Abstract
Investigation of the local sample elasticity is of high importance in many scientific domains. In 2014, Herruzo et al. published a new method based on frequency-modulation atomic force microscopy to locally determine the elasticity of samples (Nat. Commun. 2014, 5, 3126). This method gives evidence for the linearity of the relation between the frequency shift of the cantilever first flexural mode Δf1 and the square of the frequency shift of the second flexural mode Δf22. In the present work, we showed that a similar linear relation exists when measuring in contact mode with a certain load FN and propose a new method for determining the elastic modulus of samples from this relation. The measurements were performed in non-dry air at ambient temperature on three different polymers (polystyrene, polypropylene and linear low-density polyethylene) and a self-assembled monolayer of 1H,1H,2H,2H-perfluorodecyltrichlorosilane (FDTS) on a silicon oxide substrate perforated with circular holes prepared by polymer blend lithography. For all samples the relation was evidenced by recording Δf1, Δf2 and FN as a function of the Z-displacement curves of the piezoelectric scanner. The occurence of a plastic deformation followed by an elastic deformation is shown and explained. The necessary load FN for measuring in the elastic domain was assessed for each sample, used for mapping the frequency shifts Δf1 and Δf2 and for determining the elastic modulus from Δf22/Δf1. The method was used to give an estimate of the Young’s modulus of the FDTS thin film.
Introduction
Knowledge of the local elasticity of samples is of high interest in many scientific domains, as many processes and physical quantities are correlated with the elastic modulus. In biology, for instance, studies showed that the elasticity of cells depends on their age, the stage of the cell cycle and the degree of differentiation [1]. In physics, the band gap size of nanocrystals and the presence of planar defects on nanotubes are a function of the Young’s modulus [2,3]. Probing local elasticity requires an instrumentation capable of operating with high resolution and under different conditions, such as variable temperature, pressure or humidity. Since its invention, the atomic force microscope (AFM) [4] has confirmed its value for locally determining nanomechanical properties, such as the Young’s modulus, of the sample surface. Initially, the measures were done qualitatively, with the cantilever operated in intermittent-contact mode by showing the phase-shift contrast between regions of different elasticities [5]. This was followed by quantitative measurements using various static and dynamic methods [6,7]. Although the results obtained with these methods are in good agreement with theoretical data and data obtained from macroscopical experiments, difficulties in the precise determination of the elastic modulus based on the theoretical model, or during the use of the method may be encountered when using dynamic-mode AFM. This is the case with the methods devised by Hurley and Turner [6] and Herruzo and co-workers [7]. In Hurley and Turner’s [6] method, the stated equations for the computation of the normal sample stiffness by numerical methods (analytical expression for normal sample stiffness formulated by Bubendorf [8] and given in Supporting Information File 1) used to determine sample elasticity are based on the equations established by Rabe [9] and Rabe et al. [10] for atomic force acoustic microscopy (AFAM) [11-14]. They describe the dynamics of a clamped cantilever elastically coupled with the sample surface at its tip end. These equations have the disadvantage of strongly depending on the dimensions of an ideal beam-shaped cantilever. However, most cantilevers used for measurements are not ideal. Thus, to achieve consistent results, the values of length and tip height have to be corrected. When measurements using the multifrequency AFM [15,16] method of Herruzo et al., which is based on the excitation of two cantilever eigenmodes [17-21], are performed in non-dry air, the instability of the tip–sample distance feedback loop, due to the use of the frequency shift as control parameter, makes the application of the method difficult if not impossible. However, despite these disadvantages, both methods are particularly interesting because of the complementarity of their advantages. The method of Hurley and Turner [6], which is based on tracking the first flexural and torsional contact resonances, has the advantage of staying stable even if the measurements are performed in non-dry air. In contrast, the method of Herruzo et al. [7] uses a simple theoretical model that depends only weakly on the dimensions of the cantilever. We present a new dynamic method for measuring the sample elasticity [8] that combines the simplicity of the theoretical model of Herruzo et al. [7] with the robustness of the measuring method based on contact resonances. Since the range of elasticity values of polymers is covered by the domain of validity of the theoretical model, those values were used for testing the new method.
Principle of the Method
Theoretical model
The method is based on the theoretical model developed by Herruzo et al. [7] for the computation of the effective elastic modulus of samples Eeff ranging from 1 MPa to 3 GPa from the measured frequency shifts of the two flexural modes of a cantilever operated in intermittent-contact mode:
where R is the effective tip radius and ki, Ai, f0,i and Δfi(dm) are, respectively, the force constant, the oscillation amplitude, the resonance frequency and the frequency shift of the i-th flexural mode in free space as a function of dm, the closest distance between tip and sample in an oscillation cycle.
The validity of the relation and the stability of the microscope during its operation is ensured by the use of an amplitude A2 small enough compared to A1 (ideally, A1 is at least one order of magnitude larger than A2). The applied normal force on the sample is controlled by controlling Δf1, so that Δf2 changes as a function of sample elasticity. In our method based on tracking the contact resonances, the applied normal force FN is directly controlled by controlling the vertical deflection of the cantilever. We can hence rewrite the previous equation as
where α is a parameter that depends on amplitude A1, determined from a sample of known elastic modulus. Δf1 and Δf2 are the frequency shifts that depend on the applied normal force, FN. They are determined from the measured contact resonances through the relation Δfi = fi − f0,i, where fi is the contact resonance of the i-th flexural mode.
