcholin esterase (AChE) activity was enhanced in brain tissues. These changes indicated that OS was involved in neurological lesions induced by TiO2 NPs. In further studies, Krawczynska et al. [22] injected rats with TiO2 NPs intravenously. Twenty-eight days after injection, the level of aromatase was reduced and glutathione peroxidase and reductase activities were suppressed, implying that the regulation of oxidative stress in the brain was disturbed. In this study, the angiotensin system was disrupted as well. In addition, after Shrivastava et al. [23] treated male mice with TiO2 NPs through oral administration for 21 days, ROS increased, and the activities of anti-oxidant enzymes (such as SOD, and CAT, among others) were affected in the brain tissues. These changes were associated with neurotoxicity of NPs. TiO2 NPs were also shown to induce elevated levels of H2O2 and MDA in the brain after mice were put in chambers with a steady flow of TiO2 NPs (mimicking inhalation exposure), for 8 h per day, for 3 weeks [24]. Ma et al. [25] found that exposure to TiO2 NPs, through delivery in the abdominal cavity, can lead to histopathological changes in mouse brain. This was accompanied by elevated levels of ROS, MDA, constitutive nitric oxide synthase (cNOS), induced nitric oxide synthase (iNOS), and nitric oxide (NO), and inhibited activities of SOD, CAT, ascorbate peroxidase (APX), glutathione peroxidase (GSH-Px), total antioxidant capacity (T-AOC), and AChE, as well as through reduced ratios of ascorbic acid (AsA) to oxidized AsA (DAsA) and glutathione (GSH) to oxidized glutathione (GSSG). These changes implied that OS, induced by TiO2 NPs, mainly contributed to neurotoxicity. In addition, maternal exposure to TiO2 NPs was also shown to affect the OS status in the fetal brain. After pregnant rats were administrated with TiO2 NPs through subcutaneous injection, neonates showed down-regulated expression of CAT, GSH-Px, T-AOC, and an increase in both MDA expression and oxidative impairment of the DNA. When neonates matured, their performance was poor in behavioral tests (novel object recognition test, forced swim test, and sucrose preference test) [26].
Inflammatory response
Inflammatory response induced by TiO2 NPs is another major mechanism of neurotoxicity. When TiO2 NPs are transported to the brain, they interact with neurons and glial cells. Microglia are considered to be innate immune cells residing in brain. Once they are activated by exogenous substances, pro-inflammatory cytokines are released to induce neuro-inflammation [27,28]. TiO2 NPs acting as a stimulus were able to activate microglia cells. Su et al. [29] treated mice with TiO2 NPs by nasal administration for nine months, after which the glial cells showed over-proliferation and tissue necrosis was found in hippocampal area. Meanwhile, the expression of genes associated with neurotrophin signaling pathways, such as nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF), were altered in the hippocampal area, indicating that these genes were related to the neuro-inflammatory responses induced by TiO2 NPs. After mice were exposed to TiO2 NPs through intranasal administration for 90 days, expression of toll-like receptor (TLR2), TLR4, nuclear factor-kappa B (NF-κB), and tumor necrosis factor-α (TNF-α) in the mouse hippocampus was promoted. At the same time, histopathological changes were observed in the hippocampus; over-proliferation of glial cells, impaired nuclei, and cellular degeneration were observed, all of which contributed to neuro-inflammation. In addition, locomotor activity in these mice was affected. These changes suggested that inflammatory responses play a role in TiO2 NPs-induced neurotoxicity [30]. Ze et al. [31] treated mice with TiO2 NPs by intranasal administration for 90 days to determine if the p38-nuclear factor-E2-related factor-2 (p38-Nrf-2) signaling pathway was implicated in OS. Data showed that the expression of p38, Jun N-terminal kinase (JNK), NF-κB, Nrf-2, and heme oxygenase (HO-1) was promoted in the brain of TiO2 NPs-treated groups. Simultaneously, levels of O2−, H2O2, MDA, carbonyl, and 8-hydroxy-2'-deoxyguanosine (8-OHdG) were also enhanced. These findings indicated that the activated p38-Nrf-2 signaling pathway could induce excessive OS, leading to over-proliferation of spongiocytes and hematencephalon. Exposure to TiO2 NPs through tracheal administration has also been shown to induce the expression of interleukin-1β (IL-1β), TNF-α, and IL-10 in the brain. Herein, an impairment of the the blood–brain barrier and damage of astrocytes was observed [32].
Apoptosis dysfunction
Apoptosis, also called programmed cell death, is defined as the genetically determined elimination of cells. The activation of caspase plays a pivotal role in apoptosis. Human health and disease can be modulated by apoptosis [33,34]. Sheng et al. [35] found that TiO2 NPs induced apoptosis in primary hippocampal neurons. Elevated levels of Ca2+, cytochrome c, Bax, caspase-3, and caspase-12, as well as a reduction in mitochondrial membrane potential (MMP) and blc-2 levels, indicated that mitochondria- and endoplasmic reticulum-mediated signaling pathways were involved in the apoptotic process. TiO2 NPs were also shown to decrease cell viability by inducing apoptosis in the microglia N9 [36] and human astrocytes-like astrocytoma U87 cell lines [37].
Direct toxic effects on cell structures
Cell components, such as the cell membrane and mitochondria, can be targets of TiO2 NPs. TiO2 NPs can decrease cell viability of primary rat astrocytes. Herein, the mitochondrial morphology was changed and mitochondrial membrane potential (MMP) was reduced, suggesting mitochondrial impairment. At the same time, glutamate uptake was down-regulated, and ROS was promoted [38]. Coccini et al. [39] found that when D384 (human glial cell line) and SH-SY5Y (human neuronal cell line) cells were treated with TiO2 NPs, mitochondrial dysfunction, impaired cell membrane, and changes in cell morphology were detected. Mao et al. [40] discovered that a dose of TiO2 NPs, having no effect on viability in SH-SY5Y cells could cause changes in cell morphology and disruptions to the microtubule structure, both of which are associated with neurotoxicity. In addition, Ben Younes et al. [41] treated rats with TiO2 NPs through intraperitoneal injection. After which, rats exhibited altered emotional behavior in a plus maze test; however, histopathological examination demonstrated no significant differences between treated and control groups. The study failed to discuss other mechanisms associated with nanoneurotoxicity.
Genotoxicity
Genotoxicity is simply defined as the induction of DNA damage, in a direct or indirect manner, caused by substances such as benzopyrene in cigarettes or some chemotherapeutic drugs. In vivo and in vitro studies typically measure genotoxicity using the comet assay, the micronucleus test, the Ames test, and the chromosome aberration assay [42]. Golbamaki et al. [42] summarized genotoxicity data of NPs from available studies in their review, concluding that NPs can induce genotoxicity, and the mechanisms through which this occurs can be divided into direct primary genotoxicity, indirect primary genotoxicity, and secondary genotoxicity (for a comprehensive review, see Golbamaki et al. [42]). TiO2 NPs, like other types of engineered NPs, can induce genotoxicity. However, Golbamaki et al. did not report on TiO2 NPs-induced genotoxicity in the brain or in brain cells. Obviously, the relationship between neurotoxicity of TiO2 NPs and genotoxicity should be investigated comprehensively. Recently, El-Ghor et al. [43] determined that TiO2 NPs could cause DNA damage in the mouse brain. This genotoxicity could be alleviated by co-treatment with chlorophyllin (CHL). CHL is a free radical scavenger and is able to reduce the harmful effects of OS [44,45]. These findings suggest that oxidative stress induced by TiO2 NPs can cause genotoxicity to the brain.
Multiple mechanisms
Usually, activation of glia cells and mitochondrial injury are able to initiate excessive ROS production [27,46]. Meanwhile, ROS can lead to apoptosis and genotoxicity [43,47]. Therefore, the above-mentioned mechanisms, including OS, apoptosis, inflammatory responses, genotoxicity, and direct impairments on cell components may be jointly implicated in TiO2 NPs-induced neurotoxicity. OS (which promotes ROS and affects the activities of SOD, GPx, CAT) and mitochondrial impairments were observed in TiO2 NPs-treated glial cells (C6 and U373) [48]. After glial cells are damaged by TiO2 NPs, inflammatory responses would presumably occur. This might exacerbate brain damage further. Wang et al. [49] found that intranasal instillation of TiO2 NPs can induce histopathological changes in the mouse brain, in which OS (MDA increases) and inflammatory responses (elevated expressions of TNF-α and IL-1β) were involved. Shimizu et al. [50] analyzed the brains of mouse offspring by cDNA microarray and found that prenatal exposure to TiO2 NPs could alter the expression of neurotransmitter genes as well as genes associated with apoptosis, OS, and psychiatric disorders. TiO2 NPs decreased cell viability in PC12 cells in a dose- and time-dependent manner by increasing the level of ROS and proportion of apoptotic cells. Pretreating PC12 with a ROS scavenger could alleviate these harmful effects induced by TiO2 NPs [51]. In addition, an inhibition in cell proliferation, altered cell morphology (assessed by decreased F-actin), and apoptosis could be induced by TiO2 NPs in C6 and U373 cells. TiO2 NPs were also internalized by C6 and U373 cells [52]. Valdiglesias et al. [53] found that NPs were internalized by SH-SY5Y neuronal cells exposed to TiO2 NPs, which coincided with alterations in the cell cycle and an elevation in the proportion of apoptotic cells. Damage of the DNA was induced and NO oxidative stress was observed in these experimental groups. The treatment of mice with TiO2 NPs by intragastric administration resulted in an impairment of their spatial recognition memory. This impairment was mainly due to elevated expression of caspase-3 and caspase-9, Bax, and cytochrome c, and suppressed Bcl-2, in the hippocampal area. Meanwhile, the ROS levels were enhanced, and the activities of antioxidant enzymes such as SOD, CAT, ascorbate peroxidase (Apx), and GSH-Px were inhibited. In addition, the ratios of AsA to DAsA and GSH to GSSG were decreased. These changes suggested that apoptosis and OS were involved in TiO2 NPs-induced neurotoxicity [54]. Meena et al. [55] found that after rats were administrated TiO2 NPs by intravenous injection, once a week for four weeks, the ROS in the brain were significantly enhanced. This elevation of ROS promoted an inflammatory responses and led to decreased activities of SOD and GPx, elevated MDA and DNA damage, as well as an increased proportion of apoptotic cells.
