Abstract
An efficient solid-phase-supported peptide synthesis (SPPS) of morpholinoglycine oligonucleotide (MorGly) mimics has been developed. The proposed strategy includes a novel specially designed labile linker group containing the oxalyl residue and the 2-aminomethylmorpholino nucleoside analogues as first subunits.
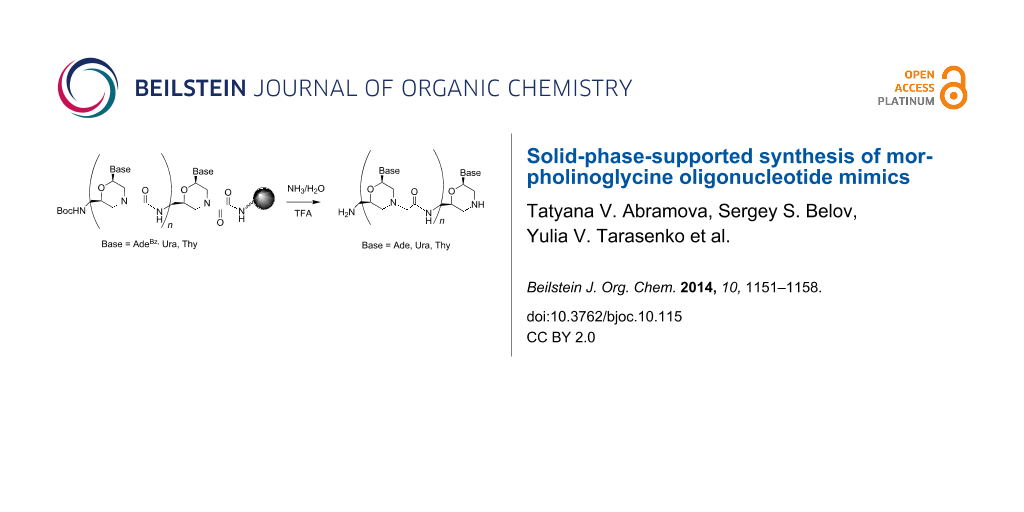
Graphical Abstract
Introduction
The phosphorodiamidate morpholino oligomers (PMO) and peptide conjugated PMO (PPMO) are currently promising candidates for antisense therapy of a number of infectious and hereditary diseases [1-4] despite of some difficulties and limitations. They also proved to be valuable tools to study fundamental problems of gene expression in the course of embryogenesis [5,6]. A few examples of morpholino oligomers containing other types of internucleoside bonds were described. An attractive feature of these new morpholino oligonucleotide analogues is the absence of additional chiral centers. Unlike commercially available PMO and PPMO, which are mixtures of diastereomers, oligomers constructed with the use of phosphoromonoamidate [7], oxalyl diamide [8], amidine [9] and methylene caxboxamide [10] groups represent the individual compounds. It means that their physicochemical properties, such as thermostability of complementary complexes, do not depend on the diastereomeric composition of the mixtures, which can vary in different preparations. The difference in the spatial structure, in the extent of base stacking, and in the stability of complementary duplexes for individual diastereomers has been demonstrated for phosphotriester [11] and methylphosphonate [12] oligonucleotide analogues and PMO [13].
The degree of protonation at physiological pH and the presence of positively charged centers in oligonucleotide analogues results in increased water solubility and higher thermal stability of complementary duplexes of such oligonucleotide mimics with nucleic acids [14,15]. Some conformationally restricted protonated PNA or pyrrolidine oligonucleotide mimics exhibit selective binding with RNA but not with DNA [16,17]. It has been shown that conjugation of PMO with cationic peptides or other cationic residues facilitates penetration of PMO into cells and efficiently prevents the growth of E. coli in vitro and in vivo [18].
Taking the above considerations into account, methylenecarboxamide (glycine) oligomers (MorGly) (Figure 1A), being protonated at physiological pH, seem to be promising candidates for the development of novel antisense oligonucleotide mimics.
Figure 1: Morpholinoglycine oligomers (A) and protected monomers 1a–e (B) for their synthesis.
Figure 1: Morpholinoglycine oligomers (A) and protected monomers 1a–e (B) for their synthesis.
A convenient synthetic procedure was previously described for protected monomers 1a–d (Figure 1B) necessary for the synthesis of the MorGly oligonucleotide mimics [19]. When studying the tandem complementary complexes of MorGly homohexa- and pentamers, we found out that the adenine-containing MorGly oligomers formed more stable complexes with poly(U) than native oligodeoxyriboadenylates of the same length. Moreover, it was revealed that the MorGly oligomers preferably bind with RNA than with DNA [20]. At the same time, the stability of complementary complexes of modified oligomers was shown to depend on the residues dangling the 4-end of the MorGly oligomers. In this study, the type of the residue dangling the 4-end of the oligomer chain (Figure 2) depended on the utilized synthetic method. We found that the MorGly oligomers containing the residues of type A formed less stable complementary complexes than oligomers containing residues of type B.
Figure 2: Dangling residues of MorGly oligomers synthesized by solid phase supported (A) and liquid phase synthesis (B); the blunt end of MorGly oligomer (C).
Figure 2: Dangling residues of MorGly oligomers synthesized by solid phase supported (A) and liquid phase syn...
Another interesting fact demonstrated in the article [20] was the dependence of the thermal stability of complementary complexes formed by the MorGly oligomers on the heterocyclic base composition (uracil or adenine) of the modified chain. It was shown while studying their tandem complexes that the impact of cooperative interactions at oligomer junctions on the thermal stability was higher for modified oligomers than for native oligodeoxyriboadenylates. This result may indicate that the contribution of nucleobase stacking to the thermal stability of complementary complexes is more important for the oligonucleotide mimics than for native oligonucleotides. The substitution of the uracil nucleobases in the MorGly oligomers by thymines with better stacking properties may solve this problem. The synthesis of the thymine containing morpholino monomer 1e was necessary to prove this hypothesis.
The promising properties of the MorGly oligomers as potential antisense agents motivated us to develop the SPPS method for the synthesis of these oligonucleotide mimics. We focused our efforts on the development of a new linker group to produce oligomers without dangling residues (Figure 2C), the synthesis of the corresponding Thy-containing monomers and the improvement of the SPPS protocol for the MorGly oligomers.
Results and Discussion
For the synthesis of the thymine-containing monomer 1e we applied the procedure published for obtaining 1d [19] using 5-methyluridine as parent compound. Synthetic schemes, procedures and physicochemical data for intermediates in the synthesis of monomer 1e are given in Supporting Information File 1.