Herruzo et al. [7] showed that if the Young’s modulus of the tip Etip is at least two orders of magnitude larger than that of the sample Esample then
Feedback controls
As introduced by Herruzo et al. [7], five different feedback loops are used as feedback controls, as shown in Figure 1: two feedback loops for keeping the amplitudes A1 and A2 constant, two feedback loops for keeping the phase shifts 1 and
2 constant in order to track the contact resonances f1 and f2, and the last feedback loop as main feedback for controlling the applied normal force FN.
![[2190-4286-9-1-1]](/bjnano/content/figures/2190-4286-9-1-1.png?scale=1.68&max-width=1024&background=FFFFFF)
Figure 1:
Feedback loops necessary to track the flexural contact resonances of the cantilever. The main feedback loop ensures that the measurements are performed with a constant normal force. The other feedback loops are used to maintain constant values of phase 1 and amplitude A1 of the first flexural mode, and of phase
2 and amplitude A2 of the second flexural mode.
Figure 1: Feedback loops necessary to track the flexural contact resonances of the cantilever. The main feedb...
Experimental
Microscope and data acquisition
The measurements were performed with a flex AFM head from Nanosurf and the Nanonis scanning probe microscope controller. The system integrates the phase-locked loops (PLLs) necessary for tracking the contact resonances and all the controlling and signal generation modules for measuring and mapping the physical quantities. Square areas of 2.5 μm × 2.5 μm were scanned at a resolution of 256 × 256 pixels and a scan speed of 2 μm·s−1.
Cantilevers
The measurements were performed with a PPP-CONT cantilever from NanosensorsTM (Si tip of Young’s modulus Etip ≈ 169 GPa) characterized by f0,1 = 16.35 kHz, k1 = 0.324 N·m−1, Q1 = 68, f0,2 = 102.98 kHz, k2 = 13.1 N·m−1, Q2 = 208, where Qi is the Q factor of the i-th flexural mode in free space. The force constants were computed for k1 from the theoretical formula k1 = (Ewt3)/(4l3) [22], where E, t, w and l are, respectively, Young’s modulus, thickness, width and length of the cantilever beam, and for k2 by using the relation k2 = 40.4k1 established by Rast and co-workers [23]. The thickness t = 2.35 μm was determined from f0,1 and the formula t ≈ 6.1911 (ρ/E)(1/2)f0,1l2 [22]. Width w = 52 μm and length l = 445 μm of the cantilever were determined by optical microscopy. The values E = 169 GPa and ρ = 2,330 kg·m−3 were taken for the Young’s modulus of the silicon beam and its mass density. The measured normal force FN,meas, which corresponds to the vertical deflection of the cantilever and gives the value of the applied normal force FN on the sample surface was calibrated with a factor obtained by dividing the cantilever spring constant by the cantilever optical sensitivity Sz. The optical sensitivity of value 229 nm·V−1 was determined by measuring a vertical deflection–distance curve on an n-type silicon(111) sample of electrical resistivity = 10 Ω·m and by taking the inverse of the slope. The two first flexural modes of the cantilever in contact were excited with the amplitudes A1 = 22 mV and A2 = 5 mV for all samples.
Samples and measurement conditions
Three different polymers (polystyrene (PS), polypropylene (PP) and linear low-density polyethylene (LLDPE)) and a self-assembled monolayer (SAM) of 1H,1H,2H,2H-perfluorodecyltrichlorosilane (FDTS) on a silicon oxide (SiOx) substrate were investigated. The SAM was prepared with circular holes obtained by polymer blend lithography (PBL) [24]. A reference sample consisting of polytetrafluoroethylene (PTFE), commonly called Teflon, with a nominal Young’s modulus of 500 MPa was used to determine parameter α(A1). The measurements were performed in non-dry air with a relative humidity of 36% at an ambient temperature of 27 °C.
Results and Discussion
Force–distance and frequency shift–distance curves
Before starting the investigation of the sample elasticity, a force–distance curve and a frequency shift–distance curve (Figure 2 and Figure 3, left) for both flexural modes were recorded for each sample to determine for which values of FN the relation between Δf1 and is linear and to compute the parameter α(A1). The measured applied normal force FN,meas and frequency shifts Δf1, Δf2 were recorded for a displacement of the piezoelectric scanner in the normal Z-direction from 0 to 350 nm. To measure the curves, the cantilever tip first indented the sample to a depth corresponding to a displacement of the Z-scanner of 350 nm and both PLLs were then switched on. The curves were recorded during retraction of the tip in order to avoid unlocking the PLLs. This would happen if the tip indented the sample from a starting position out of contact, as contact resonances are quite far from those in free space.
![[2190-4286-9-1-2]](/bjnano/content/figures/2190-4286-9-1-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2:
Left: Force–distance and frequency shift–distance curves of LLDPE, PP, PS and FDTS for the determination of the minimal normal force FN used as setpoint for the elasticity measurements. The curves for Δf1 and Δf2 as functions of the Z-displacement evidence the existence of a plastic deformation phase followed by an elastic deformation phase. Right: The fitting of the Z(FN,meas) curves gave a value close to the cantilever spring constant showing the excessive compliance of the cantilever. The curves show quasi-invariance of the ratio
in the elastic deformation phase and in the highest region of the plastic deformation phase.
