Search results
Search for "enthalpy" in Full Text gives 85 result(s) in Beilstein Journal of Nanotechnology.
Stereodiscrimination of guests in chiral organosilica aerogels studied by ESR spectroscopy
Beilstein J. Nanotechnol. 2025, 16, 2034–2054, doi:10.3762/bjnano.16.140

- temperature until almost all spin probes are mobile at room temperature (Figure 4b). The interaction enthalpy of the spin probe with the pore walls of the aerogel is relatively low, and, together with the relatively large size of the pores, a higher temperature easily induces detachment from the surfaces. If
Beyond the shell: exploring polymer–lipid interfaces in core–shell nanofibers to carry hyaluronic acid and β-caryophyllene
Beilstein J. Nanotechnol. 2025, 16, 2015–2033, doi:10.3762/bjnano.16.139

- the nanofibers. Key parameters evaluated included: glass transition temperature (Tg), crystallization temperature upon cooling (Tcc), melting temperature (Tm), melting enthalpy (ΔHm), and crystallization enthalpy (ΔHcc). Furthermore, the degree of crystallinity (Xc%) of PLA within the nanofibers was
- following equation: where ΔHm0 is the melting enthalpy of fully crystalline PLA (93.1 J/g), and XPLA is the mass fraction of PLA in the fiber (Vidal et al., 2022) [68]. Fourier transform infrared spectroscopy FTIR analysis was conducted to examine the surface composition of the nanofibers and confirm core
Dendrimer-modified carbon nanotubes for the removal and recovery of heavy metal ions from water
Beilstein J. Nanotechnol. 2025, 16, 1522–1532, doi:10.3762/bjnano.16.107

- Information File 1, Table S4) showed relatively low enthalpy changes (ΔH < 80 kJ/mol), which are more consistent with physisorption rather than chemisorption (typically 80–400 kJ/mol). Thus, the adsorption of Pb2+ and Cd2+ onto CNTs-G5 is better interpreted as being dominated by physisorption, potentially
- ). Furthermore, thermodynamic parameters such as changes of enthalpy (ΔH), entropy (ΔS), and Gibbs free energy (ΔG) were calculated by plotting the graph of ln Ke vs 1/T and using the van't Hoff equation (Supporting Information File 1, Figure S2 and Table S4). Positive ΔH and negative ΔG values confirm that
Multifunctional properties of bio-poly(butylene succinate) reinforced with multiwalled carbon nanotubes
Beilstein J. Nanotechnol. 2025, 16, 1014–1024, doi:10.3762/bjnano.16.76

- ) allow for the determination of melting temperature (Tm), glass transition temperature (Tg), crystallization temperature (Tc), and enthalpy (ΔH) of the studied materials (Table 2). During the first heating cycle, neat PBS exhibited a cold crystallization peak at 95.1 °C and a melting peak at 115.2 °C
- enthalpy of 110.3 J·g−1 for 100% crystalline PBS [31]. SEM images of PBS (a), CNTs (b), PBS/CNT_10 (c), and PBS/CNT_0.5 (d). MFR of pure PBS and PBS/CNT_0.5 nanocomposite as a function of temperature and applied load. TGA of PBS (green curves), CNTs (black curves), and PBS/CNT_10 masterbatch (red curves
Retrieval of B1 phase from high-pressure B2 phase for CdO nanoparticles by electronic excitations in CdxZn1−xO composite thin films
Beilstein J. Nanotechnol. 2025, 16, 551–560, doi:10.3762/bjnano.16.43

- method to render this phase transition reversible, allowing for the subsequent recovery of the B1 phase at atmospheric pressure. From a thermodynamic perspective, the enthalpy difference (ΔH) in the phase transition process can be subdivided into two contributions: the pressure (ΔPV) and the change in
TiO2 immobilized on 2D mordenite: effect of hydrolysis conditions on structural, textural, and optical characteristics of the nanocomposites
Beilstein J. Nanotechnol. 2025, 16, 128–140, doi:10.3762/bjnano.16.12