We have previously shown that the morpholino oxalyldiamide oligomers were cleaved by aqueous ammonia treatment, which resulted in the formation of shortened oligomers with the blunt end of type C (Figure 2) [8]. So, the oxalyl residue can be used as a labile linker between the solid support and the growing oligomer chain (Figure 3) during SPPS in combination with the tert-butyloxycarbonyl (Boc)- and acyl-protected morpholino nucleosides.
Figure 3: Cleavage of MorGly oligonucleotide mimics from solid support and deprotection of nucleobases by aqueous ammonia treatment: i) NH3/H2O.
Figure 3: Cleavage of MorGly oligonucleotide mimics from solid support and deprotection of nucleobases by aqu...
After completion of the synthesis, the cleavage of the oligomer from the solid support and the deprotection of nucleobases can be performed simultaneously by treatment with aqueous ammonia as in the solid phase synthesis of native oligonucleotides (ODN) [21]. The oxalyl-mediated attachment of the growing chain to the solid support is usual in the synthesis of base sensitive ODN derivatives [22].
Figure 3 indicates the necessity of using monomers 2 (Scheme 1) as first subunits bound to the support through the oxalyl linker. We synthesized Boc-protected aminomethylmorpholino nucleosides 2a,d,e as shown in Scheme 1 starting from aminomethylmorpholino nucleosides 3a,d,e. The synthesis of adenine and uracil containing compounds 3a,d was published earlier [8]. Thymine containing morpholino nucleoside 3e was obtained similarly to 3d starting from the 5-methyluridine. See Supporting Information File 1 for the synthetic schemes, procedures and physicochemical data for intermediates in the synthesis of monomer 3e.
Scheme 1: Synthesis of Boc-protected 2-aminomethylmorpholino nucleosides: i) di-tert-butyl dicarbonate ((Boc)2O), triethylamine (TEA), pyridine (Py); ii) AcOH/H2O.
Scheme 1: Synthesis of Boc-protected 2-aminomethylmorpholino nucleosides: i) di-tert-butyl dicarbonate ((Boc)2...
Our next goal was to attach morpholino monomers 2a,d,e to the Boc-Gly-PAM resin through the oxalyl linker. We tried different ways to do this including to change the order in assembling the elements of the whole construction, the direct attachment of the monomer unit to the support without additional 6-aminohexanoic acid and the application of more active bis(2-cyanoethyl)oxalate instead of dimethyl oxalate. However, only the route shown in Scheme 2 was successful and gave the loading of the polymer with the monomers 5a,d,e close to that of the parent resin. It is interesting that the attachment of the oxalyl-containing residue to the morpholine nitrogen in monomers 5a,d,e resulted in the appearance of duplicate signals of the nucleobases and morpholine protons in the NMR spectra (see Experimental) similar to morpholinooxalyl nucleosides [8].
Scheme 2: Loading of the Boc-Gly-PAM resin with the morpholino nucleosides 5a,d,e: i) Dimethyl oxalate, TEA, MeOH; ii) 4, TEA, Py; iii) trifluoroacetic acid (TFA)/CH2Cl2/PhSH; iv) coupling with 5a,d,e. See Table 1 for detailed protocol.
Scheme 2: Loading of the Boc-Gly-PAM resin with the morpholino nucleosides 5a,d,e: i) Dimethyl oxalate, TEA, ...
After completion of the loading, the unreacted amino groups were capped by diethyl pyrocarbonate (DEPC) as described earlier [23]. The extent of the loading and the rate of the cleavage of loaded monomers were determined after the aqueous ammonia/iPrOH treatment of aliquots of the support. The capacity of loaded supports 6a,d,e was achieved as much as 0.61–0.65 mmol/g after 4 h of the coupling reaction between monomers 5a,d,e and the Boc-Gly-PAM polymer resin. It was found that the cleavage of the monomers from the solid support in the course of the ammonia treatment was completed within two days at room temperature (see Supporting Information File 1). The structure of the monomers obtained after the ammonia treatment (Figure 3, n = 0, Base = Ade, Ura, Thy) was confirmed by thin-layer chromatography (TLC), reversed phase chromatography (RPC), and mass spectrometry (see Supporting Information File 1).
The cleavable linker containing the S–S bond has been previously used in the synthesis of PMO in the 2→4 direction [23]. This corresponds to the 5’→3’ direction of native oligonucleotides. In our case, the MorGly oligomers were synthesized in the opposite direction (4→2) [20] similar to the standard direction (3’→5’) of native ODN and peptide nucleic acid (PNA) synthesis. Probably, the same S–S-containing linker could be used in the synthesis of MorGly oligomers, but it should be examined. In our work we propose another approach, which has fewer steps and does not require the additional thiol treatment to cleave the oligomer from the support.
In our previous work [20], the reaction yields of the monomer attachment to the growing chain have dropped to 50% after the two first couplings although the addition of the first and the second monomer units provided a good yield (95%). The change of the solvents in SPPS of MorGly oligomers (see Table 1) similarly to the synthesis protocol for PMO [23] allowed us to achieve high yields in the coupling reaction. The synthesis of MorGly pentamers was performed manually in the mini spin receiver columns (0.7 mL) according to the improved protocol of the SPPS of MorGly oligomers (Table 1). The receiver column was shaken to mix the support and reactants. Swelling and capping steps were omitted beginning the second cycle. After each cycle, a small sample of the support was subjected to aqueous ammonia treatment to analyse resulting oligomers. The homogeneity and the structure of the products formed after each cycle were confirmed by TLC, RPC and mass spectrometry before and after removal of the Boc protective group (see Supporting Information File 1 for details).
Table 1: SPPS protocol for MorGLy oligomers using Boc-Gly-PAM resin (10–30 mg).
| Entry | Step | Volume | Reagents and solvents | Time |
|---|---|---|---|---|
| 1 | swelling | 0.7 mL | 1,3-dimethyl-2-imidazolidinone (DMI) | 1 h |
| 2 | washing | 2 × 0.5 mL | dichloromethane (DCM) | 2 × 3 min |
| 3 | deprotection | 2 × 0.5 mL | 0.4 M PhSH in DCM/TFA, 1:4 | 2 × 10 min |
| 4 | washing | 5 × 0.5 mL | DCM, DMI, DCM, DMI, DCM | 5 × 3 min |
| 5 | neutralization | 2 × 0.5 mL | 1-methyl-2-pyrrolidinone (NMP)/N,N-diisopropylethylamine (DIPEA), 9:1 | 2 × 1 min |
| 6 | activation | 0.04–0.12 mL | 0.2 M 5a,d,e at the first cycle, 1a,d,e at subsequent cycles; 0.18 M O-(benzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium tetrafluoroborate (TBTU); 0.18 M DIPEA; in DMI | 5–8 min |
| 7 | coupling | 0.04 mL of the solution of activated monomer per 10 mg of the resin | solution of activated monomer obtained at step 6 | 4 h |
| 8 | washing | 4 × 0.5 mL | DMI, DCM, DMI, DCM | 4 × 3 min |
| 9 | capping | 2 × 0.3 mL | DEPC/DCM, 1:9 | 2 × 5 min |
| 10 | washing | 2 × 0.5 mL | DCM | 2 × 3 min |
It is interesting to note that the mobility of the homooligomers in TLC decreased with increasing of their length (Table S1, Supporting Information File 1). This fact helped us to estimate the comparative length and purity of homooligomers. The yield in each cycle was 96–98%.