Figure 2: Left: Force–distance and frequency shift–distance curves of LLDPE, PP, PS and FDTS for the determin...
![[2190-4286-9-1-3]](/bjnano/content/figures/2190-4286-9-1-3.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 3:
Left: Force–distance and frequency shift–distance curves of PTFE. The curves of Δf1 and Δf2 as a function of the Z-displacement evidence the existence of a plastic deformation phase followed by an elastic deformation phase. Right: The fitting of the Z(FN,meas) curve gave a value close to the cantilever spring constant showing the excessive compliance of the cantilever. The curves show quasi-invariance of the ratio
in the elastic deformation phase and in the highest region of the plastic deformation phase. The parameter α(A1) determined by dividing the nominal value of the elasticity of the reference sample by the mean value of the ratio in the elastic deformation phase gave a value of 7,355 Pa·Hz−1.
Figure 3: Left: Force–distance and frequency shift–distance curves of PTFE. The curves of Δf1 and Δf2 as a fu...
In the frequency shift–displacement curves, we observe first a nonlinear relation between the measured frequency shifts, Δf1 and Δf2, and the displacement of the scanner, followed by a linear relation. This suggests a plastic deformation phase of the sample surface during the first step of the indentation, followed by an elastic deformation phase. Analysis of the force–displacement curve evidences the same relations with, however, a linear relation in the nonlinear domain of the frequency shift–displacement curves. This is explained by the low spring constant of the cantilever in comparison to the normal sample stiffness, beginning at a certain Z-displacement value.
A good model of the cantilever in contact with the sample surface is two springs in series, k1 and ksample,norm, representing the spring constant of the cantilever and the normal sample stiffness (of constant value in the elastic phase), respectively (Figure 4a). As shown in Figure 4b, the applied normal force generated by the Z-displacement Δz, is FN = keffΔz, where keff is the effective spring of value k1ksample,norm/(k1 + ksample,norm). As the normal stiffness of sample increases during indentation, the value of keff approaches k1, so that beyond a certain value of ksample,norm, the variation of ksample,norm cannot be observed anymore in the FN,meas(Z) curve. This effect is evidenced in Figure 2 and Figure 3 (right) with the FN,meas(Z) curve (black dots), where the inverse of the slope of the linear fit (red line) shows a value close to the cantilever spring constant as given in Table 1.
![[2190-4286-9-1-4]](/bjnano/content/figures/2190-4286-9-1-4.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 4: (a) Cantilever in contact with the sample surface modeled by two springs in series, k1 and ksample,norm, representing the cantilever spring constant and the normal sample stiffness, respectively. The arrow FN represents the applied normal force. (b) Effective spring constant keff and values of the ratio between measured applied normal force FN,meas and Z-displacement Δz as a function of the sample stiffness.
Figure 4: (a) Cantilever in contact with the sample surface modeled by two springs in series, k1 and ksample,...
Sample surface deformations and evolution of stress during indentation
The existence of a plastic and an elastic deformation phase is explained by the evolution of stress σ (ratio between the applied normal force FN and the cross section A of the cantilever tip) during indentation (Figure 5). At the beginning of the indentation, after setting the tip on the sample surface, stress is increased from a normal force with an initial value 0 nN. The evolution of stress can be split into three phases. In the first phase, if the applied normal force increases gently or if the contact surface between the tip apex and the surface is large enough, the generated stress increases gently enough to deform the sample surface elastically. This elastic phase does not occur if the steps of the Z-scanner, which are responsible for increasing the applied normal force, are too big. In this case, the generated stress is strong enough to deform the surface plastically, and phase 2 is initiated. In the second phase, due to the assumed pyramidal geometry of the tip, the contact area is small enough and the applied normal force is strong enough to create a stress that deforms the sample surface plastically. In the third phase, the tip geometry and the increase of the indentation depth result in a larger increase in the contact area than that produced by the applied normal force, thus decreasing the stress on the sample surface. When the stress decreases below a threshold value, σ0, the sample starts deforming elastically.
![[2190-4286-9-1-5]](/bjnano/content/figures/2190-4286-9-1-5.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 5: Three phases characterizing the evolution of stress σ during indentation. This evolution is responsible for the plastic and elastic deformation of the sample. In phase 1, in the presence of a sufficiently weak applied normal force FN or a sufficiently large cross section A, the generated stress is small enough to deform the surface elastically. This initial elastic phase can only be observed, if the steps used for the Z-scanner are small enough. If the steps are too big, the evolution of σ is directly initiated with phase 2. In phase 2, the large value of FN and its fast increase compared to A generate a stress large enough to deform the surface plastically. In phase 3, the geometry of the tip results in a fast increase of the contact area, and hence of A, compared to the moderate increase in FN, which decreases the stress. When the stress decreases below a threshold value σ0, the deformation becomes elastic. The red area of the tip apex describes the contact area of the tip. The striped area shows the region of the sample plastically deformed during indentation. The arrow at the tip apex represents the applied normal force FN; the arrows on the right of σ, FN and A characterize the increase speed (bold for fast) and magnitude (length).