Minor mechanisms
In addition to major mechanisms of TiO2 NPs-induced neurotoxicity, other minor mechanisms exist. Disdier et al. [6] discovered that after rats received TiO2 NPs through intravenous injection, neuro-inflammation was not directly induced by Ti accumulation in the brain, but instead was indirectly stimulated by cytokines or pro-inflammatory mediators in systemic circulation. Hong et al. [56] demonstrated that the decreased cell viability of primary hippocampal neurons was associated with inhibited dendritic growth, disrupted glutamate metabolism, dysregulated levels of N-methyl-D-aspartate receptors (NMDA receptors), increased Ca2+ levels and voltage of Ik in cells, and reduced activity of ATPase. However, additional experiments were needed to further discuss how each of these parameters contributed to neurotoxicity. Ze et al. [57] found that after mice were treated with TiO2 NPs by oral exposure, histopathological changes in the hippocampus were occurred. Meanwhile, affected neuron ultrastructures, such as swollen mitochondria and impaired nuclear membrane were detected. Long-term potentiation (LTP) in the hippocampus was reduced and the expressions of NMDA receptors were down-regulated as well. These changes contributed to impaired spatial memory. Prenatal exposure to TiO2 NPs was also shown to result in decreased cell proliferation in the hippocampus of rat offspring, which was associated with poor performance in the Morris water maze test and passive avoidance test [58]. Maternal exposure to TiO2 NPs can also affect the production of dopamine and its metabolites, as determined by high performance liquid chromatography (HPLC) [59], and alter gene expression related to dopamine systems, as measured by DNA microarray in neonatal mouse brain [60]. Mice performed poorly in the Y-maze test after a 60 d exposure to TiO2 NPs, and histopathological changes were observed in brain. Meanwhile, the intracellular content of trace elements (Ca, Mg, Na, K, Zn, and Fe) was disturbed. The activities of Na+/K+-ATPase, Ca2+-ATPase, and Ca2+/Mg2+-ATPase also decreased, and the levels of neurotransmitters, including acetyl choline (Ach), glutamic acid (Glu), and NO were elevated. The expression of monoamine neurotransmitters consisting of norepinephrine (NE), dopamine (DA), dihydroxyphenylacetic acid (DOPAC), 5-hydroxytryptamine (5-HT), and 5-hydroxyindoleacetic acid (5-HIAA) was down-regulated. These changes impaired spatial recognition memory [61]. When mother rats were exposed to TiO2 NPs during the lactation period, their offspring exhibited dysregulated synaptic plasticity that included the input/output (I/O) function, paired-pulse reaction, and long-term potentiation in the hippocampal zone. These developmental changes in the hippocampus could impair learning ability and memory [62]. TiO2 NPs also decreased PC12 viability through activation of JNK- and p53-mediated pathways, which disrupted cell cycle, leading to apoptosis and excessive ROS [63].
Uncertain mechanisms underlying TiO2 NPs-induced neurotoxicity
Autophagy
Autophagy can be divided into three types, 1) microautophagy, 2) macroautophagy, and 3) identified-chaperone-mediated autophagy. It is believed that macroautophagy (referred to as autophagy) is the main degradative pathway among these subtypes of autophagy [64,65]. Autophagy also acts as a dynamic recycling system in which new building blocks, and energy for cellular renovation and homeostasis, are produced [12]. However, excessive autophagy can contribute to cell death. Autophagy has been determined to be a potential mechanism of nanotoxicity [9,66]. However, few studies have described the relationship between neurotoxicity and nanomaterials. It was revealed that gold nanoparticles can increase the levels of autophagy-related proteins in human lung fibroblasts (MRC-5), concomitant with excessive MDA production [67]. After lung epithelial cancer cells (A549) were exposed to iron oxide nanoparticles, ROS production, mitochondrial impairments and autophagy were detected [68]. Autophagy in human peripheral blood monocytes can be induced by cerium dioxide nanoparticles [69]. In addition, copper oxide NPs [70], silica NPs [71], zinc oxide NPs [72], and silver NPs [73] were shown to induce autophagy in in vitro studies. TiO2 NPs were also capable of inducing autophagy. Studies showed that TiO2 NPs could induce autophagy in normal lung cells [74] and in primary human keratinocytes [75]. Based on the studies illustrated above, we hypothesize that TiO2 NPs can induce autophagy dysfunctions in brain tissues and cells. Therefore, autophagy could be a potential mechanism underlying TiO2 NPs-induced neurotoxicity. However, more studies are needed to further investigate the relationship between brain damage and TiO2 NPs-mediated autophagy dysfunction.
Epigenetics
Another potential mechanism of TiO2 NPs is epigenetic regulations. Epigenetic changes were reported to be implicated in nanotoxicity [10,11]. Epigenetics refers to all heritable alternations in gene expression not caused through changes to the DNA sequence itself, but rather arise during development and cell proliferation [13,14]. In other words, the epigenetics represents the interactions between gene expression and environmental factors, and the relationship between genes, which were modulated during development and adult life [76]. In most situations, epigenetic modifications modulate DNA transcription through mechanisms such as DNA methylation [77], histone modifications [78], and non-coding RNA (ncRNA) regulation [79].
Among them, DNA methylation is the most extensively studied epigenetic mechanism, wherein a methyl group (-CH3) from S-adenosylmethionine (SAM) is transferred to the 5-position of cytosines, in certain CpG dinucleotides, by a family of DNA methyltransferase enzymes (DNMTs). The DNMTs can be classified into three major types based on their different structures and functions, DNMT1, DNMT3a, and DNMT3b [77,80-82]. DNMT1 was identified as having a role in the maintenance of methylation during each cellular replication when DNA is duplicated [83]. DNMT3a and DNMT3b have de novo methylation ability, wherein new 5-methylcytosines are introduced in initially non-methylated genome sites [84,85]. In most cases, DNA methylation not only induces gene silencing [86], but also is related to the initiation of DNA replication [87], DNA mismatch repair [88] and inactivation of transposons [89].
Recently, more and more studies have discovered that CNS dysfunction may be potentially affected by DNA methylation. DNA methylation was associated with expression of neurotransmitters. In an in vitro study, hypermethylation of the excitatory amino acid transporter (EAAT2) promoter in glioma cells led to a deficiency in astroglial EAAT2 expression, which was related to the pathogenesis of CNS disorders with remarkable excitatory toxicity elements. Furthermore, the transcription of EAAT2 could be recovered by suppression of DNA methyltransferases [90]. Neurodegenerative diseases, such as Alzheimer's disease (AD) [91], Huntington’s disease (HD) [92], and amyotrophic lateral sclerosis (ALS) [93], are regulated by DNA methylation. Meanwhile, psychiatric disorders have been associated with an abnormal DNA methylation status in brain [94-96].
Few reports have described abnormal DNA methylation induced by NPs. SiO2 NPs can reduce global DNA methylation levels and change the methylation status of the PARP-1 promoter in human keratinocytes (HaCat) [97,98]. Silver NPs had the ability to change DNA methylation as well [99]. In a recent study, DNA methylation status, regulated by DNMTs in the A549 cell line, could be altered by oxidative stress induced by TiO2 NPs [100]. As OS is a main mechanism of nanotoxicity, we hypothesize that TiO2 NPs might be able to alter DNA methylation status in the brain through an OS-mediated pathway. More studies are needed to further investigate the role of DNA methylation in TiO2 NPs-induced neurotoxicity.
Conclusion
TiO2 NPs possess unique characteristics due to their tiny size and are widely used in many fields. The rapid development of nanotechnology and widespread applications of products based on nanomaterials are possible causes of neurological disorders in humans. Therefore, numerous in vivo and in vitro studies have been conducted to assess the neurotoxicity of TiO2 NPs. However, the exact underlying mechanisms of TiO2 NPs-induced neurotoxicity are unclear. Through summarizing studies describing the neurotoxicity of TiO2 NPs, we found that these mechanisms mainly consisted of oxidative stress (OS), apoptosis, inflammatory responses, genotoxicity, and direct impairment of cell components. Meanwhile, other mechanisms, including disturbed distributions of trace elements, disrupted signaling pathways, dysregulated levels of neurotransmitters, and synaptic plasticity also contribute to the neurotoxicity of TiO2 NPs. Furthermore, recent studies implicated autophagy and DNA methylation as results of nanotoxicity. Therefore, we hypothesized that these two mechanisms could be potentially involved in the neurotoxicity of TiO2 NPs. Further studies are needed to test these hypotheses.
In summary, to fully understand the health threats to the brain posed by TiO2 NPs, and improve the bio-safety of TiO2 NPs-based products, every possible molecular mechanism of TiO2 NPs-induced neurotoxicity must be investigated comprehensively.
Author contributions
Bin Song collected and reviewed the data and drafted the manuscript. All authors helped in drafting the first version of the manuscript and in revisions. All authors read and approved the final manuscript.
Acknowledgements
This work was supported by the Science and Technology Foundation of the Health and Family Planning Commission of Guizhou province, China (gzwjkj2015-1-026), the National Natural Science Foundation of China (81550011), and the Natural Science Foundation of Guangdong Province of China (2015A030313299).