After completion of the pentamer synthesis and cleavage of the oligomer from the support, the Boc protective group was removed by trifluoroacetic acid (TFA). Target homopentamers 7a,d,e (Figure 4) were purified by cation exchange chromatography and RPC.
Figure 4: MorGly homopentamers containing adenine, uracil, and thymine nucleobases.
Figure 4: MorGly homopentamers containing adenine, uracil, and thymine nucleobases.
Conclusion
We developed a novel type of the labile linker for the synthesis of MorGly oligomers and improved the SPPS protocol for MorGly oligomers providing high yields at each cycle. Pentamers 7a,d,e obtained by the proposed strategy will be used in the further study of the structure and the thermal stability of complementary complexes of the MorGly oligomers with native DNA and RNA to elucidate the role of the hydrogen bonds and base stacking in their formation and stability. The use of highly sophisticated constructs containing short modified oligonucleotides is nowadays a promising strategy in the antisense therapy [24]. Recently, short cationic morpholinoguanidinium oligomers were shown to penetrate living cells and to facilitate the inhibition of Gli1 [25].
The unique properties of MorGly oligomers, such as the protonated backbone at physiological pH, stability to nucleases, and more preferable complex formation with RNA than with DNA, make them an attractive alternative in comparison to native oligonucleotides and other derivatives or analogues.
Experimental
General
We used 5-methyluridine, O-(benzotriazol-1-yl)-N,N,N’,N’-tetramethyluronium tetrafluoroborate, 1-methyl-2-pyrrolidinone, 1,3-dimethyl-2-imidazolidinone (Sigma-Aldrich, USA); Boc-Gly-PAM resin (substitution 0.76 mmol/g, NovaBiochem, Germany); sodium azide, glycine (Serva, Germany); sodium periodate, bromotrichloromethane, di-tert-butyl dicarbonate (Acros Organics, USA). Receiver columns (REF 740522, Macherey–Nagel, Germany) were used for SPPS of the MorGly oligomers in manual mode. All other reagents and solvents were from Sigma–Aldrich (USA) and Reachem (Russia). NMR spectra were recorded on a Bruker AV400 spectrometer (Bruker, Germany) in appropriate deuterated solvents at 30 °C. Chemical shifts (δ) are reported in ppm relative to TMS signals. Coupling constants J are reported in Hertz. MALDI–TOF mass spectra were registered on an Autoflex III mass spectrometer (Bruker Daltonics, Germany) using 2,5-dihydroxybenzoic acid as a matrix (MALDI–TOF) in positive or negative mode in The Center of Cooperative Use (“Proteomics”, Russian Academy of Sciences). IR spectra were recorded on a Vector 22 spectrometer (Bruker Optics, Germany) in KBr.
Quantitative analytical RPC were performed on a Milichrom A02 chromatograph system (Econova, Russia) on a ProntoSIL 125 C18 column (2 × 75 mm) in a gradient of buffer B (0.1 M TEA–AcOH, pH 7.0, 80% acetonitrile) in buffer A (0.1 M TEA–AcOH, pH 7.0, water) (0–100 % over 15 min) with an elution rate of 0.2 mL/min and UV detection at 250, 260, 280, and 300 nm unless otherwise noted. TLC was carried out on Kieselgel 60 F254 plates (Merck, Germany) in the proper solvent systems (see below) and visualized by UV irradiation, ninhydrin (amine groups) or cystein/aqueous sulfuric acid (nucleoside and trityl groups). Preparative silica gel column chromatography, RPC, and cation exchange chromatography were performed using silica gel (35–70 μm, Acros Organics, USA), Porasil C 18 (55–105 μm, 125 A) (Waters, USA), and, Servacel P-23 (Serva, Germany) or SP Sepharose fast flow (GE Healthcare, USA), respectively. The compositions of all liquid mixtures are indicated as v/v percent. All evaporations were performed under reduced pressure. Compounds 1a,d and 3a,d were synthesized according to the published procedures [8,19].
Synthesis of thymine containing monomers 1e and 3e
{2-[N-(tert-Butyloxycarbonyl)aminomethyl]-6-(thymin-1-yl)morpholin-4-yl}acetic acid (1e): Thymine containing monomer 1e was synthesized similarly to uracil analogue 1d [19] starting from 5-methyluridine (1.29 g, 5 mmol). Yield 1.0 g (2.50 mmol, 50%). Rf 0.68 (iPrOH/H2O, 4:1); 1H NMR (DMSO-d6) 7.53 (s, 1H, H6-Thy), 6.95 (t, J = 5.6 Hz, 1H, Boc-NH), 5.58 (dd, J = 10.1, 1.8 Hz, 1H, H6), 3.74–3.64 (m, 1H, H2), 3.11–2.74 (m, 6H, H3, H5, Boc-HNCH2, CH2C(O)OH), 2.30 (app. t, J = 10.3 Hz, 1H, H3), 2.01 (app. t, J = 10.6 Hz, 1H, H5), 1.77 (s, 3H, СН3-Thy), 1.37 (s, 9H, CH3-Boc); MALDI–TOFMS (m/z): [M + H]+ calcd for C17H27N4O7, 399.19; found, 399.77; [M + Na]+ calcd for C17H26N4NaO7, 421.17; found, 421.64. See Supporting Information File 1 for the synthetic scheme and physicochemical data of intermediate compounds.
2-Aminomethyl-4-trityl-6-(thymin-1-yl)morpholine (3e): Thymine containing monomer 3e was synthesized similarly to uracil analogue 3d [8] starting from the corresponding 2-hydromethyl derivative (2.16 g, 4.24 mmol). Yield 1.05 g, (2.20 mmol, 52%). Rf 0.12 (EtOH/DCM, 1:9); 1H NMR (CDCl3) 7.50–7.38 (br.s, 6H, o-H-Tr), 7.28 (app.t, J = 7.5 Hz, 6H, m-H-Tr), 7.17 (t, J = 7.2 Hz, 3H, p-H-Tr), 6.98 (q, J = 0.8 Hz, 1H, H6-Thy), 6.15–6.07 (dd, J = 9.8 Hz, 1H, 2.2, H6), 4.14–4.04 (m, 1H, H2), 3.31 (dt, J = 11.2, 2.3 Hz, 1H, H3), 3.05 (dt, J = 11.8, 2.1 Hz, 1H, H5), 2.75–2.64 (m, 2H, NH2CH2), 1.81 (d, J = 0.8 Hz, 3H, CH3), 1.39 (dd, J = 11.1, 10.0 Hz, 1H, H3), 1.35–1.30 (dd, J = 11.6, 10.6 Hz, 1H, H5); MALDI–TOFMS (m/z): [M – Tr + 2H]+ (the Tr protective group was removed during the sample preparation) calcd for C10H17N4O3 241.13; found, 241.45. Physicochemical characteristics for intermediate compounds see in Supporting Information File 1.