- calorimetric hydrolysis study of Ti(OR)4 at different concentrations, the values of reaction enthalpy of the first hydrolysis stage were measured for R = Et, iPr, and n-Bu [29]. It turned out that further hydrolysis proceeds much slower and with very little heat release (for R = Et its value is zero within the
Heterogeneous reactions in a HFCVD reactor: simulation using a 2D model
Beilstein J. Nanotechnol. 2024, 15, 1627–1638, doi:10.3762/bjnano.15.128

- ) + H° (g) was developed to obtain the extensive properties of this reaction using FactSage in the temperature range from 500 to 1500 °C. Enthalpy (H), Gibbs energy (G), entropy (S), heat capacity (Cp), and Helmholtz energy (A) were obtained to calculate Keq through Equation 5 [27]. The thermodynamic
Effect of radiation-induced vacancy saturation on the first-order phase transformation in nanoparticles: insights from a model
Beilstein J. Nanotechnol. 2024, 15, 1453–1472, doi:10.3762/bjnano.15.117

- enthalpy and entropy of vacancy formation in Fe. First-order phase transformations are accompanied by nucleation and the overcoming of energy barriers. To our knowledge, the consideration of nucleation energy barriers and the alteration of surface energies during transformation under irradiation has been
- , respectively) and can be expressed as follows [17]: where, ΔHf is the enthalpy change for forming of a vacancy, ΔSf is the entropy change for vacancy formation, and ΔHmix is the ideal entropy of vacancy mixing, which may be given as: Here, T is the absolute temperature, and kB is the Boltzmann constant
- transformations from bcc to fcc and from fcc to bcc that occur in an iron-like nanomaterial. We detail the findings for pure iron at the end of the paper. The enthalpy change for vacancy formation can be estimated from the equilibrium melting temperature, Tm, and is ΔHfα = 3.76·10−19 J for the α phase and ΔHfβ
Facile synthesis of Fe-based metal–organic frameworks from Fe2O3 nanoparticles and their application for CO2/N2 separation
Beilstein J. Nanotechnol. 2024, 15, 897–908, doi:10.3762/bjnano.15.74

- . These findings have been compared with the CO2 uptake capacities of MIL-100(Fe)-based adsorbents created through different methods, as well as other MOFs and conventional materials, all of which are detailed in Table S2 (Supporting Information File 1). Isosteric enthalpy of CO2 adsorption over M-100Fe
- determine the isosteric enthalpy of CO2 adsorption (Supporting Information File 1). As anticipated, the capture of CO2 molecules using M-100Fe@Fe2O3#1.80 releases approximately 20 to 17 kJ·mol−1 when the CO2 uptake is increased up to 1.0 mmol·g−1 (Figure 10). The obtained results fall within the value range
- selectivities over M-100Fe@Fe2O3 samples for a mixture with 10% N2 and 90% CO2 at 298 K. Enthalpy of CO2 adsorption over M-100Fe@Fe2O3#1.80 sample. CO2 adsorption/desorption isotherms over M-100Fe@Fe2O3#1.80 sample for five cycles at 298 K. Textural properties of Fe2O3 and the as-prepared samples. Supporting
A review on the structural characterization of nanomaterials for nano-QSAR models
Beilstein J. Nanotechnol. 2024, 15, 854–866, doi:10.3762/bjnano.15.71

- reference parameters by empirical formulas [31]. Additionally, QM calculations can be performed in very simplified models that only describe a part of the material, such as single metal atom, to calculate the enthalpy of formation of the cation [26]. However, there is an alternative, simplified way of
Synthesis of silver–palladium Janus nanoparticles using co-sputtering of independent sources: experimental and theorical study
Beilstein J. Nanotechnol. 2024, 15, 808–816, doi:10.3762/bjnano.15.67

- offered by the same two metals in the bulk, such as Au and Ni [4]. Alloying immiscible elements is feasible in the nanoscale regime because the enthalpy of the mixture decreases as the size of the nanoparticles decreases, and it generally becomes negative below a certain particle size [5]. Silver
Laser synthesis of nanoparticles in organic solvents – products, reactions, and perspectives
Beilstein J. Nanotechnol. 2024, 15, 638–663, doi:10.3762/bjnano.15.54