Figure 5: Three phases characterizing the evolution of stress σ during indentation. This evolution is respons...
Linear relation between Δf1 and Δf22 and determination of α(A1)
The linearity relation between Δf1 and is shown in Figure 2 and Figure 3 (curves on the right) where the quasi-invariance of the ratio
in the elastic deformation phase and in the upper part of the plastic deformation phase can be observed. The mean value of the linear coefficient for each sample and the corresponding maximum deviation in percent are reported in Table 2. The weak deviation in the upper part of the plastic deformation phase suggests that setpoints for FN in that region can be used to investigate the sample elasticity. The parameter α(A1) was determined by dividing the nominal value of the Young’s modulus of PTFE by the mean value of
of PTFE. The computation yielded a value of 7,355 Pa·Hz−1.
Table 2:
Mean value of and corresponding maximum deviation (in percent) in the elastic deformation phase and the upper region of the plastic deformation phase.
| sample | mean Δf22/Δf1 (Hz) | max. dev. elastic phase (%) | max. dev. plastic phase (%) |
|---|---|---|---|
| LLDPE | 48,720 | 0.89 | 3 |
| PP | 239,738 | 1.77 | 5 |
| PS | 417,224 | 1.38 | 6.7 |
| FDTS | 61,614 | 0.1 | 0.8 |
| PTFE | 67,981 | 0.52 | 1 |
Investigation of sample elastic modulus
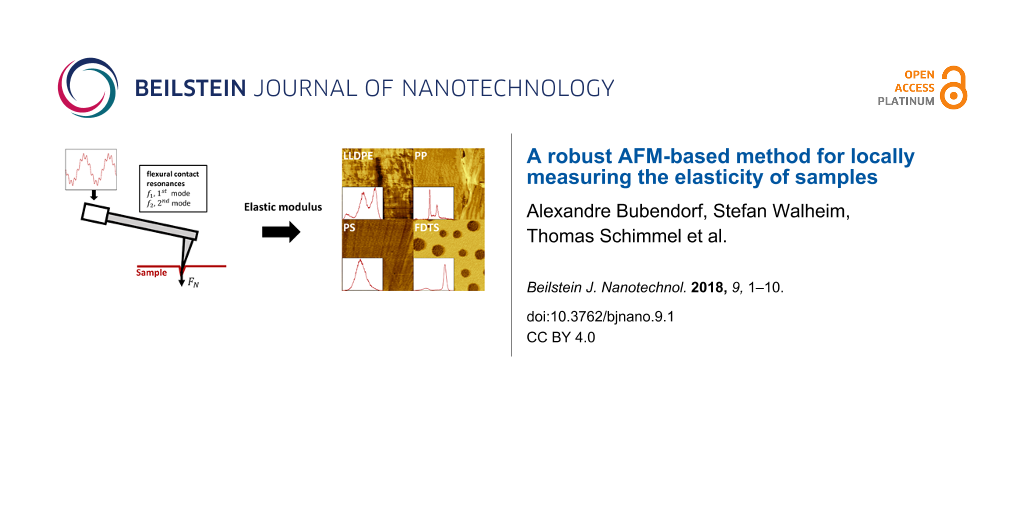
The elastic modulus of the sample was then investigated with setpoints of the main feedback in the highest region of the plastic phase of 44.4 nN for LLDPE, 29.6 nN for PP, 29.6 nN for PS and 37 nN for FDTS. The results of the scannings are shown in Figure 6 and Figure 7 for topography (panel a) and elastic modulus (panel b). The histograms in Figure 6c and Figure 7c correspond to the distribution of the elastic modulus for each map in Figure 6b and Figure 7b. Because polymers are viscoelastic materials, the elastic modulus Eeff,meas measured on the investigated samples in dynamic mode corresponds to the storage modulus. As the measured contact resonances are quite large compared to the inverse of typical material relaxation time, we can assume that the measured storage modulus is independent on the frequency. The measurements yielded values in the range of the Young’s moduli of bulk LLDPE, PP and PS as seen in Table 3. The investigation also evidenced regions of different elastic moduli, as seen in the histograms of LLDPE, PP and the SAM: LLDPE shows three different peaks centered at 362, 380 and 393 MPa, PP shows two peaks centered at 1.468 GPa and 1.565 GPa and the SAM shows two peaks centered at 472 and 478.5 MPa. Finally, the value of the storage modulus of FDTS gives an estimate of the Young’s modulus of the FDTS monolayer.
![[2190-4286-9-1-6]](/bjnano/content/figures/2190-4286-9-1-6.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 6: Mapping of (a) topography and (b) elastic modulus of 2.5 μm × 2.5 μm areas of the LLDPE, PP and PS samples. The measurements were performed in contact by applying a constant normal force FN of 44.4 nN for LLDPE and 29.6 nN for PP and PS, and by exciting the two first flexural modes of the cantilever with an amplitude of A1 = 22 mV and A2 = 5 mV, respectively, for the first and second mode. The histograms in panel (c) show the distribution of elastic modulus Eeff,meas in the maps in panel (b).
Figure 6: Mapping of (a) topography and (b) elastic modulus of 2.5 μm × 2.5 μm areas of the LLDPE, PP and PS ...