General procedure for the synthesis of Boc-protected 2-aminomethylmorpholino monomers 5a,d,e
2-[N-(tert-Butyloxycarbonyl)aminomethyl]-6-(N6-benzoyladenin-9-yl)morpholine (2a), 2-[N-(tert-butyloxycarbonyl)aminomethyl]-6-(uracil-1-yl)morpholine (2d), 2-[N-(tert-butyloxycarbonyl)aminomethyl]-6-(thymin-1-yl)morpholine (2e): The morpholino nucleoside 3a,d,e (0.72 mmol), TEA (0.35 mL, 2.1 mmol), and (Boc)2O (0.19 g, 0.87 mmol) in pyridine (1.5 mL) were stirred for 1 h. The reaction mixture was diluted with DCM (30 mL) and washed with 5% aqueous NaHCO3 (2 × 25 mL). The organic layer was evaporated with toluene several times to remove traces of pyridine. The residue was dissolved in 80% aqueous acetic acid (AcOH) (5 mL). After 30 min of stirring, the mixture was poured over ice (50 g) and carefully neutralized by adding the calculated amount of dry NaHCO3 (5.6 g) under vigorous stirring. The aqueous suspension was then washed with diethyl ether (50 mL). The aqueous layer was concentrated to 10 mL; the target product was purified by RPC in the gradient of EtOH in water (0–60%). The appropriate fractions were evaporated to give after drying the title compounds (0.36 mmol, yield 50%).
Compound 2a Rf 0.14 (EtOH/DCM, 1:9), 0.52 (iPrOH/H2O, 4:1); 1H NMR (CDCl3) 9.01 (s, 1H, Bz-NH), 8.78 (s, 1H, H8-Ade), 8.17 (s, 1H, H2-Ade), 8.01 (dt, J = 7.6, 1.2 Hz, 2H, o-H-Bz), 7.60 (tt, J = 7.5, 1.2 Hz, 1H, p-H-Bz), 7.50 (app. t, J = 7.5 Hz, 2H, m-H-Bz), 5.91 (dd, J = 10.1, 2.7 Hz, 1H, H6), 4.85 (t, J = 4.6 Hz, 1H, Boc-HNCH2), 3.9–3.86 (m, 1H, H2), 3.47–3.37 (m, 1H, Boc-HNCH2), 3.31 (dd, J = 12.2, 2.4 Hz, 1H, H3), 3.20–3.11 (m, 1H, Boc-HNCH2), 3.08 (dd, J = 10.5, 12.1 Hz, 1H, H5), 3.00 (dd, J = 12.6, 1.9 Hz, 1H, H3), 2.70 (dd, J = 12.6, 11.0 Hz, 1H, H5), 1.41 (s, 9H, CH3); MALDI–TOFMS (m/z): [M + H]+ calcd for C22H28N7O4, 454.22; found, 454.62; [M + Na]+ calcd for C22H27N7NaO4, 476.20; found, 476.68.
Compound 2d: Rf 0.12 (EtOH/DCM, 1:9), 0.53 (iPrOH/H2O, 4:1); 1H NMR (CD3OD) 7.76 (d, J = 8.0 Hz, 1H, H6-Ura), 5.69 (d, J = 8.0 Hz, 1H, H5-Ura), 5.67 (dd, J = 10.2, 2.3 Hz, 1H, H6), 3.86–3.77 (m, 1Н, H2), 3.20 (br.d, J = 5.7 Hz, 2H, Boc-NHCH2), 3.04 (dd, J = 12.6, 2.3, 1H, H3), 2.88 (dd, J = 13.0, 2.0 Hz, 1H, H5), 2.64 (dd, J = 12.6, 10.4 Hz, 1H, H3), 2.51 (dd, J = 13.0, 11.0 Hz, 1H, H5), 1.43 (s, 9H, CH3); MALDI–TOFMS (m/z): [M + H]+ calcd for C14H23N4O5, 327.17; found, 327.02; [M + Na]+ calcd for C14H22N4NaO5, 349.15; found, 349.02.
Compound 2e: Rf 0.19 (EtOH/DCM, 1:9), 0.77 (iPrOH/H2O, 4:1); 1H NMR (CDCl3) 7.21 (q, J = 1.1 Hz, 1H, H6-Thy), 5.67 (dd, J = 10.0, 2.6 Hz, 1H, H6), 4.84 (t, J = 5.2 Hz, 1H, Boc-NHCH2), 3.82–3.74 (m, 1H, H2), 3.44–3.35 (m, 1H, Boc-NHCH2), 3.13–3.02 (m, 2Н, Boc-NHCH2, H3), 2.91 (dd, J = 12.8, 1.7 Hz, 1H, H5), 2.61 (dd, J = 10.2, 12.2 Hz, 1H, H3), 2.52 (dd, J = 12.8, 11.3 Hz, 1H, H5), 1.92 (d, J = 1.1 Hz, 3H, CH3-Thy), 1.42 (s, 9H, CH3-Boc); MALDI–TOFMS (m/z): [M + H]+ calcd 341.18 for C15H25N4O5, found, 340.94; [M + Na]+ calcd for C15H24N4NaO5, 363.16; found, 362.96.
6-[N-(2-methoxy-2-oxoacetyl)]aminohexanoic acid (4): A mixture of dimethyl oxalate (1.20 g, 10 mmol), 6-aminohexanoic acid (1.31 g, 10 mmol) and TEA (10 mmol, 1.40 mL) in dry MeOH (20 mL) was stirred at room temperature for 24 h. The reaction mixture was evaporated; the residue was triturated with diethyl ether (25 mL) and cooled in an ice bath. Diethyl ether was decanted, and the residue was dried in vacuum to give the title compound 4 as a partial TEA salt (2.62 g, 8.2 mmol, colourless semisolid at room temperature). 1H NMR (DMSO-d6) 8.92 (t, J = 5.8 Hz, 1H, NH), 3.76 (s, 3H, OCH3), 3.10 (app. q, J = 6.7 Hz, 2H, NHCH2CH2), 2.65 (q, J = 7.2 Hz, 2H, NCH2CH3), 2.17 (t, J = 7.4 Hz, 2H, CH2CH2C(O)OH), 1.54–1.38 (m, 4H, NHCH2CH2CH2, CH2CH2CH2C(O)OH), 1.30–1.18 (m, 2H, CH2CH2CH2CH2CH2), 1.01 (t, J = 7.2 Hz, 3H, NCH2CH3); 13C NMR (DMSO-d6) 175.0, 161.6, 157.1, 53.1, 45.8, 39.1, 34.2, 28.6, 26.2, 24.6, 10.6.