- -heptane (Figure 5). While the overall gas formation was ruled by the solvent’s molar enthalpy of vaporization, the correlation of hydrogen formation with the physical properties of the solvents was not possible. Instead, the hydrogen formation depends on the liquid’s pyrolysis behavior. As such, 1-hexene
Experimental investigation of usage of POE lubricants with Al2O3, graphene or CNT nanoparticles in a refrigeration compressor
Beilstein J. Nanotechnol. 2023, 14, 1041–1058, doi:10.3762/bjnano.14.86

- and condensation temperatures. To validate the study, the actual refrigeration cycle with R1234yf is presented as in previous studies [36]. The pressure (P) – specific enthalpy (h) diagram obtained from the validation tests was given in Figure 11. Accordingly, it was seen that the actual P–h diagram
Fragmentation of metal(II) bis(acetylacetonate) complexes induced by slow electrons
Beilstein J. Nanotechnol. 2023, 14, 980–987, doi:10.3762/bjnano.14.81

- constant. Taking into account the sublimation enthalpy, ΔHsub, for MnL2 and CoL2 (i.e., 139.3 and 130.1 kJ/mol, respectively [24]), and the working temperature (i.e., 390 and 420 K, respectively), we estimate the value of PCoL2/PMnL2 to be about 38, assuming C to be constant. This ratio of pressures, also
- of the precursor anion leads to a substantial increase in the M···O bond length. Thus, stabilization of the [ML2]− anion prevents the dissociation of the TNI and, hence, the production of [L]− near 0 eV. Furthermore, the formation enthalpy of this fragment has been calculated to be 2.01, 1.72, 0.95
Antibody-conjugated nanoparticles for target-specific drug delivery of chemotherapeutics
Beilstein J. Nanotechnol. 2023, 14, 912–926, doi:10.3762/bjnano.14.75

- binding is a highly selective interaction because both enthalpy and entropy are involved in the binding thermodynamics [73]. In case of multivalent particles, the entropy loss on binding is less than that of the two molecules in free solution. The multivalent NPs are very specific for the corresponding
Two-dimensional molecular networks at the solid/liquid interface and the role of alkyl chains in their building blocks
Beilstein J. Nanotechnol. 2023, 14, 872–892, doi:10.3762/bjnano.14.72

- were partially desorbed from the surface of HOPG. Desorption from the surface is unfavorable regarding enthalpy. However, the detached alkyl chain is mobile in the solution phase; thus, desorption from the surface is favorable concerning entropy. Therefore, the peculiar 2D structural change can be
Nanostructured lipid carriers containing benznidazole: physicochemical, biopharmaceutical and cellular in vitro studies
Beilstein J. Nanotechnol. 2023, 14, 804–818, doi:10.3762/bjnano.14.66

- thermograms are shown in Figure 2, whereas the melting temperature (Tm), the enthalpy of fusion (ΔHf), and crystallinity index (CI) are presented in Table 1. Whereas BNZ showed an endothermic peak at its melting point (191.2 °C) [23], the formulation showed two endothermic peaks in the range of 40–50 °C
- the nanoparticle thermogram, indicating that BNZ was dispersed within the lipid matrix [24]. Correlating with the lower enthalpy of fusion, the CI (%) value of the nanoparticles was lower than that of the bulk myristyl myristate. Lipid molecules could be less ordered in the nanoparticles than in the
- to identify significant differences against the control group. *p < 0.05, **p < 0.01. Thermal properties of benznidazole (BNZ), myristyl myristate, poloxamer 188, and nanoparticles (NLC-VEHICLE and NLC-BNZ). Abbreviations: Tm, melting temperature; ΔHf, fusion enthalpy; CI (%), crystallinity index
Conjugated photothermal materials and structure design for solar steam generation
Beilstein J. Nanotechnol. 2023, 14, 454–466, doi:10.3762/bjnano.14.36