References
-
Chen, X.; Mao, S. S. Chem. Rev. 2007, 107, 2891–2959. doi:10.1021/cr0500535
Return to citation in text: [1] -
Montazer, M.; Seifollahzadeh, S. Photochem. Photobiol. 2011, 87, 877–883. doi:10.1111/j.1751-1097.2011.00917.x
Return to citation in text: [1] -
Adam, V.; Loyaux-Lawniczak, S.; Quaranta, G. Environ. Sci. Pollut. Res. 2015, 22, 11175–11192. doi:10.1007/s11356-015-4661-x
Return to citation in text: [1] -
Shi, H.; Magaye, R.; Castranova, V.; Zhao, J. Part. Fibre Toxicol. 2013, 10, No. 15. doi:10.1186/1743-8977-10-15
Return to citation in text: [1] -
Geraets, L.; Oomen, A. G.; Krystek, P.; Jacobsen, N. R.; Wallin, H.; Laurentie, M.; Verharen, H. W.; Brandon, E. F. A.; de Jong, W. H. Part. Fibre Toxicol. 2014, 11, 30. doi:10.1186/1743-8977-11-30
Return to citation in text: [1] -
Disdier, C.; Devoy, J.; Cosnefroy, A.; Chalansonnet, M.; Herlin-Boime, N.; Brun, E.; Lund, A.; Mabondzo, A. Part. Fibre Toxicol. 2015, 12, 27. doi:10.1186/s12989-015-0102-8
Return to citation in text: [1] [2] [3] -
Pelclova, D.; Zdimal, V.; Fenclova, Z.; Vlckova, S.; Turci, F.; Corazzari, I.; Kacer, P.; Schwarz, J.; Zikova, N.; Makes, O.; Syslova, K.; Komarc, M.; Belacek, J.; Navratil, T.; Machajova, M.; Zakharov, S. Occup. Environ. Med. 2016, 73, 110–118. doi:10.1136/oemed-2015-103161
Return to citation in text: [1] -
Song, B.; Liu, J.; Feng, X.; Wei, M.; Shao, L. Nanoscale Res. Lett. 2015, 10, 342. doi:10.1186/s11671-015-1042-9
Return to citation in text: [1] -
Stern, S. T.; Adiseshaiah, P. P.; Crist, R. M. Part. Fibre Toxicol. 2012, 9, 20. doi:10.1186/1743-8977-9-20
Return to citation in text: [1] [2] -
Shyamasundar, S.; Ng, C. T.; Yung, L. Y. L.; Dheen, S. T.; Bay, B. H. Epigenomics 2015, 7, 395–411. doi:10.2217/epi.15.3
Return to citation in text: [1] [2] -
Stoccoro, A.; Karlsson, H. L.; Coppedè, F.; Migliore, L. Toxicology 2013, 313, 3–14. doi:10.1016/j.tox.2012.12.002
Return to citation in text: [1] [2] -
Mizushima, N.; Komatsu, M. Cell 2011, 147, 728–741. doi:10.1016/j.cell.2011.10.026
Return to citation in text: [1] [2] -
Jaenisch, R.; Bird, A. Nat. Genet. 2003, 33, 245–254. doi:10.1038/ng1089
Return to citation in text: [1] [2] -
Egger, G.; Liang, G.; Aparicio, A.; Jones, P. A. Nature 2004, 429, 457–463. doi:10.1038/nature02625
Return to citation in text: [1] [2] -
Armstrong, J. S.; Rajasekaran, M.; Chamulitrat, W.; Gatti, P.; Hellstrom, W. J.; Sikka, S. C. Free Radical Biol. Med. 1999, 26, 869–880. doi:10.1016/s0891-5849(98)00275-5
Return to citation in text: [1] -
Starkov, A. A. Ann. N. Y. Acad. Sci. 2008, 1147, 37–52. doi:10.1196/annals.1427.015
Return to citation in text: [1] -
Jaiswal, A. K. Free Radical Biol. Med. 2004, 36, 1199–1207. doi:10.1016/j.freeradbiomed.2004.02.074
Return to citation in text: [1] -
Okuda, M.; Li, K.; Beard, M. R.; Showalter, L. A.; Scholle, F.; Lemon, S. M.; Weinman, S. A. Gastroenterology 2002, 122, 366–375. doi:10.1053/gast.2002.30983
Return to citation in text: [1] -
Long, T. C.; Saleh, N.; Tilton, R. D.; Lowry, G. V.; Veronesi, B. Environ. Sci. Technol. 2006, 40, 4346–4352. doi:10.1021/es060589n
Return to citation in text: [1] [2] -
Long, T. C.; Tajuba, J.; Sama, P.; Saleh, N.; Swartz, C.; Parker, J.; Hester, S.; Lowry, G. V.; Veronesi, B. Environ. Health Perspect. 2007, 115, 1631–1637. doi:10.1289/ehp.10216
Return to citation in text: [1] [2] -
Wang, J.; Chen, C.; Liu, Y.; Jiao, F.; Li, W.; Lao, F.; Li, Y.; Li, B.; Ge, C.; Zhou, G.; Gao, Y.; Zhao, Y.; Chai, Z. Toxicol. Lett. 2008, 183, 72–80. doi:10.1016/j.toxlet.2008.10.001
Return to citation in text: [1] [2] -
Krawczyńska, A.; Dziendzikowska, K.; Gromadzka-Ostrowska, J.; Lankoff, A.; Herman, A. P.; Oczkowski, M.; Królikowski, T.; Wilczak, J.; Wojewódzka, M.; Kruszewski, M. Food Chem. Toxicol. 2015, 85, 96–105. doi:10.1016/j.fct.2015.08.005
Return to citation in text: [1] [2] -
Shrivastava, R.; Raza, S.; Yadav, A.; Kushwaha, P.; Flora, S. J. S. Drug Chem. Toxicol. 2014, 37, 336–347. doi:10.3109/01480545.2013.866134
Return to citation in text: [1] [2] -
Yin, J.; Kang, C.; Li, Y.; Li, Q.; Zhang, X.; Li, W. Toxicol. Res. (Cambridge, U. K.) 2014, 3, 367–374. doi:10.1039/c4tx00040d
Return to citation in text: [1] [2] -
Ma, L.; Liu, J.; Li, N.; Wang, J.; Duan, Y.; Yan, J.; Liu, H.; Wang, H.; Hong, F. Biomaterials 2010, 31, 99–105. doi:10.1016/j.biomaterials.2009.09.028
Return to citation in text: [1] [2] -
Cui, Y.; Chen, X.; Zhou, Z.; Lei, Y.; Ma, M.; Cao, R.; Sun, T.; Xu, J.; Huo, M.; Cao, R.; Wen, C.; Che, Y. Chemosphere 2014, 96, 99–104. doi:10.1016/j.chemosphere.2013.07.051
Return to citation in text: [1] [2] -
Block, M. L.; Zecca, L.; Hong, J.-S. Nat. Rev. Neurosci. 2007, 8, 57–69. doi:10.1038/nrn2038
Return to citation in text: [1] [2] -
Olson, J. K.; Miller, S. D. J. Immunol. 2004, 173, 3916–3924. doi:10.4049/jimmunol.173.6.3916
Return to citation in text: [1] -
Su, M.; Sheng, L.; Zhao, X.; Wang, L.; Yu, X.; Hong, J.; Xu, B.; Liu, D.; Jiang, H.; Ze, X.; Zhu, Y.; Long, Y.; Zhou, J.; Cui, J.; Li, K.; Ze, Y.; Hong, F. Toxicol. Res. (Cambridge, U. K.) 2015, 4, 344–350. doi:10.1039/c4tx00106k
Return to citation in text: [1] [2] -
Ze, Y.; Sheng, L.; Zhao, X.; Hong, J.; Ze, X.; Yu, X.; Pan, X.; Lin, A.; Zhao, Y.; Zhang, C.; Zhou, Q.; Wang, L.; Hong, F. PLoS One 2014, 9, No. e92230. doi:10.1371/journal.pone.0092230
Return to citation in text: [1] [2] -
Ze, Y.; Zheng, L.; Zhao, X.; Gui, S.; Sang, X.; Su, J.; Guan, N.; Zhu, L.; Sheng, L.; Hu, R.; Cheng, J.; Cheng, Z.; Sun, Q.; Wang, L.; Hong, F. Chemosphere 2013, 92, 1183–1189. doi:10.1016/j.chemosphere.2013.01.094
Return to citation in text: [1] [2] -
Liu, Y.; Xu, Z.; Li, X. Brain Inj. 2013, 27, 934–939. doi:10.3109/02699052.2013.793401
Return to citation in text: [1] [2] -
Elmore, S. Toxicol. Pathol. 2007, 35, 495–516. doi:10.1080/01926230701320337
Return to citation in text: [1] -
Wirawan, E.; Vande Walle, L.; Kersse, K.; Cornelis, S.; Claerhout, S.; Vanoverberghe, I.; Roelandt, R.; De Rycke, R.; Verspurten, J.; Declercq, W.; Agostinis, P.; Vanden Berghe, T.; Lippens, S.; Vandenabeele, P. Cell Death Dis. 2010, 1, No. e18. doi:10.1038/cddis.2009.16
Return to citation in text: [1] -
Sheng, L.; Ze, Y.; Wang, L.; Yu, X.; Hong, J.; Zhao, X.; Ze, X.; Liu, D.; Xu, B.; Zhu, Y.; Long, Y.; Lin, A.; Zhang, C.; Zhao, Y.; Hong, F. J. Biomed. Mater. Res., Part A 2015, 103, 1141–1149. doi:10.1002/jbm.a.35263
Return to citation in text: [1] [2] -
Li, X.; Xu, S.; Zhang, Z.; Schluesener, H. J. Chin. Sci. Bull. 2009, 54, 3830–3836. doi:10.1007/s11434-009-0548-x
Return to citation in text: [1] [2] -
Lai, J. C. K.; Lai, M. B.; Jandhyam, S.; Dukhande, V. V.; Bhushan, A.; Daniels, C. K.; Leung, S. W. Int. J. Nanomed. 2008, 3, 533–545. doi:10.2147/IJN.S3234
Return to citation in text: [1] [2] -
Wilson, C. L.; Natarajan, V.; Hayward, S. L.; Khalimonchuk, O.; Kidambi, S. Nanoscale 2015, 7, 18477–18488. doi:10.1039/c5nr03646a
Return to citation in text: [1] [2] -
Coccini, T.; Grandi, S.; Lonati, D.; Locatelli, C.; De Simone, U. NeuroToxicology 2015, 48, 77–89. doi:10.1016/j.neuro.2015.03.006
Return to citation in text: [1] [2] -
Mao, Z.; Xu, B.; Ji, X.; Zhou, K.; Zhang, X.; Chen, M.; Han, X.; Tang, Q.; Wang, X.; Xia, Y. Nanoscale 2015, 7, 8466–8475. doi:10.1039/c5nr01448d
Return to citation in text: [1] [2] -
Ben Younes, N. R.; Amara, S.; Mrad, I.; Ben-Slama, I.; Jeljeli, M.; Omri, K.; El Ghoul, J.; El Mir, L.; Ben Rhouma, K.; Abdelmelek, H.; Sakly, M. Environ. Sci. Pollut. Res. 2015, 22, 8728–8737. doi:10.1007/s11356-014-4002-5