![[2190-4286-9-1-7]](/bjnano/content/figures/2190-4286-9-1-7.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 7: Mapping of (a) topography and (b) elastic modulus of a 2.5 μm × 2.5 μm area of the FDTS + SiOx SAM sample. The measurements were performed in contact by applying a constant normal force FN of 37 nN, and by exciting the two first flexural modes of the cantilever with an amplitude of A1 = 22 mV and A2 = 5 mV respectively for the first and second mode. The histogram in panel (c) shows the distribution of elastic modulus Eeff,meas in panel (b).
Figure 7: Mapping of (a) topography and (b) elastic modulus of a 2.5 μm × 2.5 μm area of the FDTS + SiOx SAM ...
Table 3: Measured storage moduli Eeff,meas and literature Young’s modulus values of bulk materials Esample,lit of LLDPE, PP, PS and FDTS. The values of Eeff,meas are in the range of the Young’s moduli of the bulk materials. No literature value is available for FDTS.
Effect of tip functionalization on the SiOx elasticity peak of the SAM sample
The proximity of the peaks of FDTS and SiOx holes can be explained by the fact that during scanning, some material of FDTS has gathered around the tip, so that the relatively soft FDTS-coating of the tip is sensed (see also Figure 8). The effect of collecting Teflon-like molecules with AFM tips has been known for a long time. It has been successfully used for the topographic imaging of the Si(111) surface with atomic resolution. Howald et al. [27] studied the Si(111) 7 × 7 reconstruction in UHV by contact force microscopy. They observed adhesive forces of up to 103 nN between the Si tip and the Si(111) surface. By coating the tip with PTFE (Teflon), they could reduce the sticking forces to 10 nN. A short scan on a PTFE sample led to a reliable coating of the tip for the subsequent AFM scan on silicon. Since our sample consists of a perforated Teflon-like monolayer, the cantilever tip is most likely constantly functionalized with a thin layer of FDTS. This leads to the observed soft elasticity in- and outside the holes. The stability of this functionalization during the scan can be verified with topographic images. The holes show a depth slightly below 1.2 nm, which is the height of the FDTS monolayer, as seen in Figure 8. If the tip coating was worn-off during the scan, larger or at least partially deeper holes would appear.
![[2190-4286-9-1-8]](/bjnano/content/figures/2190-4286-9-1-8.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 8: Line profile of the SiOx holes in the topographical mapping of the SAM showing their relative height and width due to the functionalization of the cantilever tip with FDTS material. On the right, a schematic of the tip–sample interaction is depicted. Loosely bound FDTS molecules are able to migrate to the AFM tip and can therefore functionalize by forming a thin layer on the tip apex.
Figure 8: Line profile of the SiOx holes in the topographical mapping of the SAM showing their relative heigh...
Conclusion
We present a robust method for the quantitative determination of local elastic modulus of sample under ambient conditions (humidity and temperature). It combines the simplicity of the theoretical model developed by Herruzo et al. [7] for the determination of sample elasticity from 1 MPa to 3 GPa with a robust measuring method based on contact resonances. The measurements consist of tracking the first and second flexural contact resonances of the cantilever to determine the frequency shifts Δf1 and Δf2 relative to the corresponding resonances in free space in order to compute the elastic modulus.
The linear relation between Δf1 and established in [7] can be determined from the measurement of Δf1 and Δf2 as functions of the Z-displacement of the piezoelectric scanner. These curves were measured on four polymers, i.e., LLDPE, PP, PS, PTFE, and a Teflon-like fluorinated SAM.
Analysis of these curves evidenced the existence of plastic and elastic deformation phases. The two subsequent phases are explained by the evolution of stress σ during indentation by the cantilever tip. Plastic deformation begins when sufficient stress is applied to the surface. The slow increase in tip cross section A compared to the applied normal force FN results in an increase in σ and leads to the plastic deformation of the sample surface. The geometrical shape of the tip, assumed to be pyramidal, results in a fast increase in tip cross section compared to the increase in applied normal force FN when the tip goes deeper into the surface. This, in turn, decreases the stress. When the σ is below a threshold value, the surface begins to deform elastically.
The elastic phase is characterized by a linear relation between the frequency shifts Δf1 and Δf2 and the Z-displacement of the piezoelectric scanner. A linear relation between the measured normal force FN,meas and the displacement in both phases can also be observed if the spring constant of the cantilever is small compared to the normal sample stiffness. This linearity does not reflect the variation in normal sample stiffness but the excessive compliance of the cantilever. The use of cantilevers with higher spring constants is actually limited for technical reasons. Indeed, for cantilevers with a too high spring constant, the second flexural contact resonance is out of the bandpass of the vertical deflection channel in most of the microscope heads.
The analysis of the curve showed that setpoints for the applied normal force in the highest region of the plastic deformation phase could be used without causing a large deviation in the elasticity compared with the results we would obtain in the elastic deformation phase. The elasticity of LLDPE, PP, PS, and the SAM was then investigated. As polymers are viscoelastic materials, the elastic modulus Eeff,meas measured in dynamic mode corresponds to the storage modulus. We assumed a frequency independence of the measured storage modulus as the measured contact resonances are quite large compared to the inverse of typical material relaxation times. The measurements showed values for the storage modulus in the range of the Young’s moduli for bulk materials for LLDPE, PP and PS.