- the initial temperature of water, c is the specific heat of water (4.2 J·g−1·K−1), Q is the sensible heat of water, HLV is the enthalpy of water vaporization, and Ein is the energy of the incident light. Conjugated organic photothermal materials Conjugated organic small molecules Organic molecular
Plasmonic nanotechnology for photothermal applications – an evaluation
Beilstein J. Nanotechnol. 2023, 14, 380–419, doi:10.3762/bjnano.14.33

- water vaporisation can be calculated from Δm is the water mass loss during irradiation, is the phase change enthalpy of water to vapour, M is the molar mass of water, I is the solar power intensity at the surface of the sample, S is the irradiated area of the water surface, and τ is the irradiation
Sputtering onto liquids: a critical review
Beilstein J. Nanotechnol. 2022, 13, 10–53, doi:10.3762/bjnano.13.2
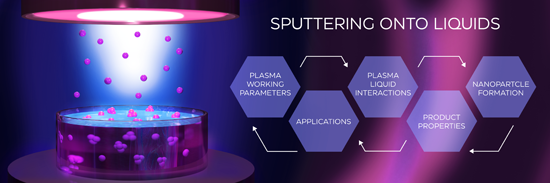
- while the distance between the target and the substrate is of the order of several centimeters. Ultimately, these atoms may be thermalized if the number of collisions is high and the atoms land on the surface with a kinetic energy of the order of the gas temperature (ca. 1/40 eV). Condensation enthalpy
Criteria ruling particle agglomeration
Beilstein J. Nanotechnol. 2021, 12, 1093–1100, doi:10.3762/bjnano.12.81

- direction of a minimum of the free enthalpy. In this context, one may observe mechanisms leading to a reduction of the surface energy or controlled by the van der Waals interaction. Additionally, the ensemble may arrange in the direction of a maximum of the entropy. Simulations based on Monte Carlo methods
- of an agglomerated ensemble, that is, an exponential function characterized by two parameters. In this context, it is important to realize that one has to take care of fluctuations of the entropy. Keywords: agglomeration; enthalpy; entropy; simulation; surface energy; van der Waals interaction
- . Synthesis of larger particles may be carried out in the gas phase, where the probability of collision is controlled by the geometry of the particles or forces among the particles [1]. Alternatively, in case of existing particles, the agglomeration is controlled by the minimum of free enthalpy. In this case
Prediction of Co and Ru nanocluster morphology on 2D MoS2 from interaction energies
Beilstein J. Nanotechnol. 2021, 12, 704–724, doi:10.3762/bjnano.12.56

- , this indicates a strong interaction with the MoS2 ML, while tetrahedral geometries have an energy gain originating from Co–Co interactions instead. If we compare with Cu, the Co–S bond enthalpy is much higher than the Cu–S bond enthalpy, at 331 kJ/mol and 274.5 kJ/mol respectively (we did not find data
Surface-enhanced Raman scattering of water in aqueous dispersions of silver nanoparticles
Beilstein J. Nanotechnol. 2021, 12, 497–506, doi:10.3762/bjnano.12.40

- -exponential factor and R is the universal gas constant. This data treatment is analogous to the procedures proposed by Walrafen et al. [46] for the calculation of the enthalpy ΔH° referring to the formation of O–H∙∙∙O units. The results presented here are consistent with the results proposed by Walrafen et al
ZnO and MXenes as electrode materials for supercapacitor devices
Beilstein J. Nanotechnol. 2021, 12, 49–57, doi:10.3762/bjnano.12.4

- abundant raw materials. Also, it is less harmful to the environment [19]. Miao et al. fabricated 3D porous MXene-rGO films by using a self-propagating approach, which involves great enthalpy change, chain reactions, and drastically propagates onto the entire film in seconds. As a result, a specific
Magnetohydrodynamic stagnation point on a Casson nanofluid flow over a radially stretching sheet
Beilstein J. Nanotechnol. 2020, 11, 1303–1315, doi:10.3762/bjnano.11.114

- enthalpy. The kinetic energy of the fluid particles increases for higher values of Ec. Hence, the temperature of the fluid rises marginally and therefore, the associated momentum and thermal boundary layer thickness are enhanced. Figure 14 and Figure 15 elucidate the effect of the radiation parameter, R















































































































































































































































































