Return to citation in text: [1] -
Golbamaki, N.; Rasulev, B.; Cassano, A.; Robinson, R. L. M.; Benfenati, E.; Leszczynski, J.; Cronin, M. T. D. Nanoscale 2015, 7, 2154–2198. doi:10.1039/c4nr06670g
Return to citation in text: [1] [2] [3] -
El-Ghor, A. A.; Noshy, M. M.; Galal, A.; Mohamed, H. R. H. Toxicol. Sci. 2014, 142, 21–32. doi:10.1093/toxsci/kfu157
Return to citation in text: [1] [2] [3] -
Kumar, S. S.; Shankar, B.; Sainis, F. B. Biochim. Biophys. Acta, Gen. Subj. 2004, 1672, 100–111. doi:10.1016/j.bbagen.2004.03.002
Return to citation in text: [1] -
Zhang, Y.; Guan, L.; Wang, X.; Wen, T.; Xing, J.; Zhao, J. Free Radical Res. 2008, 42, 362–371. doi:10.1080/10715760801993076
Return to citation in text: [1] -
Indo, H. P.; Davidson, M.; Yen, H.-C.; Suenaga, S.; Tomita, K.; Nishii, T.; Higuchi, M.; Koga, Y.; Ozawa, T.; Majima, H. J. Mitochondrion 2007, 7, 106–118. doi:10.1016/j.mito.2006.11.026
Return to citation in text: [1] -
Kizaki, M.; Xian, M.; Sagawa, M.; Ikeda, Y. Curr. Pharm. Biotechnol. 2006, 7, 323–329. doi:10.2174/138920106778521541
Return to citation in text: [1] -
Huerta-García, E.; Pérez-Arizti, J. A.; Márquez-Ramírez, S. G.; Delgado-Buenrostro, N. L.; Chirino, Y. I.; Iglesias, G. G.; López-Marure, R. Free Radical Biol. Med. 2014, 73, 84–94. doi:10.1016/j.freeradbiomed.2014.04.026
Return to citation in text: [1] [2] -
Wang, J.; Liu, Y.; Jiao, F.; Lao, F.; Li, W.; Gu, Y.; Li, Y.; Ge, C.; Zhou, G.; Li, B.; Zhao, Y.; Chai, Z.; Chen, C. Toxicology 2008, 254, 82–90. doi:10.1016/j.tox.2008.09.014
Return to citation in text: [1] [2] -
Shimizu, M.; Tainaka, H.; Oba, T.; Mizuo, K.; Umezawa, M.; Takeda, K. Part. Fibre Toxicol. 2009, 6, No. 20. doi:10.1186/1743-8977-6-20
Return to citation in text: [1] [2] -
Liu, S.; Xu, L.; Zhang, T.; Ren, G.; Yang, Z. Toxicology 2010, 267, 172–177. doi:10.1016/j.tox.2009.11.012
Return to citation in text: [1] [2] -
Márquez-Ramírez, S. G.; Delgado-Buenrostro, N. L.; Chirino, Y. I.; Iglesias, G. G.; López-Marure, R. Toxicology 2012, 302, 146–156. doi:10.1016/j.tox.2012.09.005
Return to citation in text: [1] [2] -
Valdiglesias, V.; Costa, C.; Sharma, V.; Kiliç, G.; Pásaro, E.; Teixeira, J. P.; Dhawan, A.; Laffon, B. Food Chem. Toxicol. 2013, 57, 352–361. doi:10.1016/j.fct.2013.04.010
Return to citation in text: [1] [2] -
Hu, R.; Zheng, L.; Zhang, T.; Gao, G.; Cui, Y.; Cheng, Z.; Cheng, J.; Hong, M.; Tang, M.; Hong, F. J. Hazard. Mater. 2011, 191, 32–40. doi:10.1016/j.jhazmat.2011.04.027
Return to citation in text: [1] [2] -
Meena, R.; Kumar, S.; Paulraj, R. J. Nanopart. Res. 2015, 17, 49. doi:10.1007/s11051-015-2868-x
Return to citation in text: [1] [2] -
Hong, F.; Sheng, L.; Ze, Y.; Hong, J.; Zhou, Y.; Wang, L.; Liu, D.; Yu, X.; Xu, B.; Zhao, X.; Ze, X. Biomaterials 2015, 53, 76–85. doi:10.1016/j.biomaterials.2015.02.067
Return to citation in text: [1] [2] -
Ze, Y.; Sheng, L.; Zhao, X.; Ze, X.; Wang, X.; Zhou, Q.; Liu, J.; Yuan, Y.; Gui, S.; Sang, X.; Sun, Q.; Hong, J.; Yu, X.; Wang, L.; Li, B.; Hong, F. J. Hazard. Mater. 2014, 264, 219–229. doi:10.1016/j.jhazmat.2013.10.072
Return to citation in text: [1] [2] -
Mohammadipour, A.; Fazel, A.; Haghir, H.; Motejaded, F.; Rafatpanah, H.; Zabihi, H.; Hosseini, M.; Bideskan, A. E. Environ. Toxicol. Pharmacol. 2014, 37, 617–625. doi:10.1016/j.etap.2014.01.014
Return to citation in text: [1] [2] -
Takahashi, Y.; Mizuo, K.; Shinkai, Y.; Oshio, S.; Takeda, K. J. Toxicol. Sci. 2010, 35, 749–756. doi:10.2131/jts.35.749
Return to citation in text: [1] [2] -
Umezawa, M.; Tainaka, H.; Kawashima, N.; Shimizu, M.; Takeda, K. J. Toxicol. Sci. 2012, 37, 1247–1252. doi:10.2131/jts.37.1247
Return to citation in text: [1] [2] -
Hu, R.; Gong, X.; Duan, Y.; Li, N.; Che, Y.; Cui, Y.; Zhou, M.; Liu, C.; Wang, H.; Hong, F. Biomaterials 2010, 31, 8043–8050. doi:10.1016/j.biomaterials.2010.07.011
Return to citation in text: [1] [2] -
Gao, X.; Yin, S.; Tang, M.; Chen, J.; Yang, Z.; Zhang, W.; Chen, L.; Yang, B.; Li, Z.; Zha, Y.; Ruan, D.; Wang, M. Biol. Trace Elem. Res. 2011, 143, 1616–1628. doi:10.1007/s12011-011-8990-4
Return to citation in text: [1] [2] -
Wu, J.; Sun, J.; Xue, Y. Toxicol. Lett. 2010, 199, 269–276. doi:10.1016/j.toxlet.2010.09.009
Return to citation in text: [1] [2] -
Levine, B.; Kroemer, G. Cell 2008, 132, 27–42. doi:10.1016/j.cell.2007.12.018
Return to citation in text: [1] -
Mizushima, N.; Levine, B.; Cuervo, A. M.; Klionsky, D. J. Nature 2008, 451, 1069–1075. doi:10.1038/nature06639
Return to citation in text: [1] -
Cohignac, V.; Landry, M. J.; Boczkowski, J.; Lanone, S. Nanomaterials 2014, 4, 548–582. doi:10.3390/nano4030548
Return to citation in text: [1] -
Li, J. J.; Hartono, D.; Ong, C.-N.; Bay, B.-H.; Yung, L.-Y. L. Biomaterials 2010, 31, 5996–6003. doi:10.1016/j.biomaterials.2010.04.014
Return to citation in text: [1] -
Khan, M. I.; Mohammad, A.; Patil, G.; Naqvi, S. A. H.; Chauhan, L. K. S.; Ahmad, I. Biomaterials 2012, 33, 1477–1488. doi:10.1016/j.biomaterials.2011.10.080
Return to citation in text: [1] -
Hussain, S.; Al-Nsour, F.; Rice, A. B.; Marshburn, J.; Yingling, B.; Ji, Z. X.; Zink, J. I.; Walker, N. J.; Garantziotis, S. ACS Nano 2012, 6, 5820–5829. doi:10.1021/nn302235u
Return to citation in text: [1] -
Sun, T.; Yan, Y.; Zhao, Y.; Guo, F.; Jiang, C. PLoS One 2012, 7, No. e43442. doi:10.1371/journal.pone.0043442
Return to citation in text: [1] -
Yu, Y.; Duan, J.; Yu, Y.; Li, Y.; Liu, X.; Zhou, X.; Ho, K.-F.; Tian, L.; Sun, Z. J. Hazard. Mater. 2014, 270, 176–186. doi:10.1016/j.jhazmat.2014.01.028
Return to citation in text: [1] -
Yu, K.-N.; Yoon, T.-J.; Minai-Tehrani, A.; Kim, J.-E.; Park, S. J.; Jeong, M. S.; Ha, S.-W.; Lee, J.-K.; Kim, J. S.; Cho, M.-H. Toxicol. In Vitro 2013, 27, 1187–1195. doi:10.1016/j.tiv.2013.02.010
Return to citation in text: [1] -
Lee, Y.-H.; Cheng, F.-Y.; Chiu, H.-W.; Tsai, J.-C.; Fang, C.-Y.; Chen, C.-W.; Wang, Y.-J. Biomaterials 2014, 35, 4706–4715. doi:10.1016/j.biomaterials.2014.02.021
Return to citation in text: [1] -
Yu, K.-N.; Chang, S.-H.; Park, S. J.; Lim, J.; Lee, J.; Yoon, T.-J.; Kim, J.-S.; Cho, M.-H. PLoS One 2015, 10, No. e0131208. doi:10.1371/journal.pone.0131208
Return to citation in text: [1] [2] -
Zhao, Y.; Howe, J. L. C.; Yu, Z.; Leong, D. T.; Chu, J. J. H.; Loo, J. S. C.; Ng, K. W. Small 2013, 9, 387–392. doi:10.1002/smll.201201363
Return to citation in text: [1] [2] -
Qureshi, I. A.; Mehler, M. F. Mol. Aspects Med. 2013, 34, 875–882. doi:10.1016/j.mam.2012.06.011
Return to citation in text: [1] -
Bird, A. P. Nature 1986, 321, 209–213. doi:10.1038/321209a0
Return to citation in text: [1] [2] -
Strahl, B. D.; Allis, C. D. Nature 2000, 403, 41–45. doi:10.1038/47412
Return to citation in text: [1] -
Mattick, J. S.; Makunin, I. V. Hum. Mol. Genet. 2006, 15 (Suppl. 1), R17–R29. doi:10.1093/hmg/ddl046
Return to citation in text: [1] -
Robertson, K. D.; Uzvolgyi, E.; Liang, G.; Talmadge, C.; Sumegi, J.; Gonzales, F. A.; Jones, P. A. Nucleic Acids Res. 1999, 27, 2291–2298. doi:10.1093/nar/27.11.2291
Return to citation in text: [1] -
Fujii, S.; Wang, A. H.-J.; van der Marel, G.; van Boom, J. H.; Rich, A. Nucleic Acids Res. 1982, 10, 7879–7892. doi:10.1093/nar/10.23.7879
Return to citation in text: [1] -
Chiang, P. K.; Gordon, R. K.; Tal, J.; Zeng, G. C.; Doctor, B. P.; Pardhasaradhi, K.; McCann, P. P. FASEB J. 1996, 10, 471–480.