9-{2-[N-(tert-Butyloxycarbonyl)aminomethyl]-6-(N6-benzoyladenin-9-yl)morpholin-4-yl}-8,9-dioxo-7-azanonanoic acid (5a), 9-{2-[N-(tert-butyloxycarbonyl)aminomethyl]-6-(uracil-1-yl)morpholin-4-yl}-8,9-dioxo-7-azanonanoic acid (5d), 9-{2-[N-(tert-butyloxycarbonyl)aminomethyl]-6-(thymin-1-yl)morpholin-4-yl}-8,9-dioxo-7-azanonanoic acid (5e): Boc-protected morpholino nucleosides 3a,d,e (0.35 mmol), the acid 4 (0.76 g, 3.5 mmol), and TEA (0.42 mL, 3 mmol) in pyridine (3 mL) were heated at 50 °C for 48 h. The reaction mixture was then cooled and diluted with DCM (30 mL). The solution was washed with water (30 mL). The aqueous layer was extracted with DCM (2 × 30 mL). The organic layers were combined and evaporated several times with water to remove pyridine. The target product was purified by RPC in the gradient of MeCN in water (0–50%). The appropriate fractions were evaporated to give after drying the title compounds as a light cream powder. Yield 0.28 mmol, 80%.
Compound 5a: Rf 0.43 (EtOH/DCM, 1:9), 0.73 (iPrOH/H2O, 4:1); 1H NMR (CD3OD) 8.76, 8.75 (2s, 0.5H each, H8-Ade), 8.67, 8.66 (2s, 0.5H each, H2-Ade), 8.10 (br.dt, J = 8.4, 1.2, 2H, o-H-Bz), 7.68 (br.tt, J = 7.5, 1.2 Hz, 1H, p-H-Bz), 7.59 (br.tt, J = 7.8, 1.3 Hz, 2H, m-H-Bz), 6.09, 6.02 (2dd, J = 10.5, 2.8 Hz, 0.5H each, H6), 4.82–4.78 (br.d, J = 11.8 Hz, 0.5H, H3), 4.66–4.58 (m, 2.5H, H5, Boc-NHCH2), 4.52 (dt, J = 13.2, 2.2 Hz, 0.5H, H3), 4.21 (br.d, J = 13.2 Hz, 0.5H, H5), 4.05–3.93 (m, 1H, H2), 3.94 (dd, J = 12.6, 10.6 Hz, 0.5H, H3), 3.58 (dd, J = 12.2, 10.6 Hz, 0.5H, H5), 3.28–3.23 (m, 2H, NHCH2CH2-), 2.89 (dd, J = 13.5, 11.4 Hz, 0.5H, H3), 2.32 (t, J = 7.2 Hz, 1H, CH2CH2C(O)OH), 2.32–2.28 (m, 0.5H, H5), 2.23 (t, J = 7.2 Hz, 1H, CH2CH2C(O)OH), 1.72–1.55 (m, 4H, NHCH2CH2, CH2CH2C(O)OH), 1.44, 1.43 (2s, 4.5H each, CH3), 1.43–1.36 (m, 2H, CH2CH2CH2C(O)OH); MALDI–TOFMS (m/z): [M + H]+ calcd for C30H39N8O8, 639.29; found, 639.23; [M + Na]+ calcd for C30H38N8NaO8, 661.27; found, 661.22; [M + K]+ calcd for C30H38KN8O8, 677.24; found, 676.19.
Compound 5d: Rf 0.45 (EtOH/DCM, 1:9), 0.79 (iPrOH/H2O, 4:1); 1H NMR (CD3OD) 7.83, 7.80 (2d, J = 8.2 Hz, 0.5H each, H6-Ura), 5.76, 5.74 (2d, J = 8.2 Hz, 0.5H each, H5-Ura), 5.74, 5.71 (2dd, J = 10.2, 2.9 Hz, 0.5 each, H6), 4.75–4.59 (m, 2H, Boc-NHCH2), 4.56 (br.d, J = 12.9 Hz, 0.5H, H3), 4.44 (dt, J = 13.1, 2.0 Hz, 0.5H, H5), 4.26 (br.d, J = 12.9 Hz, 0.5H, H3), 4.10 (dt, J = 13.1, 1.8 Hz, 0.5H, H5), 3.91–3.81 (m, 1H, H2), 3.33–3.28 (m, 2.5H, H3, NHCH2CH2), 3.14 (dd, J = 13.4, 11.5 Hz, 0.5H, H5), 2.94 (dd, J = 13.2, 10.4 Hz, 0.5H, H3), 2.76 (dd, J = 13.3, 11.4 Hz, 0.5H, H5), 2.33, 2.31 (2t, J = 7.5 Hz, 2H, CH2CH2C(O)OH), 1.72–1.56 (m, 4H, NHCH2CH2, CH2CH2C(O)OH), 1.47, 1.46 (2s, 4.5H each, CH3), 1.47–1.37 (m, 2H, CH2CH2CH2C(O)OH); MALDI–TOFMS (m/z): [M + H]+ calcd for C22H34N5O9, 512.24; found, 512.07; [M + Na]+ calcd for C22H33N5NaO9, 534.22; found, 534.20; [M + K]+ calcd for C22H33KN5O9, 550.19; found, 550.16.