The method can also distinguish variations in the elasticity of surface such as LLDPE, PP and the FDTS SAM. The investigated SAM consists of a monolayer of FDTS patterned with circular 1.2 nm deep holes. Due to the functionalization of the cantilever tip by SAM molecules, a similar stiffness is measured inside and outside the holes. Finally, the measured value of the storage modulus of 478.5 ± 0.5 MPa, which is close to the value for bulk PTFE (500 MPa), can be used as an estimation of the Young’s modulus of the FDTS monolayer.
Supporting Information
| Supporting Information File 1: Supporting information features the analytical expression for normal sample stiffness based on Hurley and Turner’s equations. | ||
| Format: PDF | Size: 306.4 KB | Download |
Acknowledgements
We would like to thank the Swiss National Science Foundation (SNF), the Swiss Nanoscience Institute (SNI) and the Commission for Technology and Innovation (CTI) for financing the project. Thomas Schimmel and Stefan Walheim acknowledge the financial support by the Baden-Württemberg Stiftung within the Research Network ”Functional Nanostructures”. We especially thank Alexis Baratoff for rewarding discussions about contact AFM and polymer elasticity.
References
-
Kasas, S.; Dietler, G. Probing the Nanomechanical Properties of Viruses, Cells and Cellular Structures. In Life at the Nanoscale: Atomic Force Microscopy of Live Cells; Dufrêne, Y., Ed.; Pan Stanford Publishing: Singapore, 2011; pp 335–352.
Return to citation in text: [1] -
Dai, S.; Zhao, J.; He, M.-r.; Wang, X.; Wan, J.; Shan, Z.; Zhu, J. Nano Lett. 2015, 15, 8–15. doi:10.1021/nl501986d
Return to citation in text: [1] -
Lu, H.; Meng, X. Sci. Rep. 2015, 5, 16939. doi:10.1038/srep16939
Return to citation in text: [1] -
Binnig, G.; Quate, C. F.; Gerber, C. Phys. Rev. Lett. 1986, 56, 930–934. doi:10.1103/PhysRevLett.56.930
Return to citation in text: [1] -
Tamayo, J.; Garcia, R. Appl. Phys. Lett. 1997, 71, 2394–2396. doi:10.1063/1.120039
Return to citation in text: [1] -
Hurley, D. C.; Turner, J. A. J. Appl. Phys. 2007, 102, 033509. doi:10.1063/1.2767387
Return to citation in text: [1] [2] [3] [4] -
Herruzo, E. T.; Perrino, A. P.; Garcia, R. Nat. Commun. 2014, 5, 3126. doi:10.1038/ncomms4126
Return to citation in text: [1] [2] [3] [4] [5] [6] [7] [8] [9] -
Bubendorf, A. Elasticity of Polymers Investigated by Atomic Force Microscopy. Ph.D. Thesis, University of Basel, Basel, Switzerland, 2015.
Return to citation in text: [1] [2] -
Rabe, U. Atomic Force Acoustic Microscopy. In Applied Scanning Probe Methods II; Bhushan, B.; Fuchs, H., Eds.; Springer: Berlin, Germany, 2006; pp 37–90.
Return to citation in text: [1] -
Rabe, U.; Turner, J.; Arnold, W. Appl. Phys. A 1998, 66, S277–S282. doi:10.1007/s003390051145
Return to citation in text: [1] -
Rabe, U.; Arnold, W. Appl. Phys. Lett. 1994, 64, 1493–1495. doi:10.1063/1.111869
Return to citation in text: [1] -
Rabe, U.; Amelio, S.; Kester, E.; Scherer, V.; Hirsekorn, S.; Arnold, W. Quantitative determination of contact stiffness using atomic force acoustic microscopy. Ultrasonics; Elsevier: Amsterdam, Netherlands, 2000; Vol. 48, pp 430–437.
Return to citation in text: [1] -
Rabe, U.; Amelio, S.; Kopycinska, M.; Hirsekorn, S.; Kempf, M.; Göken, M.; Arnold, W. Surf. Interface Anal. 2002, 33, 65–70. doi:10.1002/sia.1163
Return to citation in text: [1] -
Rabe, U.; Kopycinska-Müller, M.; Hirsekorn, S. Atomic Force Acoustic Microscopy. In Acoustic Scanning Probe Microscopy; Marinello, F.; Passeri, D.; Savio, E., Eds.; Springer: Berlin, Germany, 2012; pp 123–153.
