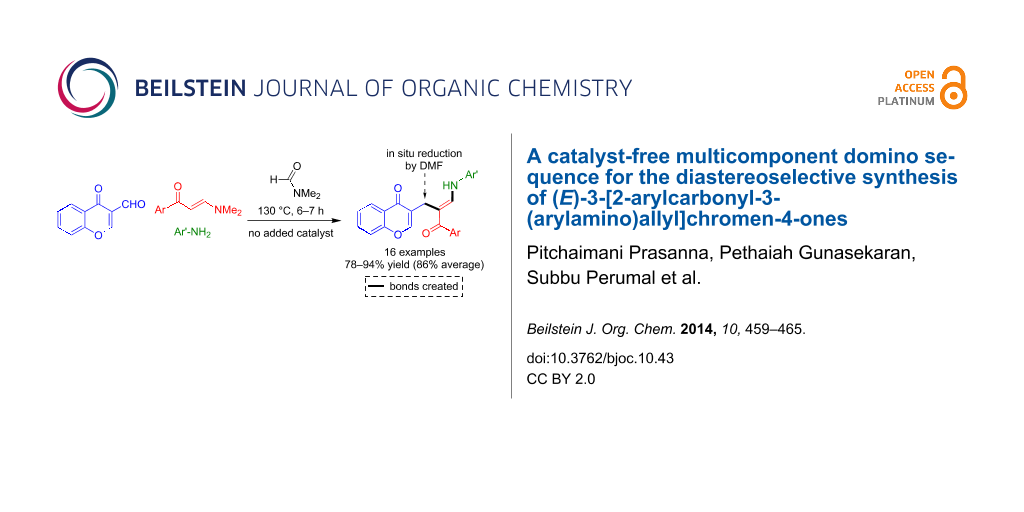
Abstract
The three-component domino reactions of (E)-3-(dimethylamino)-1-arylprop-2-en-1-ones, 3-formylchromone and anilines under catalyst-free conditions afforded a library of novel (E)-3-(2-arylcarbonyl-3-(arylamino)allyl)-4H-chromen-4-ones in good to excellent yields and in a diastereoselective transformation. This transformation generates one C–C and one C–N bond and presumably proceeds via a reaction sequence comprising a Michael-type addition–elimination reaction, a nucleophilic attack of an enamine to a carbonyl reminiscent of one of the steps of the Bayllis–Hilman condensation, and a final deoxygenation. The deoxygenation is assumed to be induced by carbon monoxide resulting from the thermal decomposition of the dimethylformamide solvent.
Graphical Abstract
Introduction
Chromones are widely present in nature, especially in the plant kingdom, and a wide variety of useful biological properties are associated with them [1,2]. Chromone derivatives act as effective tyrosine and protein kinase C inhibitors [3] and display antifungal [4,5], antimycobacterial [6], antiviral [7], antihypertensive [8], anti-oxidant [9-12], HIV-inhibitory [13], anti-inflammatory [14,15], immunomodulatory [16], antithrombotic [17], and anticancer [18-20] activities. Furthermore, some chromone derivatives have been identified as suitable fluorophores for live cell imaging [21]. In view of its importance chromone has emerged as a pharmacophore in drug discovery programmes, leading to several drugs in the market (e.g. cromolyn and nedocromil) and thereby continuing to draw the attention of synthetic organic and medicinal chemists [22-24]. However, there are relatively few methods, which allow the preparation of hybrid structures containing chromone derivatives attached to other heterocyclic systems due to the lack of suitable building blocks. In order to come up with such building blocks, we planned the preparation of chromones containing a β-enaminone structural fragment, since enaminones are versatile starting materials in organic synthesis and are notably important for the synthesis of nitrogen heterocycles [25-27]. In particular, β-enaminoketones are endowed with electrophilic and nucleophilic reaction centers and have a versatile reactivity that allows their application in the synthesis of important heterocycles such as indole [28], dihydropyridine [29], quinoline [30], pyrrole [31] and pyridinone [32]. Furthermore, they can take part in one-pot multicomponent reactions with both nucleophilic and electrophilic reactants, leading to a fast access to structurally diverse carbocycles and heterocycles, an area in which we have recently become interested [33-42]. Thus, the present study ia a continuation of our research program on the construction of novel heterocycles employing one-pot green domino-multicomponent transformations [43-54].