Return to citation in text: [1] -
Robert, M.-F.; Morin, S.; Beaulieu, N.; Gauthier, F.; Chute, I. C.; Barsalou, A.; MacLeod, A. R. Nat. Genet. 2002, 33, 61–65. doi:10.1038/ng1068
Return to citation in text: [1] -
Okano, M.; Bell, D. W.; Haber, D. A.; Li, E. Cell 1999, 99, 247–257. doi:10.1016/s0092-8674(00)81656-6
Return to citation in text: [1] -
Bachman, K. E.; Rountree, M. R.; Baylin, S. B. J. Biol. Chem. 2001, 276, 32282–32287. doi:10.1074/jbc.M104661200
Return to citation in text: [1] -
Herman, J. G.; Latif, F.; Weng, Y.; Lerman, M. I.; Zbar, B.; Liu, S.; Samid, D.; Duan, D. S.; Gnarra, J. R.; Linehan, W. M. Proc. Natl. Acad. Sci. U. S. A. 1994, 91, 9700–9704. doi:10.1073/pnas.91.21.9700
Return to citation in text: [1] -
Schübeler, D.; Lorincz, M. C.; Cimbora, D. M.; Telling, A.; Feng, Y.-Q.; Bouhassira, E. E.; Groudine, M. Mol. Cell. Biol. 2000, 20, 9103–9112. doi:10.1128/mcb.20.24.9103-9112.2000
Return to citation in text: [1] -
Hare, J. T.; Taylor, J. H. Proc. Natl. Acad. Sci. U. S. A. 1985, 82, 7350–7354. doi:10.1073/pnas.82.21.7350
Return to citation in text: [1] -
Reinders, J.; Wulff, B. B. H.; Mirouze, M.; Marí-Ordóñez, A.; Dapp, M.; Rozhon, W.; Bucher, E.; Theiler, G.; Paszkowski, J. Genes Dev. 2009, 23, 939–950. doi:10.1101/gad.524609
Return to citation in text: [1] -
Zschocke, J.; Allritz, C.; Engele, J.; Rein, T. Glia 2007, 55, 663–674. doi:10.1002/glia.20497
Return to citation in text: [1] -
De Jager, P. L.; Srivastava, G.; Lunnon, K.; Burgess, J.; Schalkwyk, L. C.; Yu, L.; Eaton, M. L.; Keenan, B. T.; Ernst, J.; McCabe, C.; Tang, A.; Raj, T.; Replogle, J.; Brodeur, W.; Gabriel, S.; Chai, H. S.; Younkin, C.; Younkin, S. G.; Zou, F.; Szyf, M.; Epstein, C. B.; Schneider, J. A.; Bernstein, B. E.; Meissner, A.; Ertekin-Taner, N.; Chibnik, L. B.; Kellis, M.; Mill, J.; Bennett, D. A. Nat. Neurosci. 2014, 17, 1156–1163. doi:10.1038/nn.3786
Return to citation in text: [1] -
Ng, C. W.; Yildirim, F.; Yap, Y. S.; Dalin, S.; Matthews, B. J.; Velez, P. J.; Labadorf, A.; Housman, D. E.; Fraenkel, E. Proc. Natl. Acad. Sci. U. S. A. 2013, 110, 2354–2359. doi:10.1073/pnas.1221292110
Return to citation in text: [1] -
Wong, M.; Gertz, B.; Chestnut, B. A.; Martin, L. J. Front. Cell. Neurosci. 2013, 7, No. 279. doi:10.3389/fncel.2013.00279
Return to citation in text: [1] -
Mill, J.; Tang, T.; Kaminsky, Z.; Khare, T.; Yazdanpanah, S.; Bouchard, L.; Jia, P.; Assadzadeh, A.; Flanagan, J.; Schumacher, A.; Wang, S.-C.; Petronis, A. Am. J. Hum. Genet. 2008, 82, 696–711. doi:10.1016/j.ajhg.2008.01.008
Return to citation in text: [1] -
Chen, C.; Zhang, C.; Cheng, L.; Reilly, J. L.; Bishop, J. R.; Sweeney, J. A.; Chen, H.-Y.; Gershon, E. S.; Liu, C. Bipolar Disord. 2014, 16, 790–799. doi:10.1111/bdi.12255
Return to citation in text: [1] -
Haghighi, F.; Xin, Y.; Chanrion, B.; O'Donnell, A. H.; Ge, Y.; Dwork, A. J.; Arango, V.; Mann, J. J. Dialogues Clin. Neurosci 2014, 16, 430–438.
Return to citation in text: [1] -
Gong, C.; Tao, G.; Yang, L.; Liu, J.; Liu, Q.; Li, W.; Zhuang, Z. Toxicol. Lett. 2012, 209, 264–269. doi:10.1016/j.toxlet.2012.01.007
Return to citation in text: [1] -
Gong, C.; Tao, G.; Yang, L.; Liu, J.; Liu, Q.; Zhuang, Z. Biochem. Biophys. Res. Commun. 2010, 397, 397–400. doi:10.1016/j.bbrc.2010.05.076
Return to citation in text: [1] -
Zhang, X.-F.; Park, J.-H.; Choi, Y.-J.; Kang, M.-H.; Gurunathan, S.; Kim, J.-H. Int. J. Nanomed. 2015, 10, 7057–7071. doi:10.2147/ijn.s95694
Return to citation in text: [1] -
Bai, W.; Chen, Y.; Gao, A. Int. J. Nanomed. 2015, 10, 5561–5569. doi:10.2147/ijn.s88059
Return to citation in text: [1] [2]
| 42. | Golbamaki, N.; Rasulev, B.; Cassano, A.; Robinson, R. L. M.; Benfenati, E.; Leszczynski, J.; Cronin, M. T. D. Nanoscale 2015, 7, 2154–2198. doi:10.1039/c4nr06670g |
| 43. | El-Ghor, A. A.; Noshy, M. M.; Galal, A.; Mohamed, H. R. H. Toxicol. Sci. 2014, 142, 21–32. doi:10.1093/toxsci/kfu157 |
| 42. | Golbamaki, N.; Rasulev, B.; Cassano, A.; Robinson, R. L. M.; Benfenati, E.; Leszczynski, J.; Cronin, M. T. D. Nanoscale 2015, 7, 2154–2198. doi:10.1039/c4nr06670g |
| 42. | Golbamaki, N.; Rasulev, B.; Cassano, A.; Robinson, R. L. M.; Benfenati, E.; Leszczynski, J.; Cronin, M. T. D. Nanoscale 2015, 7, 2154–2198. doi:10.1039/c4nr06670g |
| 41. | Ben Younes, N. R.; Amara, S.; Mrad, I.; Ben-Slama, I.; Jeljeli, M.; Omri, K.; El Ghoul, J.; El Mir, L.; Ben Rhouma, K.; Abdelmelek, H.; Sakly, M. Environ. Sci. Pollut. Res. 2015, 22, 8728–8737. doi:10.1007/s11356-014-4002-5 |
| 49. | Wang, J.; Liu, Y.; Jiao, F.; Lao, F.; Li, W.; Gu, Y.; Li, Y.; Ge, C.; Zhou, G.; Li, B.; Zhao, Y.; Chai, Z.; Chen, C. Toxicology 2008, 254, 82–90. doi:10.1016/j.tox.2008.09.014 |
| 43. | El-Ghor, A. A.; Noshy, M. M.; Galal, A.; Mohamed, H. R. H. Toxicol. Sci. 2014, 142, 21–32. doi:10.1093/toxsci/kfu157 |
| 47. | Kizaki, M.; Xian, M.; Sagawa, M.; Ikeda, Y. Curr. Pharm. Biotechnol. 2006, 7, 323–329. doi:10.2174/138920106778521541 |
| 48. | Huerta-García, E.; Pérez-Arizti, J. A.; Márquez-Ramírez, S. G.; Delgado-Buenrostro, N. L.; Chirino, Y. I.; Iglesias, G. G.; López-Marure, R. Free Radical Biol. Med. 2014, 73, 84–94. doi:10.1016/j.freeradbiomed.2014.04.026 |
| 44. | Kumar, S. S.; Shankar, B.; Sainis, F. B. Biochim. Biophys. Acta, Gen. Subj. 2004, 1672, 100–111. doi:10.1016/j.bbagen.2004.03.002 |
| 45. | Zhang, Y.; Guan, L.; Wang, X.; Wen, T.; Xing, J.; Zhao, J. Free Radical Res. 2008, 42, 362–371. doi:10.1080/10715760801993076 |
| 27. | Block, M. L.; Zecca, L.; Hong, J.-S. Nat. Rev. Neurosci. 2007, 8, 57–69. doi:10.1038/nrn2038 |
| 46. | Indo, H. P.; Davidson, M.; Yen, H.-C.; Suenaga, S.; Tomita, K.; Nishii, T.; Higuchi, M.; Koga, Y.; Ozawa, T.; Majima, H. J. Mitochondrion 2007, 7, 106–118. doi:10.1016/j.mito.2006.11.026 |
| 54. | Hu, R.; Zheng, L.; Zhang, T.; Gao, G.; Cui, Y.; Cheng, Z.; Cheng, J.; Hong, M.; Tang, M.; Hong, F. J. Hazard. Mater. 2011, 191, 32–40. doi:10.1016/j.jhazmat.2011.04.027 |
| 75. | Zhao, Y.; Howe, J. L. C.; Yu, Z.; Leong, D. T.; Chu, J. J. H.; Loo, J. S. C.; Ng, K. W. Small 2013, 9, 387–392. doi:10.1002/smll.201201363 |
| 55. | Meena, R.; Kumar, S.; Paulraj, R. J. Nanopart. Res. 2015, 17, 49. doi:10.1007/s11051-015-2868-x |
| 74. | Yu, K.-N.; Chang, S.-H.; Park, S. J.; Lim, J.; Lee, J.; Yoon, T.-J.; Kim, J.-S.; Cho, M.-H. PLoS One 2015, 10, No. e0131208. doi:10.1371/journal.pone.0131208 |
| 52. | Márquez-Ramírez, S. G.; Delgado-Buenrostro, N. L.; Chirino, Y. I.; Iglesias, G. G.; López-Marure, R. Toxicology 2012, 302, 146–156. doi:10.1016/j.tox.2012.09.005 |
| 13. | Jaenisch, R.; Bird, A. Nat. Genet. 2003, 33, 245–254. doi:10.1038/ng1089 |
| 14. | Egger, G.; Liang, G.; Aparicio, A.; Jones, P. A. Nature 2004, 429, 457–463. doi:10.1038/nature02625 |
| 53. | Valdiglesias, V.; Costa, C.; Sharma, V.; Kiliç, G.; Pásaro, E.; Teixeira, J. P.; Dhawan, A.; Laffon, B. Food Chem. Toxicol. 2013, 57, 352–361. doi:10.1016/j.fct.2013.04.010 |