Return to citation in text: [1] -
Lozano, J. R.; Garcia, R. Phys. Rev. Lett. 2008, 100, 076102. doi:10.1103/PhysRevLett.100.076102
Return to citation in text: [1] -
Garcia, R.; Herruzo, E. T. Nat. Nanotechnol. 2012, 7, 217–226. doi:10.1038/nnano.2012.38
Return to citation in text: [1] -
Rodriguez, T. R.; Garcia, R. Appl. Phys. Lett. 2004, 84, 449–451. doi:10.1063/1.1642273
Return to citation in text: [1] -
Proksch, R. Appl. Phys. Lett. 2006, 89, 113121. doi:10.1063/1.2345593
Return to citation in text: [1] -
Martínez, N. F.; Lozano, J. R.; Herruzo, E. T.; Garcia, F.; Richter, C.; Sulzbach, T.; Garcia, R. Appl. Phys. Lett. 2008, 19, 384011. doi:10.1088/0957-4484/19/38/384011
Return to citation in text: [1] -
Herruzo, E. T.; Garcia, R. Beilstein J. Nanotechnol. 2012, 3, 198–206. doi:10.3762/bjnano.3.22
Return to citation in text: [1] -
Garcia, R.; Proksch, R. Eur. Polym. J. 2013, 49, 1897–1906. doi:10.1016/j.eurpolymj.2013.03.037
Return to citation in text: [1] -
Meyer, E.; Gyalog, T.; Overney, R. M.; Dransfeld, K., Eds. Friction and Rheology on the Nanometer Scale; World Scientific Publishing Co Pte Ltd: Singapore, 1998.
Return to citation in text: [1] [2] -
Rast, S.; Wattinger, C.; Gysin, U.; Meyer, E. Rev. Sci. Instrum. 2000, 71, 2772–2775. doi:10.1063/1.1150690
Return to citation in text: [1] -
Huang, C.; Moosmann, M.; Jin, J.; Heiler, T.; Walheim, S.; Schimmel, T. Beilstein J. Nanotechnol. 2012, 3, 620–628. doi:10.3762/bjnano.3.71
Return to citation in text: [1] -
Lechner, M. D. Polymers. In Springer Handbook of Condensed Matter and Materials Data; Martienssen, W.; Warlimont, H., Eds.; Springer: Berlin, Germany, 2005; pp 477–552.
Return to citation in text: [1] -
Materials Data Book. Cambridge University Engineering Department: Cambridge, United Kingdom, 2003; http://www-mdp.eng.cam.ac.uk/web/library/enginfo/cueddatabooks/materials.pdf (accessed June 21, 2017).
Return to citation in text: [1] [2] -
Howald, L.; Lüthi, R.; Meyer, E.; Güntherodt, H.-J. J. Phys.: Condens. Matter 1994, 93, 267–268. doi:10.1007/BF01312696
Return to citation in text: [1]
| 26. | Materials Data Book. Cambridge University Engineering Department: Cambridge, United Kingdom, 2003; http://www-mdp.eng.cam.ac.uk/web/library/enginfo/cueddatabooks/materials.pdf (accessed June 21, 2017). |
| 27. | Howald, L.; Lüthi, R.; Meyer, E.; Güntherodt, H.-J. J. Phys.: Condens. Matter 1994, 93, 267–268. doi:10.1007/BF01312696 |
| 7. | Herruzo, E. T.; Perrino, A. P.; Garcia, R. Nat. Commun. 2014, 5, 3126. doi:10.1038/ncomms4126 |
| 1. | Kasas, S.; Dietler, G. Probing the Nanomechanical Properties of Viruses, Cells and Cellular Structures. In Life at the Nanoscale: Atomic Force Microscopy of Live Cells; Dufrêne, Y., Ed.; Pan Stanford Publishing: Singapore, 2011; pp 335–352. |
| 6. | Hurley, D. C.; Turner, J. A. J. Appl. Phys. 2007, 102, 033509. doi:10.1063/1.2767387 |
| 7. | Herruzo, E. T.; Perrino, A. P.; Garcia, R. Nat. Commun. 2014, 5, 3126. doi:10.1038/ncomms4126 |
| 6. | Hurley, D. C.; Turner, J. A. J. Appl. Phys. 2007, 102, 033509. doi:10.1063/1.2767387 |
| 5. | Tamayo, J.; Garcia, R. Appl. Phys. Lett. 1997, 71, 2394–2396. doi:10.1063/1.120039 |
| 7. | Herruzo, E. T.; Perrino, A. P.; Garcia, R. Nat. Commun. 2014, 5, 3126. doi:10.1038/ncomms4126 |
| 4. | Binnig, G.; Quate, C. F.; Gerber, C. Phys. Rev. Lett. 1986, 56, 930–934. doi:10.1103/PhysRevLett.56.930 |
| 15. | Lozano, J. R.; Garcia, R. Phys. Rev. Lett. 2008, 100, 076102. doi:10.1103/PhysRevLett.100.076102 |
| 16. | Garcia, R.; Herruzo, E. T. Nat. Nanotechnol. 2012, 7, 217–226. doi:10.1038/nnano.2012.38 |
| 2. | Dai, S.; Zhao, J.; He, M.-r.; Wang, X.; Wan, J.; Shan, Z.; Zhu, J. Nano Lett. 2015, 15, 8–15. doi:10.1021/nl501986d |
| 3. | Lu, H.; Meng, X. Sci. Rep. 2015, 5, 16939. doi:10.1038/srep16939 |