In order to achieve our goal, we embarked on the study of the three-component reactions between 3-formylchromone (1), (E)-3-(dimethylamino)-1-arylprop-2-en-1-ones 2 and anilines 3, which we expected to furnish novel (E)-3-[2-arylcarbonyl-3-(arylamino)allyl]-4H-chromen-4-ones 5 comprising the desired chromonone and β-enaminoketone moieties via intermediate species 4. The overall synthetic strategy is summarized in Scheme 1.
Scheme 1: Summary of the transformations involved in the synthesis of compounds 5, containing chromone and β-enaminoketone moieties.
Scheme 1: Summary of the transformations involved in the synthesis of compounds 5, containing chromone and β-...
Results and Discussion
We started our study with the optimization of a model reaction between 3-formylchromone (1, 1 mmol), (E)-3-(dimethylamino)-1-(3-nitrophenyl)prop-2-en-1-one (1 mmol) and 4-methoxyaniline (1 mmol). This three-component reaction was initially examined in solvents such as acetonitrile, dioxane, dimethyl sulfoxide, toluene and ethanol under heating. However, the only isolable product was compound 4d, which originated from a reaction between the last two components without participation of the chromone substrate (Table 1, entries 1–5). The use of dimethylformamide as a solvent, on the other hand, allowed the isolation of the target (E)-3-[3-(4-methoxyphenylamino]-2-(3-nitrobenzoyl)allyl)-4H-chromen-4-one (5f). After temperature fine-tuning (Table 1, entries 6–11), we identified heating in DMF at 130 °C for 6 h as the optimal conditions, which afforded 5f in 94% yield (Table 1, entry 11).
Table 1: Solvent screen and temperature optimization for the synthesis of compound 5f.
|
|
||||
| Entry | Solvent | Time (h) | Temp. (°C) | Yield (%)a |
|---|---|---|---|---|
| 1 | CH3CN | 12 | 80 | –b |
| 2 | dioxane | 10 | 102 | –b |
| 3 | DMSO | 10 | 140 | –b |
| 4 | toluene | 10 | 110 | –b |
| 5 | EtOH | 10 | 78 | –b |
| 6 | DMF | 6 | 60 | 27 |
| 7 | DMF | 6 | 80 | 34 |
| 8 | DMF | 6 | 100 | 43 |
| 9 | DMF | 6 | 110 | 61 |
| 10 | DMF | 6 | 120 | 74 |
| 11 | DMF | 6 | 130 | 94 |
aIsolated yield after purification by column chromatography. bCompound 4d was obtained predominantly.
All the subsequent reactions of 3-formylchromone (1), (E)-3-(dimethylamino)-1-arylprop-2-en-1-ones 2 and substituted anilines 3 were performed under the optimal conditions (equimolecular amounts, DMF at 130 °C) and were completed in 6–7 h. After completion of the reaction, removal of the solvent and purification of the residue by column chromatography, (E)-3-[2-arylcarbonyl-3-(arylamino)allyl]-4H-chromen-4-ones 5 were obtained in pure form in 78–94% yields (Scheme 2 and Table 2). It is noteworthy, that a similar reaction, in which the less hindered, more reactive formaldehyde was employed as the aldehyde component, took a completely divergent course and afforded 5-arylcarbonyl-1,3-diarylhexahydropyrimidines arising from pseudo five-component reactions of (E)-3-(dimethylamino)-1-arylprop-2-en-1-ones, two molecules of formaldehyde and two molecules of aniline [55].
Table 2: Scope and yields of the synthesis of compounds 5.