| 10. | Shyamasundar, S.; Ng, C. T.; Yung, L. Y. L.; Dheen, S. T.; Bay, B. H. Epigenomics 2015, 7, 395–411. doi:10.2217/epi.15.3 |
| 11. | Stoccoro, A.; Karlsson, H. L.; Coppedè, F.; Migliore, L. Toxicology 2013, 313, 3–14. doi:10.1016/j.tox.2012.12.002 |
| 50. | Shimizu, M.; Tainaka, H.; Oba, T.; Mizuo, K.; Umezawa, M.; Takeda, K. Part. Fibre Toxicol. 2009, 6, No. 20. doi:10.1186/1743-8977-6-20 |
| 71. | Yu, Y.; Duan, J.; Yu, Y.; Li, Y.; Liu, X.; Zhou, X.; Ho, K.-F.; Tian, L.; Sun, Z. J. Hazard. Mater. 2014, 270, 176–186. doi:10.1016/j.jhazmat.2014.01.028 |
| 51. | Liu, S.; Xu, L.; Zhang, T.; Ren, G.; Yang, Z. Toxicology 2010, 267, 172–177. doi:10.1016/j.tox.2009.11.012 |
| 70. | Sun, T.; Yan, Y.; Zhao, Y.; Guo, F.; Jiang, C. PLoS One 2012, 7, No. e43442. doi:10.1371/journal.pone.0043442 |
| 73. | Lee, Y.-H.; Cheng, F.-Y.; Chiu, H.-W.; Tsai, J.-C.; Fang, C.-Y.; Chen, C.-W.; Wang, Y.-J. Biomaterials 2014, 35, 4706–4715. doi:10.1016/j.biomaterials.2014.02.021 |
| 72. | Yu, K.-N.; Yoon, T.-J.; Minai-Tehrani, A.; Kim, J.-E.; Park, S. J.; Jeong, M. S.; Ha, S.-W.; Lee, J.-K.; Kim, J. S.; Cho, M.-H. Toxicol. In Vitro 2013, 27, 1187–1195. doi:10.1016/j.tiv.2013.02.010 |
| 69. | Hussain, S.; Al-Nsour, F.; Rice, A. B.; Marshburn, J.; Yingling, B.; Ji, Z. X.; Zink, J. I.; Walker, N. J.; Garantziotis, S. ACS Nano 2012, 6, 5820–5829. doi:10.1021/nn302235u |
| 57. | Ze, Y.; Sheng, L.; Zhao, X.; Ze, X.; Wang, X.; Zhou, Q.; Liu, J.; Yuan, Y.; Gui, S.; Sang, X.; Sun, Q.; Hong, J.; Yu, X.; Wang, L.; Li, B.; Hong, F. J. Hazard. Mater. 2014, 264, 219–229. doi:10.1016/j.jhazmat.2013.10.072 |
| 58. | Mohammadipour, A.; Fazel, A.; Haghir, H.; Motejaded, F.; Rafatpanah, H.; Zabihi, H.; Hosseini, M.; Bideskan, A. E. Environ. Toxicol. Pharmacol. 2014, 37, 617–625. doi:10.1016/j.etap.2014.01.014 |
| 6. | Disdier, C.; Devoy, J.; Cosnefroy, A.; Chalansonnet, M.; Herlin-Boime, N.; Brun, E.; Lund, A.; Mabondzo, A. Part. Fibre Toxicol. 2015, 12, 27. doi:10.1186/s12989-015-0102-8 |
| 56. | Hong, F.; Sheng, L.; Ze, Y.; Hong, J.; Zhou, Y.; Wang, L.; Liu, D.; Yu, X.; Xu, B.; Zhao, X.; Ze, X. Biomaterials 2015, 53, 76–85. doi:10.1016/j.biomaterials.2015.02.067 |
| 76. | Qureshi, I. A.; Mehler, M. F. Mol. Aspects Med. 2013, 34, 875–882. doi:10.1016/j.mam.2012.06.011 |
| 19. | Long, T. C.; Saleh, N.; Tilton, R. D.; Lowry, G. V.; Veronesi, B. Environ. Sci. Technol. 2006, 40, 4346–4352. doi:10.1021/es060589n |
| 20. | Long, T. C.; Tajuba, J.; Sama, P.; Saleh, N.; Swartz, C.; Parker, J.; Hester, S.; Lowry, G. V.; Veronesi, B. Environ. Health Perspect. 2007, 115, 1631–1637. doi:10.1289/ehp.10216 |
| 87. | Schübeler, D.; Lorincz, M. C.; Cimbora, D. M.; Telling, A.; Feng, Y.-Q.; Bouhassira, E. E.; Groudine, M. Mol. Cell. Biol. 2000, 20, 9103–9112. doi:10.1128/mcb.20.24.9103-9112.2000 |
| 21. | Wang, J.; Chen, C.; Liu, Y.; Jiao, F.; Li, W.; Lao, F.; Li, Y.; Li, B.; Ge, C.; Zhou, G.; Gao, Y.; Zhao, Y.; Chai, Z. Toxicol. Lett. 2008, 183, 72–80. doi:10.1016/j.toxlet.2008.10.001 |
| 86. | Herman, J. G.; Latif, F.; Weng, Y.; Lerman, M. I.; Zbar, B.; Liu, S.; Samid, D.; Duan, D. S.; Gnarra, J. R.; Linehan, W. M. Proc. Natl. Acad. Sci. U. S. A. 1994, 91, 9700–9704. doi:10.1073/pnas.91.21.9700 |
| 89. | Reinders, J.; Wulff, B. B. H.; Mirouze, M.; Marí-Ordóñez, A.; Dapp, M.; Rozhon, W.; Bucher, E.; Theiler, G.; Paszkowski, J. Genes Dev. 2009, 23, 939–950. doi:10.1101/gad.524609 |
| 17. | Jaiswal, A. K. Free Radical Biol. Med. 2004, 36, 1199–1207. doi:10.1016/j.freeradbiomed.2004.02.074 |
| 18. | Okuda, M.; Li, K.; Beard, M. R.; Showalter, L. A.; Scholle, F.; Lemon, S. M.; Weinman, S. A. Gastroenterology 2002, 122, 366–375. doi:10.1053/gast.2002.30983 |
| 88. | Hare, J. T.; Taylor, J. H. Proc. Natl. Acad. Sci. U. S. A. 1985, 82, 7350–7354. doi:10.1073/pnas.82.21.7350 |
| 77. | Bird, A. P. Nature 1986, 321, 209–213. doi:10.1038/321209a0 |
| 80. | Robertson, K. D.; Uzvolgyi, E.; Liang, G.; Talmadge, C.; Sumegi, J.; Gonzales, F. A.; Jones, P. A. Nucleic Acids Res. 1999, 27, 2291–2298. doi:10.1093/nar/27.11.2291 |
| 81. | Fujii, S.; Wang, A. H.-J.; van der Marel, G.; van Boom, J. H.; Rich, A. Nucleic Acids Res. 1982, 10, 7879–7892. doi:10.1093/nar/10.23.7879 |
| 82. | Chiang, P. K.; Gordon, R. K.; Tal, J.; Zeng, G. C.; Doctor, B. P.; Pardhasaradhi, K.; McCann, P. P. FASEB J. 1996, 10, 471–480. |
| 79. | Mattick, J. S.; Makunin, I. V. Hum. Mol. Genet. 2006, 15 (Suppl. 1), R17–R29. doi:10.1093/hmg/ddl046 |
| 84. | Okano, M.; Bell, D. W.; Haber, D. A.; Li, E. Cell 1999, 99, 247–257. doi:10.1016/s0092-8674(00)81656-6 |
| 85. | Bachman, K. E.; Rountree, M. R.; Baylin, S. B. J. Biol. Chem. 2001, 276, 32282–32287. doi:10.1074/jbc.M104661200 |
| 83. | Robert, M.-F.; Morin, S.; Beaulieu, N.; Gauthier, F.; Chute, I. C.; Barsalou, A.; MacLeod, A. R. Nat. Genet. 2002, 33, 61–65. doi:10.1038/ng1068 |
| 5. | Geraets, L.; Oomen, A. G.; Krystek, P.; Jacobsen, N. R.; Wallin, H.; Laurentie, M.; Verharen, H. W.; Brandon, E. F. A.; de Jong, W. H. Part. Fibre Toxicol. 2014, 11, 30. doi:10.1186/1743-8977-11-30 |
| 6. | Disdier, C.; Devoy, J.; Cosnefroy, A.; Chalansonnet, M.; Herlin-Boime, N.; Brun, E.; Lund, A.; Mabondzo, A. Part. Fibre Toxicol. 2015, 12, 27. doi:10.1186/s12989-015-0102-8 |
| 29. | Su, M.; Sheng, L.; Zhao, X.; Wang, L.; Yu, X.; Hong, J.; Xu, B.; Liu, D.; Jiang, H.; Ze, X.; Zhu, Y.; Long, Y.; Zhou, J.; Cui, J.; Li, K.; Ze, Y.; Hong, F. Toxicol. Res. (Cambridge, U. K.) 2015, 4, 344–350. doi:10.1039/c4tx00106k |
| 4. | Shi, H.; Magaye, R.; Castranova, V.; Zhao, J. Part. Fibre Toxicol. 2013, 10, No. 15. doi:10.1186/1743-8977-10-15 |
| 3. | Adam, V.; Loyaux-Lawniczak, S.; Quaranta, G. Environ. Sci. Pollut. Res. 2015, 22, 11175–11192. doi:10.1007/s11356-015-4661-x |
| 26. | Cui, Y.; Chen, X.; Zhou, Z.; Lei, Y.; Ma, M.; Cao, R.; Sun, T.; Xu, J.; Huo, M.; Cao, R.; Wen, C.; Che, Y. Chemosphere 2014, 96, 99–104. doi:10.1016/j.chemosphere.2013.07.051 |
| 2. | Montazer, M.; Seifollahzadeh, S. Photochem. Photobiol. 2011, 87, 877–883. doi:10.1111/j.1751-1097.2011.00917.x |
| 27. | Block, M. L.; Zecca, L.; Hong, J.-S. Nat. Rev. Neurosci. 2007, 8, 57–69. doi:10.1038/nrn2038 |
| 28. | Olson, J. K.; Miller, S. D. J. Immunol. 2004, 173, 3916–3924. doi:10.4049/jimmunol.173.6.3916 |
| 29. | Su, M.; Sheng, L.; Zhao, X.; Wang, L.; Yu, X.; Hong, J.; Xu, B.; Liu, D.; Jiang, H.; Ze, X.; Zhu, Y.; Long, Y.; Zhou, J.; Cui, J.; Li, K.; Ze, Y.; Hong, F. Toxicol. Res. (Cambridge, U. K.) 2015, 4, 344–350. doi:10.1039/c4tx00106k |
| 24. | Yin, J.; Kang, C.; Li, Y.; Li, Q.; Zhang, X.; Li, W. Toxicol. Res. (Cambridge, U. K.) 2014, 3, 367–374. doi:10.1039/c4tx00040d |
| 6. | Disdier, C.; Devoy, J.; Cosnefroy, A.; Chalansonnet, M.; Herlin-Boime, N.; Brun, E.; Lund, A.; Mabondzo, A. Part. Fibre Toxicol. 2015, 12, 27. doi:10.1186/s12989-015-0102-8 |