| 17. | Rodriguez, T. R.; Garcia, R. Appl. Phys. Lett. 2004, 84, 449–451. doi:10.1063/1.1642273 |
| 18. | Proksch, R. Appl. Phys. Lett. 2006, 89, 113121. doi:10.1063/1.2345593 |
| 19. | Martínez, N. F.; Lozano, J. R.; Herruzo, E. T.; Garcia, F.; Richter, C.; Sulzbach, T.; Garcia, R. Appl. Phys. Lett. 2008, 19, 384011. doi:10.1088/0957-4484/19/38/384011 |
| 20. | Herruzo, E. T.; Garcia, R. Beilstein J. Nanotechnol. 2012, 3, 198–206. doi:10.3762/bjnano.3.22 |
| 21. | Garcia, R.; Proksch, R. Eur. Polym. J. 2013, 49, 1897–1906. doi:10.1016/j.eurpolymj.2013.03.037 |
| 8. | Bubendorf, A. Elasticity of Polymers Investigated by Atomic Force Microscopy. Ph.D. Thesis, University of Basel, Basel, Switzerland, 2015. |
| 10. | Rabe, U.; Turner, J.; Arnold, W. Appl. Phys. A 1998, 66, S277–S282. doi:10.1007/s003390051145 |
| 6. | Hurley, D. C.; Turner, J. A. J. Appl. Phys. 2007, 102, 033509. doi:10.1063/1.2767387 |
| 11. | Rabe, U.; Arnold, W. Appl. Phys. Lett. 1994, 64, 1493–1495. doi:10.1063/1.111869 |
| 12. | Rabe, U.; Amelio, S.; Kester, E.; Scherer, V.; Hirsekorn, S.; Arnold, W. Quantitative determination of contact stiffness using atomic force acoustic microscopy. Ultrasonics; Elsevier: Amsterdam, Netherlands, 2000; Vol. 48, pp 430–437. |
| 13. | Rabe, U.; Amelio, S.; Kopycinska, M.; Hirsekorn, S.; Kempf, M.; Göken, M.; Arnold, W. Surf. Interface Anal. 2002, 33, 65–70. doi:10.1002/sia.1163 |
| 14. | Rabe, U.; Kopycinska-Müller, M.; Hirsekorn, S. Atomic Force Acoustic Microscopy. In Acoustic Scanning Probe Microscopy; Marinello, F.; Passeri, D.; Savio, E., Eds.; Springer: Berlin, Germany, 2012; pp 123–153. |
| 7. | Herruzo, E. T.; Perrino, A. P.; Garcia, R. Nat. Commun. 2014, 5, 3126. doi:10.1038/ncomms4126 |
| 7. | Herruzo, E. T.; Perrino, A. P.; Garcia, R. Nat. Commun. 2014, 5, 3126. doi:10.1038/ncomms4126 |
| 6. | Hurley, D. C.; Turner, J. A. J. Appl. Phys. 2007, 102, 033509. doi:10.1063/1.2767387 |
| 9. | Rabe, U. Atomic Force Acoustic Microscopy. In Applied Scanning Probe Methods II; Bhushan, B.; Fuchs, H., Eds.; Springer: Berlin, Germany, 2006; pp 37–90. |
| 7. | Herruzo, E. T.; Perrino, A. P.; Garcia, R. Nat. Commun. 2014, 5, 3126. doi:10.1038/ncomms4126 |
| 8. | Bubendorf, A. Elasticity of Polymers Investigated by Atomic Force Microscopy. Ph.D. Thesis, University of Basel, Basel, Switzerland, 2015. |
| 7. | Herruzo, E. T.; Perrino, A. P.; Garcia, R. Nat. Commun. 2014, 5, 3126. doi:10.1038/ncomms4126 |
| 25. | Lechner, M. D. Polymers. In Springer Handbook of Condensed Matter and Materials Data; Martienssen, W.; Warlimont, H., Eds.; Springer: Berlin, Germany, 2005; pp 477–552. |
| 26. | Materials Data Book. Cambridge University Engineering Department: Cambridge, United Kingdom, 2003; http://www-mdp.eng.cam.ac.uk/web/library/enginfo/cueddatabooks/materials.pdf (accessed June 21, 2017). |
| 22. | Meyer, E.; Gyalog, T.; Overney, R. M.; Dransfeld, K., Eds. Friction and Rheology on the Nanometer Scale; World Scientific Publishing Co Pte Ltd: Singapore, 1998. |
| 24. | Huang, C.; Moosmann, M.; Jin, J.; Heiler, T.; Walheim, S.; Schimmel, T. Beilstein J. Nanotechnol. 2012, 3, 620–628. doi:10.3762/bjnano.3.71 |
| 22. | Meyer, E.; Gyalog, T.; Overney, R. M.; Dransfeld, K., Eds. Friction and Rheology on the Nanometer Scale; World Scientific Publishing Co Pte Ltd: Singapore, 1998. |
| 23. | Rast, S.; Wattinger, C.; Gysin, U.; Meyer, E. Rev. Sci. Instrum. 2000, 71, 2772–2775. doi:10.1063/1.1150690 |
| 7. | Herruzo, E. T.; Perrino, A. P.; Garcia, R. Nat. Commun. 2014, 5, 3126. doi:10.1038/ncomms4126 |
| 7. | Herruzo, E. T.; Perrino, A. P.; Garcia, R. Nat. Commun. 2014, 5, 3126. doi:10.1038/ncomms4126 |
© 2018 Bubendorf et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Nanotechnology terms and conditions: (http://www.beilstein-journals.org/bjnano)