| Entry | Comp. | Ar | Ar' | Time (h) | Yield (%)a |
|---|---|---|---|---|---|
| 1 | 5a | C6H5 | 4-MeC6H4 | 6 | 85 |
| 2 | 5b | 4-ClC6H4 | 4-MeC6H4 | 6 | 87 |
| 3 | 5c | 3-NO2C6H4 | 4-MeC6H4 | 6 | 90 |
| 4 | 5d | C6H5 | 4-MeOC6H4 | 6 | 89 |
| 5 | 5e | 4-ClC6H4 | 4-MeOC6H4 | 6 | 91 |
| 6 | 5f | 3-NO2C6H4 | 4-MeOC6H4 | 6 | 94 |
| 7 | 5g | 4-ClC6H4 | 4-ClC6H4 | 6 | 86 |
| 8 | 5h | 4-MeC6H4 | 4-ClC6H4 | 7 | 84 |
| 9 | 5i | 4-MeOC6H4 | 4-ClC6H4 | 7 | 82 |
| 10 | 5j | 3-NO2C6H4 | 4-ClC6H4 | 6 | 87 |
| 11 | 5k | C6H5 | 4-BrC6H4 | 6 | 80 |
| 12 | 5l | 4-MeC6H4 | 4-BrC6H4 | 6 | 81 |
| 13 | 5m | 4-MeC6H4 | 4-FC6H4 | 7 | 83 |
| 14 | 5n | 4-MeC6H4 | 3-NO2C6H4 | 7 | 78 |
| 15 | 5o | 4-ClC6H4 | C6H5 | 6 | 86 |
| 16 | 5p | 3-NO2C6H4 | C6H5 | 6 | 88 |
aIsolated yield after purification by column chromatography.
The structure of compounds 5 was deduced from one and two-dimensional NMR spectroscopic data, as detailed in Supporting Information File 1 for 5h as a representative example. The structure of 5h deduced from the NMR spectroscopic studies was subsequently confirmed by single-crystal X-ray crystallographic data, as shown in Figure 1 [56].
![[1860-5397-10-43-1]](/bjoc/content/figures/1860-5397-10-43-1.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 1: X-ray structure of compound 5h.
Figure 1: X-ray structure of compound 5h.
Our initial mechanistic hypothesis accounting for the formation of compounds 5 is depicted in Scheme 3. An initial Michael addition–elimination reaction, leading to the exchange between the starting arylamine 3 and dimethylamine, explains the formation of intermediates 4, which were the final reaction products in most investigated solvents. However, in DMF, the enamine moiety of 4 attacks the aldehyde group in 1 giving rise to 6. This reaction resembles one of the steps of the Bayllis–Hilman condensation and is presumably promoted by the presence of traces of formic acid as a contaminant of the solvent [57].
Scheme 3: Initial mechanistic proposal to explain the formation of compounds 5 that was ruled out by deuteration experiments.
Scheme 3: Initial mechanistic proposal to explain the formation of compounds 5 that was ruled out by deuterat...
We established the feasibility of the first step by showing that the reaction between two of our starting enaminones, namely 2a (Ar = Ph) and 2c (Ar = 3-NO2C6H4) with aniline under our standard reaction conditions (DMF, 130 °C) affords the corresponding compounds 4 after 3 h in 94% and 90% yields, respectively. As previously mentioned (Table 1), the nature of the solvent was found to be of critical importance, and the transformation of intermediate 4 into the final products 5 was found to work only in DMF, among many investigated solvents. Since the reaction between 4 and 1 is proposed to be catalyzed by acid, we carried out the multicomponent reaction in a selection of solvents (dioxane, acetonitrile, dimethyl sulfoxide, ethanol, ethylene glycol) in the presence of one equivalent of HCl, but all these attempts failed, while the same conditions were successful in DMF. These results can be explained by assuming that the initial aldol-type reaction between 1 and 4 to give 6 is reversible and is driven to completion by the reduction step, which takes place in DMF only. Thus, interestingly, the reaction does not stop at compound 6, but instead undergoes a reductive termination step, which leads to the final products 5. This reduction step is intriguing, and a first mechanistic possibility could be a hydride transfer from formate anion. While this mechanism might partly account for the observed reduction, we acknowledge that it is problematic to rely on solvent impurities to account for a stoichiometric reduction. Alternatively, dimethylformamide itself might have been the reducing agent. There are a few scattered literature reports on the use of DMF as a reducing agent, the first of which seems to be the reduction of diazonium tetrafluoroborate salts to arenes [58]. DMF has also been described as a reducing agent acting by hydride transfer in Pd-catalyzed processes, where the metal plays a critical role in the reduction by decomposing it and facilitating hydride transfer from a Pd intermediate [59-61]. However, it is not clear whether the same type of reaction may take place under our conditions. In order to test this ideas experimentally, we carried out the reaction between 3-formylchromone (1), (E)-3-(dimethylamino)-1-(4-chlorophenyl)prop-2-en-1-one and p-toluidine in DMF-d7. If the mechanistic hypothesis was correct, this reaction should lead to a d-7 intermediate and hence to a mixture of 5 and d-5, with the latter being major due to isotope effects in the tautomeric equilibrium. However, this reaction failed to show any incorporation of deuterium into 5, and therefore we had to abandon the hydride-transfer hypothesis.