| 25. | Ma, L.; Liu, J.; Li, N.; Wang, J.; Duan, Y.; Yan, J.; Liu, H.; Wang, H.; Hong, F. Biomaterials 2010, 31, 99–105. doi:10.1016/j.biomaterials.2009.09.028 |
| 8. | Song, B.; Liu, J.; Feng, X.; Wei, M.; Shao, L. Nanoscale Res. Lett. 2015, 10, 342. doi:10.1186/s11671-015-1042-9 |
| 22. | Krawczyńska, A.; Dziendzikowska, K.; Gromadzka-Ostrowska, J.; Lankoff, A.; Herman, A. P.; Oczkowski, M.; Królikowski, T.; Wilczak, J.; Wojewódzka, M.; Kruszewski, M. Food Chem. Toxicol. 2015, 85, 96–105. doi:10.1016/j.fct.2015.08.005 |
| 7. | Pelclova, D.; Zdimal, V.; Fenclova, Z.; Vlckova, S.; Turci, F.; Corazzari, I.; Kacer, P.; Schwarz, J.; Zikova, N.; Makes, O.; Syslova, K.; Komarc, M.; Belacek, J.; Navratil, T.; Machajova, M.; Zakharov, S. Occup. Environ. Med. 2016, 73, 110–118. doi:10.1136/oemed-2015-103161 |
| 23. | Shrivastava, R.; Raza, S.; Yadav, A.; Kushwaha, P.; Flora, S. J. S. Drug Chem. Toxicol. 2014, 37, 336–347. doi:10.3109/01480545.2013.866134 |
| 32. | Liu, Y.; Xu, Z.; Li, X. Brain Inj. 2013, 27, 934–939. doi:10.3109/02699052.2013.793401 |
| 33. | Elmore, S. Toxicol. Pathol. 2007, 35, 495–516. doi:10.1080/01926230701320337 |
| 34. | Wirawan, E.; Vande Walle, L.; Kersse, K.; Cornelis, S.; Claerhout, S.; Vanoverberghe, I.; Roelandt, R.; De Rycke, R.; Verspurten, J.; Declercq, W.; Agostinis, P.; Vanden Berghe, T.; Lippens, S.; Vandenabeele, P. Cell Death Dis. 2010, 1, No. e18. doi:10.1038/cddis.2009.16 |
| 100. | Bai, W.; Chen, Y.; Gao, A. Int. J. Nanomed. 2015, 10, 5561–5569. doi:10.2147/ijn.s88059 |
| 30. | Ze, Y.; Sheng, L.; Zhao, X.; Hong, J.; Ze, X.; Yu, X.; Pan, X.; Lin, A.; Zhao, Y.; Zhang, C.; Zhou, Q.; Wang, L.; Hong, F. PLoS One 2014, 9, No. e92230. doi:10.1371/journal.pone.0092230 |
| 31. | Ze, Y.; Zheng, L.; Zhao, X.; Gui, S.; Sang, X.; Su, J.; Guan, N.; Zhu, L.; Sheng, L.; Hu, R.; Cheng, J.; Cheng, Z.; Sun, Q.; Wang, L.; Hong, F. Chemosphere 2013, 92, 1183–1189. doi:10.1016/j.chemosphere.2013.01.094 |
| 94. | Mill, J.; Tang, T.; Kaminsky, Z.; Khare, T.; Yazdanpanah, S.; Bouchard, L.; Jia, P.; Assadzadeh, A.; Flanagan, J.; Schumacher, A.; Wang, S.-C.; Petronis, A. Am. J. Hum. Genet. 2008, 82, 696–711. doi:10.1016/j.ajhg.2008.01.008 |
| 95. | Chen, C.; Zhang, C.; Cheng, L.; Reilly, J. L.; Bishop, J. R.; Sweeney, J. A.; Chen, H.-Y.; Gershon, E. S.; Liu, C. Bipolar Disord. 2014, 16, 790–799. doi:10.1111/bdi.12255 |
| 96. | Haghighi, F.; Xin, Y.; Chanrion, B.; O'Donnell, A. H.; Ge, Y.; Dwork, A. J.; Arango, V.; Mann, J. J. Dialogues Clin. Neurosci 2014, 16, 430–438. |
| 93. | Wong, M.; Gertz, B.; Chestnut, B. A.; Martin, L. J. Front. Cell. Neurosci. 2013, 7, No. 279. doi:10.3389/fncel.2013.00279 |
| 99. | Zhang, X.-F.; Park, J.-H.; Choi, Y.-J.; Kang, M.-H.; Gurunathan, S.; Kim, J.-H. Int. J. Nanomed. 2015, 10, 7057–7071. doi:10.2147/ijn.s95694 |
| 97. | Gong, C.; Tao, G.; Yang, L.; Liu, J.; Liu, Q.; Li, W.; Zhuang, Z. Toxicol. Lett. 2012, 209, 264–269. doi:10.1016/j.toxlet.2012.01.007 |
| 98. | Gong, C.; Tao, G.; Yang, L.; Liu, J.; Liu, Q.; Zhuang, Z. Biochem. Biophys. Res. Commun. 2010, 397, 397–400. doi:10.1016/j.bbrc.2010.05.076 |
| 90. | Zschocke, J.; Allritz, C.; Engele, J.; Rein, T. Glia 2007, 55, 663–674. doi:10.1002/glia.20497 |
| 39. | Coccini, T.; Grandi, S.; Lonati, D.; Locatelli, C.; De Simone, U. NeuroToxicology 2015, 48, 77–89. doi:10.1016/j.neuro.2015.03.006 |
| 92. | Ng, C. W.; Yildirim, F.; Yap, Y. S.; Dalin, S.; Matthews, B. J.; Velez, P. J.; Labadorf, A.; Housman, D. E.; Fraenkel, E. Proc. Natl. Acad. Sci. U. S. A. 2013, 110, 2354–2359. doi:10.1073/pnas.1221292110 |
| 40. | Mao, Z.; Xu, B.; Ji, X.; Zhou, K.; Zhang, X.; Chen, M.; Han, X.; Tang, Q.; Wang, X.; Xia, Y. Nanoscale 2015, 7, 8466–8475. doi:10.1039/c5nr01448d |
| 91. | De Jager, P. L.; Srivastava, G.; Lunnon, K.; Burgess, J.; Schalkwyk, L. C.; Yu, L.; Eaton, M. L.; Keenan, B. T.; Ernst, J.; McCabe, C.; Tang, A.; Raj, T.; Replogle, J.; Brodeur, W.; Gabriel, S.; Chai, H. S.; Younkin, C.; Younkin, S. G.; Zou, F.; Szyf, M.; Epstein, C. B.; Schneider, J. A.; Bernstein, B. E.; Meissner, A.; Ertekin-Taner, N.; Chibnik, L. B.; Kellis, M.; Mill, J.; Bennett, D. A. Nat. Neurosci. 2014, 17, 1156–1163. doi:10.1038/nn.3786 |
| 37. | Lai, J. C. K.; Lai, M. B.; Jandhyam, S.; Dukhande, V. V.; Bhushan, A.; Daniels, C. K.; Leung, S. W. Int. J. Nanomed. 2008, 3, 533–545. doi:10.2147/IJN.S3234 |
| 38. | Wilson, C. L.; Natarajan, V.; Hayward, S. L.; Khalimonchuk, O.; Kidambi, S. Nanoscale 2015, 7, 18477–18488. doi:10.1039/c5nr03646a |
| 35. | Sheng, L.; Ze, Y.; Wang, L.; Yu, X.; Hong, J.; Zhao, X.; Ze, X.; Liu, D.; Xu, B.; Zhu, Y.; Long, Y.; Lin, A.; Zhang, C.; Zhao, Y.; Hong, F. J. Biomed. Mater. Res., Part A 2015, 103, 1141–1149. doi:10.1002/jbm.a.35263 |
| 36. | Li, X.; Xu, S.; Zhang, Z.; Schluesener, H. J. Chin. Sci. Bull. 2009, 54, 3830–3836. doi:10.1007/s11434-009-0548-x |
| 43. | El-Ghor, A. A.; Noshy, M. M.; Galal, A.; Mohamed, H. R. H. Toxicol. Sci. 2014, 142, 21–32. doi:10.1093/toxsci/kfu157 |
| 19. | Long, T. C.; Saleh, N.; Tilton, R. D.; Lowry, G. V.; Veronesi, B. Environ. Sci. Technol. 2006, 40, 4346–4352. doi:10.1021/es060589n |
| 20. | Long, T. C.; Tajuba, J.; Sama, P.; Saleh, N.; Swartz, C.; Parker, J.; Hester, S.; Lowry, G. V.; Veronesi, B. Environ. Health Perspect. 2007, 115, 1631–1637. doi:10.1289/ehp.10216 |
| 56. | Hong, F.; Sheng, L.; Ze, Y.; Hong, J.; Zhou, Y.; Wang, L.; Liu, D.; Yu, X.; Xu, B.; Zhao, X.; Ze, X. Biomaterials 2015, 53, 76–85. doi:10.1016/j.biomaterials.2015.02.067 |
| 52. | Márquez-Ramírez, S. G.; Delgado-Buenrostro, N. L.; Chirino, Y. I.; Iglesias, G. G.; López-Marure, R. Toxicology 2012, 302, 146–156. doi:10.1016/j.tox.2012.09.005 |
| 36. | Li, X.; Xu, S.; Zhang, Z.; Schluesener, H. J. Chin. Sci. Bull. 2009, 54, 3830–3836. doi:10.1007/s11434-009-0548-x |
| 53. | Valdiglesias, V.; Costa, C.; Sharma, V.; Kiliç, G.; Pásaro, E.; Teixeira, J. P.; Dhawan, A.; Laffon, B. Food Chem. Toxicol. 2013, 57, 352–361. doi:10.1016/j.fct.2013.04.010 |
| 48. | Huerta-García, E.; Pérez-Arizti, J. A.; Márquez-Ramírez, S. G.; Delgado-Buenrostro, N. L.; Chirino, Y. I.; Iglesias, G. G.; López-Marure, R. Free Radical Biol. Med. 2014, 73, 84–94. doi:10.1016/j.freeradbiomed.2014.04.026 |
| 39. | Coccini, T.; Grandi, S.; Lonati, D.; Locatelli, C.; De Simone, U. NeuroToxicology 2015, 48, 77–89. doi:10.1016/j.neuro.2015.03.006 |
| 40. | Mao, Z.; Xu, B.; Ji, X.; Zhou, K.; Zhang, X.; Chen, M.; Han, X.; Tang, Q.; Wang, X.; Xia, Y. Nanoscale 2015, 7, 8466–8475. doi:10.1039/c5nr01448d |
| 35. | Sheng, L.; Ze, Y.; Wang, L.; Yu, X.; Hong, J.; Zhao, X.; Ze, X.; Liu, D.; Xu, B.; Zhu, Y.; Long, Y.; Lin, A.; Zhang, C.; Zhao, Y.; Hong, F. J. Biomed. Mater. Res., Part A 2015, 103, 1141–1149. doi:10.1002/jbm.a.35263 |
| 38. | Wilson, C. L.; Natarajan, V.; Hayward, S. L.; Khalimonchuk, O.; Kidambi, S. Nanoscale 2015, 7, 18477–18488. doi:10.1039/c5nr03646a |