Compound 5e: Rf 0.68 (EtOH/DCM, 1:9), 0.83 (iPrOH/H2O, 4:1); 1H NMR (CD3OD) 7.67, 7.64 (2q, J = 1.1 Hz, 0.5 each, H6-Thy), 5.74, 5.70 (2dd, J = 10.0, 2.7 Hz, 0.5 each, H6), 4.75–4.58 (m, 2H, Boc-NHCH2), 4.51 (br.d, J = 12.9 Hz, 0.5H, H3), 4.43 (dt, J = 13.6, 2.0 Hz, 0.5H, H5), 4.21 (br.d, J = 12.8 Hz, 0.5H, H3), 4.09 (dt, J = 13.1, 2.2 Hz, 0.5H, H5), 3.89–3.80 (m, 1H, H2), 3.32–3.26 (m, 2.5H, H3, NHCH2CH2), 3.15 (dd, J = 13.6, 11.4 Hz, 0.5H, H5), 2.99 (dd, J = 13.1, 10.5 Hz 0.5H, H3), 2.76 (dd, J = 13.1, 11.4 Hz, 0.5H, H5), 2.34, 2.32 (2t, J = 7.5 Hz, 2H, CH2CH2C(O)OH), 1.93, 1.92 (2d, J = 1.1 Hz, 1.5H each, CH3-Thy), 1.71–1.56 (m, 4H, NHCH2CH2, CH2CH2C(O)OH), 1.47, 1.46 (2s, 4.5H each, CH3-Boc), 1.47–1.37 (m, 2H, CH2CH2CH2C(O)OH); MALDI–TOFMS (m/z): [M + Na]+ calcd for C23H35N5NaO9, 548.23; found, 548.27; [M + K]+ calcd for C23H35KN5O9, 564.21; found, 564.26
Loading of the support with the first monomer
Boc-Gly-PAM resin (10–30 mg) was placed in the receiver column equipped with a cap at the outlet. Loading of the resin was performed according the protocol (Table 1) using the monomers 5a,d,e for the coupling step. The column was shaken to mix resin and reactants. Changing the reactants and solvents was carried out by centrifugation of the column equipped with a collection tube at 2000 min−1. After drying, a sample of the resin (1–2 mg) was treated with a mixture of iPrOH and concentrated (25%) aqueous ammonia (0.1 mL, 1:1) for 48 h at room temperature under shaking. The substitution of the supports 6a,d,e was determined after calculating the amount of the cleaved monomer according the data of quantitative analytical HPLC using the extinction coefficients ε260 15.02 mM−1cm−1 for A, 9.66 for U and 8.56 for T [26] and was found to be 0.60–0.65 mmol/g. Physicochemical characteristics of the cleaved monomers are given in Supporting Information File 1.
Synthesis of MorGly homopentamers 7a,d,e
Pentamers 7a,d,e were synthesized manually in the receiver columns (see above) on the supports 6a,d,e (10 mg), respectively, by repeating steps 2–8 of the protocol (Table 1). Monomers 1a,d,e were used for the coupling step. Physicochemical characteristics of oligomers cleaved from the support after certain cycles are given in Supporting Information File 1. After completion of the synthesis, the oligomers were cleaved from the support by concentrated (25%) aqueous ammonia/iPrOH (1:1, 1 mL) treatment for 48 h at room temperature under shaking. The supernatants were evaporated. The Boc-protected oligomers were treated with TFA (0.5 mL) for 5 min and evaporated. Crude deprotected oligomers were dissolved in water containing 0.1% TFA (1 mL) and subjected to cation exchange chromatography (SP Sepharose) in a gradient of NaCl (0–2M) in aqueous 20% ethanol containing 0.1% TFA. The appropriate fractions were evaporated, and the target pentamers were purified by RPC in the gradient of MeCN in water (0–40%) containing 0.1% TFA. The appropriate fractions were concentrated using vacuum centrifugation. After purification, 0.5–1.0 μmol of homopentamers 7a,d,e were obtained. RPC HPLC traces of purified pentamers 7a,d,e are provided in Supporting Information File 1.
Pentamer 7a: MALDI–TOFMS: (m/z): [M + H]+ calcd for C58H76N36O9, 1406.66; found, 1406.43.
Pentamer 7d: MALDI–TOFMS (m/z): [M + H]+ calcd for C53H71N20O19, 1291.52; found, 1291.65.
Pentamer 7e: MALDI–TOFMS (m/z): [M + H]+ calcd for C58H81N20O19, 1361.60; found, 1361.71.
Supporting Information
Synthetic schemes, procedures and physicochemical data for intermediates in the synthesis of compounds 1e and 3e; kinetics of the cleavage of monomers from supports 6a,d; TLC, RPC HPLC traces and mass spectra data for oligomers cleaved from the samples of supports 6a,d,e and for purified pentamers 7a,d,e are provided in the Supporting Information.
| Supporting Information File 1: Syntheses and characteristics for selected compounds. | ||
| Format: PDF | Size: 677.1 KB | Download |
Acknowledgements
The work was supported by the President program “Leading Scientific Schools” (project no. 1205.2014.4); the grant of the Russian Foundation for Basic Research (project no. 12-04-01454-a and 14-04-01018-a); and the Interdisciplinary Integration project no. 60 of the Presidium of the Siberian Branch of the Russian Academy of Sciences. We also acknowledge support from the Ministry of Education and Science of the Russian Federation, agreement no. 14.В25.31.0028.
References
-
Panchal, R. G.; Geller, B. L.; Mellbye, B.; Lane, D.; Iversen, P. L.; Bavari, S. Nucleic Acid Ther. 2012, 22, 316–322.
Return to citation in text: [1] -
Leger, A. J.; Mosquea, L. M.; Clayton, N. P.; Wu, I. H.; Weeden, T.; Nelson, C. A.; Phillips, L.; Roberts, E.; Piepenhagen, P. A.; Cheng, S. H.; Wentworth, B. M. Nucleic Acid Ther. 2013, 23, 109–117.
Return to citation in text: [1] -
Watts, J. K.; Corey, D. R. J. Pathol. 2012, 226, 365–379. doi:10.1002/path.2993
Return to citation in text: [1] -
Deleavey, G. F.; Damha, M. J. Chem. Biol. 2012, 19, 937–954. doi:10.1016/j.chembiol.2012.07.011
Return to citation in text: [1] -
Bill, B. R.; Petzold, A. M.; Clark, K. J.; Schimmenti, L. A.; Ekker, S. C. Zebrafish 2009, 6, 69–77. doi:10.1089/zeb.2008.0555
Return to citation in text: [1] -
Anderson, J. L.; Carten, J. D.; Farber, S. A. In Zebrafish: Cellular and Developmental Biology, 3rd ed.; Detrich, H. W.; Westerfield, M.; Zon, L. I., Eds.; Methods in Cell Biology, Vol. 101; Elsevier Academic Press Inc.: San Diego, CA, USA, 2011; pp 111–141.
Return to citation in text: [1] -
Zhang, N.; Tan, C.; Cai, P.; Jiang, Y.; Zhang, P.; Zhao, Y. Tetrahedron Lett. 2008, 49, 3570–3573. doi:10.1016/j.tetlet.2008.04.035
Return to citation in text: [1] -
Abramova, T. V.; Kassakin, M. F.; Lomzov, A. A.; Pyshnyi, D. V.; Silnikov, V. N. Bioorg. Chem. 2007, 35, 258–275. doi:10.1016/j.bioorg.2006.12.003
Return to citation in text: [1] [2] [3] [4] [5] [6] -
Pérez-Rentero, S.; Alguacil, J.; Robles, J. Tetrahedron 2009, 65, 1171–1179. doi:10.1016/j.tet.2008.11.069
Return to citation in text: [1] -
Chakhmakhcheva, O.; Andrianov, M.; Buryakova, A.; Choob, M.; Efimov, V. Nucleosides Nucleotides 1999, 18, 1427–1428. doi:10.1080/07328319908044742
Return to citation in text: [1] -
Abramova, T. V.; Vorobev, Y. N.; Lebedev, A. V. Bioorg. Khim. 1986, 12, 1335–1347.