| 37. | Lai, J. C. K.; Lai, M. B.; Jandhyam, S.; Dukhande, V. V.; Bhushan, A.; Daniels, C. K.; Leung, S. W. Int. J. Nanomed. 2008, 3, 533–545. doi:10.2147/IJN.S3234 |
| 51. | Liu, S.; Xu, L.; Zhang, T.; Ren, G.; Yang, Z. Toxicology 2010, 267, 172–177. doi:10.1016/j.tox.2009.11.012 |
| 63. | Wu, J.; Sun, J.; Xue, Y. Toxicol. Lett. 2010, 199, 269–276. doi:10.1016/j.toxlet.2010.09.009 |
| 13. | Jaenisch, R.; Bird, A. Nat. Genet. 2003, 33, 245–254. doi:10.1038/ng1089 |
| 14. | Egger, G.; Liang, G.; Aparicio, A.; Jones, P. A. Nature 2004, 429, 457–463. doi:10.1038/nature02625 |
| 15. | Armstrong, J. S.; Rajasekaran, M.; Chamulitrat, W.; Gatti, P.; Hellstrom, W. J.; Sikka, S. C. Free Radical Biol. Med. 1999, 26, 869–880. doi:10.1016/s0891-5849(98)00275-5 |
| 16. | Starkov, A. A. Ann. N. Y. Acad. Sci. 2008, 1147, 37–52. doi:10.1196/annals.1427.015 |
| 75. | Zhao, Y.; Howe, J. L. C.; Yu, Z.; Leong, D. T.; Chu, J. J. H.; Loo, J. S. C.; Ng, K. W. Small 2013, 9, 387–392. doi:10.1002/smll.201201363 |
| 100. | Bai, W.; Chen, Y.; Gao, A. Int. J. Nanomed. 2015, 10, 5561–5569. doi:10.2147/ijn.s88059 |
| 12. | Mizushima, N.; Komatsu, M. Cell 2011, 147, 728–741. doi:10.1016/j.cell.2011.10.026 |
| 74. | Yu, K.-N.; Chang, S.-H.; Park, S. J.; Lim, J.; Lee, J.; Yoon, T.-J.; Kim, J.-S.; Cho, M.-H. PLoS One 2015, 10, No. e0131208. doi:10.1371/journal.pone.0131208 |
| 9. | Stern, S. T.; Adiseshaiah, P. P.; Crist, R. M. Part. Fibre Toxicol. 2012, 9, 20. doi:10.1186/1743-8977-9-20 |
| 10. | Shyamasundar, S.; Ng, C. T.; Yung, L. Y. L.; Dheen, S. T.; Bay, B. H. Epigenomics 2015, 7, 395–411. doi:10.2217/epi.15.3 |
| 11. | Stoccoro, A.; Karlsson, H. L.; Coppedè, F.; Migliore, L. Toxicology 2013, 313, 3–14. doi:10.1016/j.tox.2012.12.002 |
| 64. | Levine, B.; Kroemer, G. Cell 2008, 132, 27–42. doi:10.1016/j.cell.2007.12.018 |
| 65. | Mizushima, N.; Levine, B.; Cuervo, A. M.; Klionsky, D. J. Nature 2008, 451, 1069–1075. doi:10.1038/nature06639 |
| 12. | Mizushima, N.; Komatsu, M. Cell 2011, 147, 728–741. doi:10.1016/j.cell.2011.10.026 |
| 62. | Gao, X.; Yin, S.; Tang, M.; Chen, J.; Yang, Z.; Zhang, W.; Chen, L.; Yang, B.; Li, Z.; Zha, Y.; Ruan, D.; Wang, M. Biol. Trace Elem. Res. 2011, 143, 1616–1628. doi:10.1007/s12011-011-8990-4 |
| 63. | Wu, J.; Sun, J.; Xue, Y. Toxicol. Lett. 2010, 199, 269–276. doi:10.1016/j.toxlet.2010.09.009 |
| 60. | Umezawa, M.; Tainaka, H.; Kawashima, N.; Shimizu, M.; Takeda, K. J. Toxicol. Sci. 2012, 37, 1247–1252. doi:10.2131/jts.37.1247 |
| 61. | Hu, R.; Gong, X.; Duan, Y.; Li, N.; Che, Y.; Cui, Y.; Zhou, M.; Liu, C.; Wang, H.; Hong, F. Biomaterials 2010, 31, 8043–8050. doi:10.1016/j.biomaterials.2010.07.011 |
| 59. | Takahashi, Y.; Mizuo, K.; Shinkai, Y.; Oshio, S.; Takeda, K. J. Toxicol. Sci. 2010, 35, 749–756. doi:10.2131/jts.35.749 |
| 30. | Ze, Y.; Sheng, L.; Zhao, X.; Hong, J.; Ze, X.; Yu, X.; Pan, X.; Lin, A.; Zhao, Y.; Zhang, C.; Zhou, Q.; Wang, L.; Hong, F. PLoS One 2014, 9, No. e92230. doi:10.1371/journal.pone.0092230 |
| 26. | Cui, Y.; Chen, X.; Zhou, Z.; Lei, Y.; Ma, M.; Cao, R.; Sun, T.; Xu, J.; Huo, M.; Cao, R.; Wen, C.; Che, Y. Chemosphere 2014, 96, 99–104. doi:10.1016/j.chemosphere.2013.07.051 |
| 24. | Yin, J.; Kang, C.; Li, Y.; Li, Q.; Zhang, X.; Li, W. Toxicol. Res. (Cambridge, U. K.) 2014, 3, 367–374. doi:10.1039/c4tx00040d |
| 57. | Ze, Y.; Sheng, L.; Zhao, X.; Ze, X.; Wang, X.; Zhou, Q.; Liu, J.; Yuan, Y.; Gui, S.; Sang, X.; Sun, Q.; Hong, J.; Yu, X.; Wang, L.; Li, B.; Hong, F. J. Hazard. Mater. 2014, 264, 219–229. doi:10.1016/j.jhazmat.2013.10.072 |
| 55. | Meena, R.; Kumar, S.; Paulraj, R. J. Nanopart. Res. 2015, 17, 49. doi:10.1007/s11051-015-2868-x |
| 68. | Khan, M. I.; Mohammad, A.; Patil, G.; Naqvi, S. A. H.; Chauhan, L. K. S.; Ahmad, I. Biomaterials 2012, 33, 1477–1488. doi:10.1016/j.biomaterials.2011.10.080 |
| 23. | Shrivastava, R.; Raza, S.; Yadav, A.; Kushwaha, P.; Flora, S. J. S. Drug Chem. Toxicol. 2014, 37, 336–347. doi:10.3109/01480545.2013.866134 |
| 9. | Stern, S. T.; Adiseshaiah, P. P.; Crist, R. M. Part. Fibre Toxicol. 2012, 9, 20. doi:10.1186/1743-8977-9-20 |
| 66. | Cohignac, V.; Landry, M. J.; Boczkowski, J.; Lanone, S. Nanomaterials 2014, 4, 548–582. doi:10.3390/nano4030548 |
| 22. | Krawczyńska, A.; Dziendzikowska, K.; Gromadzka-Ostrowska, J.; Lankoff, A.; Herman, A. P.; Oczkowski, M.; Królikowski, T.; Wilczak, J.; Wojewódzka, M.; Kruszewski, M. Food Chem. Toxicol. 2015, 85, 96–105. doi:10.1016/j.fct.2015.08.005 |
| 67. | Li, J. J.; Hartono, D.; Ong, C.-N.; Bay, B.-H.; Yung, L.-Y. L. Biomaterials 2010, 31, 5996–6003. doi:10.1016/j.biomaterials.2010.04.014 |
| 50. | Shimizu, M.; Tainaka, H.; Oba, T.; Mizuo, K.; Umezawa, M.; Takeda, K. Part. Fibre Toxicol. 2009, 6, No. 20. doi:10.1186/1743-8977-6-20 |
| 58. | Mohammadipour, A.; Fazel, A.; Haghir, H.; Motejaded, F.; Rafatpanah, H.; Zabihi, H.; Hosseini, M.; Bideskan, A. E. Environ. Toxicol. Pharmacol. 2014, 37, 617–625. doi:10.1016/j.etap.2014.01.014 |
| 59. | Takahashi, Y.; Mizuo, K.; Shinkai, Y.; Oshio, S.; Takeda, K. J. Toxicol. Sci. 2010, 35, 749–756. doi:10.2131/jts.35.749 |
| 60. | Umezawa, M.; Tainaka, H.; Kawashima, N.; Shimizu, M.; Takeda, K. J. Toxicol. Sci. 2012, 37, 1247–1252. doi:10.2131/jts.37.1247 |
| 62. | Gao, X.; Yin, S.; Tang, M.; Chen, J.; Yang, Z.; Zhang, W.; Chen, L.; Yang, B.; Li, Z.; Zha, Y.; Ruan, D.; Wang, M. Biol. Trace Elem. Res. 2011, 143, 1616–1628. doi:10.1007/s12011-011-8990-4 |
| 32. | Liu, Y.; Xu, Z.; Li, X. Brain Inj. 2013, 27, 934–939. doi:10.3109/02699052.2013.793401 |
| 54. | Hu, R.; Zheng, L.; Zhang, T.; Gao, G.; Cui, Y.; Cheng, Z.; Cheng, J.; Hong, M.; Tang, M.; Hong, F. J. Hazard. Mater. 2011, 191, 32–40. doi:10.1016/j.jhazmat.2011.04.027 |
| 31. | Ze, Y.; Zheng, L.; Zhao, X.; Gui, S.; Sang, X.; Su, J.; Guan, N.; Zhu, L.; Sheng, L.; Hu, R.; Cheng, J.; Cheng, Z.; Sun, Q.; Wang, L.; Hong, F. Chemosphere 2013, 92, 1183–1189. doi:10.1016/j.chemosphere.2013.01.094 |
| 25. | Ma, L.; Liu, J.; Li, N.; Wang, J.; Duan, Y.; Yan, J.; Liu, H.; Wang, H.; Hong, F. Biomaterials 2010, 31, 99–105. doi:10.1016/j.biomaterials.2009.09.028 |
| 61. | Hu, R.; Gong, X.; Duan, Y.; Li, N.; Che, Y.; Cui, Y.; Zhou, M.; Liu, C.; Wang, H.; Hong, F. Biomaterials 2010, 31, 8043–8050. doi:10.1016/j.biomaterials.2010.07.011 |
| 21. | Wang, J.; Chen, C.; Liu, Y.; Jiao, F.; Li, W.; Lao, F.; Li, Y.; Li, B.; Ge, C.; Zhou, G.; Gao, Y.; Zhao, Y.; Chai, Z. Toxicol. Lett. 2008, 183, 72–80. doi:10.1016/j.toxlet.2008.10.001 |
| 49. | Wang, J.; Liu, Y.; Jiao, F.; Lao, F.; Li, W.; Gu, Y.; Li, Y.; Ge, C.; Zhou, G.; Li, B.; Zhao, Y.; Chai, Z.; Chen, C. Toxicology 2008, 254, 82–90. doi:10.1016/j.tox.2008.09.014 |
© 2016 Song et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Nanotechnology terms and conditions: (http://www.beilstein-journals.org/bjnano)