Return to citation in text: [1] -
Thiviyanathan, V.; Vyazovkina, K. V.; Gozansky, E. K.; Bichenchova, E.; Abramova, T. V.; Luxon, B. A.; Lebedev, A. V.; Gorenstein, D. G. Biochemistry 2002, 41, 827–838. doi:10.1021/bi011551k
Return to citation in text: [1] -
Kang, H.; Chou, P.-J.; Johnson, W. C., Jr.; Weller, D.; Huang, S.-B.; Summerton, J. E. Biopolymers 1992, 32, 1351–1363. doi:10.1002/bip.360321009
Return to citation in text: [1] -
Debart, F.; Abes, S.; Deglane, G.; Moulton, H. M.; Clair, P.; Gait, M. J.; Vasser, J.-J.; Lebleu, B. Curr. Top. Med. Chem. 2007, 7, 727–737. doi:10.2174/156802607780487704
Return to citation in text: [1] -
Yeh, J. I.; Shivachev, B.; Rapireddy, S.; Crawford, M. J.; Gil, R. R.; Du, S.; Madrid, M.; Ly, D. H. J. Am. Chem. Soc. 2010, 132, 10717–10727. doi:10.1021/ja907225d
Return to citation in text: [1] -
Kumar, V. A.; Ganesh, K. N. Curr. Top. Med. Chem. 2007, 7, 715–726. doi:10.2174/156802607780487722
Return to citation in text: [1] -
Worthington, R. J.; Micklefield, J. Chem.–Eur. J. 2011, 17, 14429–14441. doi:10.1002/chem.201101950
Return to citation in text: [1] -
Mellbye, B. L.; Weller, D. D.; Hassinger, J. N.; Reeves, M. D.; Lovejoy, C. E.; Iversen, P. L.; Geller, B. L. J. Antimicrob. Chemother. 2010, 65, 98–106. doi:10.1093/jac/dkp392
Return to citation in text: [1] -
Kasakin, M. F.; Abramova, T. V.; Silnikov, V. N. Russ. J. Bioorg. Chem. 2011, 37, 752–757. doi:10.1134/S1068162011060070
Return to citation in text: [1] [2] [3] [4] -
Abramova, T. V.; Kassakin, M. F.; Tarasenko, Yu. V.; Lomzov, A. A.; Koval, V. V.; Pyshnyi, D. V.; Silnikov, V. N. Russ. J. Bioorg. Chem. 2012, 38, 400–411. doi:10.1134/S1068162012040024
Return to citation in text: [1] [2] [3] [4] -
Abramova, T. Molecules 2013, 18, 1063–1075. doi:10.3390/molecules18011063
Return to citation in text: [1] -
Alul, R. H.; Singman, C. N.; Zhang, G.; Letsinger, R. L. Nucleic Acids Res. 1991, 19, 1527–1532. doi:10.1093/nar/19.7.1527
Return to citation in text: [1] -
Weller, D. D.; Hassinger, J. N.; Cai, B. Z. Oligonucleotide analogs having cationic intersubunit linkages. U.S. Patent 7,943,762 B2, May 17, 2011.
Return to citation in text: [1] [2] [3] -
Mallikaratchy, P.; Gardner, J.; Nordstrøm, L. U. R.; Veomett, N. J.; McDevitt, M. R.; Heaney, M. L.; Scheinberg, D. A. Nucleic Acid Ther. 2013, 23, 289–299.
Return to citation in text: [1] -
Pattanayak, S.; Khatra, H.; Saha, S.; Sinha, S. RSC Adv. 2014, 4, 1951–1954. doi:10.1039/c3ra45257c
Return to citation in text: [1] -
Cavaluzzi, M. J.; Borer, P. N. Nucleic Acids Res. 2004, 32, e13. doi:10.1093/nar/gnh015
Return to citation in text: [1]
| 25. | Pattanayak, S.; Khatra, H.; Saha, S.; Sinha, S. RSC Adv. 2014, 4, 1951–1954. doi:10.1039/c3ra45257c |
| 8. | Abramova, T. V.; Kassakin, M. F.; Lomzov, A. A.; Pyshnyi, D. V.; Silnikov, V. N. Bioorg. Chem. 2007, 35, 258–275. doi:10.1016/j.bioorg.2006.12.003 |
| 19. | Kasakin, M. F.; Abramova, T. V.; Silnikov, V. N. Russ. J. Bioorg. Chem. 2011, 37, 752–757. doi:10.1134/S1068162011060070 |
| 19. | Kasakin, M. F.; Abramova, T. V.; Silnikov, V. N. Russ. J. Bioorg. Chem. 2011, 37, 752–757. doi:10.1134/S1068162011060070 |
| 1. | Panchal, R. G.; Geller, B. L.; Mellbye, B.; Lane, D.; Iversen, P. L.; Bavari, S. Nucleic Acid Ther. 2012, 22, 316–322. |
| 2. | Leger, A. J.; Mosquea, L. M.; Clayton, N. P.; Wu, I. H.; Weeden, T.; Nelson, C. A.; Phillips, L.; Roberts, E.; Piepenhagen, P. A.; Cheng, S. H.; Wentworth, B. M. Nucleic Acid Ther. 2013, 23, 109–117. |
| 3. | Watts, J. K.; Corey, D. R. J. Pathol. 2012, 226, 365–379. doi:10.1002/path.2993 |
| 4. | Deleavey, G. F.; Damha, M. J. Chem. Biol. 2012, 19, 937–954. doi:10.1016/j.chembiol.2012.07.011 |
| 9. | Pérez-Rentero, S.; Alguacil, J.; Robles, J. Tetrahedron 2009, 65, 1171–1179. doi:10.1016/j.tet.2008.11.069 |
| 20. | Abramova, T. V.; Kassakin, M. F.; Tarasenko, Yu. V.; Lomzov, A. A.; Koval, V. V.; Pyshnyi, D. V.; Silnikov, V. N. Russ. J. Bioorg. Chem. 2012, 38, 400–411. doi:10.1134/S1068162012040024 |
| 8. | Abramova, T. V.; Kassakin, M. F.; Lomzov, A. A.; Pyshnyi, D. V.; Silnikov, V. N. Bioorg. Chem. 2007, 35, 258–275. doi:10.1016/j.bioorg.2006.12.003 |
| 19. | Kasakin, M. F.; Abramova, T. V.; Silnikov, V. N. Russ. J. Bioorg. Chem. 2011, 37, 752–757. doi:10.1134/S1068162011060070 |
| 7. | Zhang, N.; Tan, C.; Cai, P.; Jiang, Y.; Zhang, P.; Zhao, Y. Tetrahedron Lett. 2008, 49, 3570–3573. doi:10.1016/j.tetlet.2008.04.035 |
| 19. | Kasakin, M. F.; Abramova, T. V.; Silnikov, V. N. Russ. J. Bioorg. Chem. 2011, 37, 752–757. doi:10.1134/S1068162011060070 |
| 5. | Bill, B. R.; Petzold, A. M.; Clark, K. J.; Schimmenti, L. A.; Ekker, S. C. Zebrafish 2009, 6, 69–77. doi:10.1089/zeb.2008.0555 |
| 6. | Anderson, J. L.; Carten, J. D.; Farber, S. A. In Zebrafish: Cellular and Developmental Biology, 3rd ed.; Detrich, H. W.; Westerfield, M.; Zon, L. I., Eds.; Methods in Cell Biology, Vol. 101; Elsevier Academic Press Inc.: San Diego, CA, USA, 2011; pp 111–141. |
| 20. | Abramova, T. V.; Kassakin, M. F.; Tarasenko, Yu. V.; Lomzov, A. A.; Koval, V. V.; Pyshnyi, D. V.; Silnikov, V. N. Russ. J. Bioorg. Chem. 2012, 38, 400–411. doi:10.1134/S1068162012040024 |
| 13. | Kang, H.; Chou, P.-J.; Johnson, W. C., Jr.; Weller, D.; Huang, S.-B.; Summerton, J. E. Biopolymers 1992, 32, 1351–1363. doi:10.1002/bip.360321009 |
| 16. | Kumar, V. A.; Ganesh, K. N. Curr. Top. Med. Chem. 2007, 7, 715–726. doi:10.2174/156802607780487722 |
| 17. | Worthington, R. J.; Micklefield, J. Chem.–Eur. J. 2011, 17, 14429–14441. doi:10.1002/chem.201101950 |
| 12. | Thiviyanathan, V.; Vyazovkina, K. V.; Gozansky, E. K.; Bichenchova, E.; Abramova, T. V.; Luxon, B. A.; Lebedev, A. V.; Gorenstein, D. G. Biochemistry 2002, 41, 827–838. doi:10.1021/bi011551k |
| 18. | Mellbye, B. L.; Weller, D. D.; Hassinger, J. N.; Reeves, M. D.; Lovejoy, C. E.; Iversen, P. L.; Geller, B. L. J. Antimicrob. Chemother. 2010, 65, 98–106. doi:10.1093/jac/dkp392 |
| 11. | Abramova, T. V.; Vorobev, Y. N.; Lebedev, A. V. Bioorg. Khim. 1986, 12, 1335–1347. |
| 8. | Abramova, T. V.; Kassakin, M. F.; Lomzov, A. A.; Pyshnyi, D. V.; Silnikov, V. N. Bioorg. Chem. 2007, 35, 258–275. doi:10.1016/j.bioorg.2006.12.003 |
| 10. | Chakhmakhcheva, O.; Andrianov, M.; Buryakova, A.; Choob, M.; Efimov, V. Nucleosides Nucleotides 1999, 18, 1427–1428. doi:10.1080/07328319908044742 |
| 14. | Debart, F.; Abes, S.; Deglane, G.; Moulton, H. M.; Clair, P.; Gait, M. J.; Vasser, J.-J.; Lebleu, B. Curr. Top. Med. Chem. 2007, 7, 727–737. doi:10.2174/156802607780487704 |
| 15. | Yeh, J. I.; Shivachev, B.; Rapireddy, S.; Crawford, M. J.; Gil, R. R.; Du, S.; Madrid, M.; Ly, D. H. J. Am. Chem. Soc. 2010, 132, 10717–10727. doi:10.1021/ja907225d |
| 26. | Cavaluzzi, M. J.; Borer, P. N. Nucleic Acids Res. 2004, 32, e13. doi:10.1093/nar/gnh015 |
| 22. | Alul, R. H.; Singman, C. N.; Zhang, G.; Letsinger, R. L. Nucleic Acids Res. 1991, 19, 1527–1532. doi:10.1093/nar/19.7.1527 |
| 8. | Abramova, T. V.; Kassakin, M. F.; Lomzov, A. A.; Pyshnyi, D. V.; Silnikov, V. N. Bioorg. Chem. 2007, 35, 258–275. doi:10.1016/j.bioorg.2006.12.003 |
| 23. | Weller, D. D.; Hassinger, J. N.; Cai, B. Z. Oligonucleotide analogs having cationic intersubunit linkages. U.S. Patent 7,943,762 B2, May 17, 2011. |
| 24. | Mallikaratchy, P.; Gardner, J.; Nordstrøm, L. U. R.; Veomett, N. J.; McDevitt, M. R.; Heaney, M. L.; Scheinberg, D. A. Nucleic Acid Ther. 2013, 23, 289–299. |
| 20. | Abramova, T. V.; Kassakin, M. F.; Tarasenko, Yu. V.; Lomzov, A. A.; Koval, V. V.; Pyshnyi, D. V.; Silnikov, V. N. Russ. J. Bioorg. Chem. 2012, 38, 400–411. doi:10.1134/S1068162012040024 |
| 20. | Abramova, T. V.; Kassakin, M. F.; Tarasenko, Yu. V.; Lomzov, A. A.; Koval, V. V.; Pyshnyi, D. V.; Silnikov, V. N. Russ. J. Bioorg. Chem. 2012, 38, 400–411. doi:10.1134/S1068162012040024 |
| 23. | Weller, D. D.; Hassinger, J. N.; Cai, B. Z. Oligonucleotide analogs having cationic intersubunit linkages. U.S. Patent 7,943,762 B2, May 17, 2011. |
| 23. | Weller, D. D.; Hassinger, J. N.; Cai, B. Z. Oligonucleotide analogs having cationic intersubunit linkages. U.S. Patent 7,943,762 B2, May 17, 2011. |
| 8. | Abramova, T. V.; Kassakin, M. F.; Lomzov, A. A.; Pyshnyi, D. V.; Silnikov, V. N. Bioorg. Chem. 2007, 35, 258–275. doi:10.1016/j.bioorg.2006.12.003 |
| 8. | Abramova, T. V.; Kassakin, M. F.; Lomzov, A. A.; Pyshnyi, D. V.; Silnikov, V. N. Bioorg. Chem. 2007, 35, 258–275. doi:10.1016/j.bioorg.2006.12.003 |
© 2014 Abramova et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)