We came up with an alternative explanation based on the well-known fact that DMF decomposes into dimethylamine and carbon monoxide at its boiling point. Carbon monoxide can act as a deoxygenating agent [62] and thus explain the transformation of 6 into 5 as shown in Scheme 4. Because of the synthetic interest of allylic and benzylic deoxygenations, further research into this reaction is under way in our laboratories.
Scheme 4: Alternative mechanistic proposal based on a carbon monoxide-induced deoxygenation.
Scheme 4: Alternative mechanistic proposal based on a carbon monoxide-induced deoxygenation.
Conclusion
We have developed a facile three-component diastereoselective synthesis of novel (E)-3-[2-arylcarbonyl-3-(arylamino)allyl]-4H-chromen-4-ones containing chromone and β-enaminoketone structural fragments from simple, readily available starting materials in a one-pot operation and in good to excellent yields. This transformation occurs via a domino sequence of reactions, which generates one C–C and one C–N bond. Presumably, this transformation proceeds via a reaction sequence comprising a Michael-type addition–elimination reaction, a nucleophilic attack of an enamine to a carbonyl, and a final deoxygenation step. We propose that the deoxygenation step is induced by carbon monoxide resulting from thermal decomposition of the dimethylformamide solvent.
Experimental
General methods. Melting points were measured in open capillary tubes and are uncorrected. The 1H NMR, 13C NMR, DEPT, H,H-COSY, C,H-COSY and HMBC spectra were recorded on a Bruker (Avance) 300 MHz NMR instrument by using TMS as an internal standard and CDCl3 as a solvent. Standard Bruker software was used throughout. Chemical shifts are given in parts per million (δ-scale), and the coupling constants are given in Hertz. Silica gel-G plates (Merck) were used for TLC analysis with a mixture of petroleum ether (60–80 °C) and ethyl acetate as an eluent. Elemental analyses were performed on a Perkin Elmer 2400 Series II Elemental CHNS analyzer.
General procedure for the synthesis of (E)-3-(2-arylcarbonyl-3-(arylamino)allyl)-4H-chromen-4-one derivatives 5a–5p. In a similar manner as described in [55], a mixture of 3-formylchromone (1, 1 mmol), enaminone 2 (1 mmol) and aniline 3 (1 mmol) in DMF (5 mL) was heated at 130 °C for 6–7 h. The reaction progress was monitored by TLC. After completion of the reaction, the solvent was removed and the product was purified by column chromatography with a petroleum ether–ethyl acetate mixture (4:1 v/v) as an eluent to afford compounds 5. Characterization data for representative compounds are given below. The characterization data for the full library can be found in Supporting Information File 1.
(E)-3-(2-Benzoyl-3-(p-tolylamino)allyl)-4H-chromen-4-one (5a): Isolated yield 0.336 g (85%); colorless solid; mp 215–216 °C; 1H NMR (300 MHz, CDCl3, δ, ppm) 2.28 (s, 3H), 3.71 (s, 2H), 6.92 (d, J = 8.4 Hz, 2H), 7.08 (d, J = 8.1 Hz, 2H), 7.37–7.45 (m, 4H), 7.47–7.56 (m, 4H), 7.66–7.71 (m, 1H), 8.31 (d, J = 7.8 Hz, 1H), 8.52 (s, 1H), 9.93 (d, J = 12.6 Hz, 1H); 13C NMR (75 MHz, CDCl3, δ, ppm): 20.1, 20.6, 113.3, 115.8, 118.3, 123.7, 123.9, 125.0, 125.9, 128.0, 128.5, 129.8, 130.1, 132.3, 133.7, 138.9, 140.9, 146.6, 156.2, 156.7, 179.8, 195.0; HRMS–ESI (m/z): [M − H]− calcd for C26H20NO3, 394.14432; found, 394.144817; Anal. calcd for C26H21NO3: C, 78.97; H, 5.35; N, 3.54; found: C, 79.02; H, 5.29; N, 3.62.
(E)-3-(2-(3-Nitrobenzoyl)-3-(p-tolylamino)allyl)-4H-chromen-4-one (5c): Isolated yield 0.396 g (90%); yellow solid; mp 206–207 °C; 1H NMR (300 MHz, CDCl3, δ, ppm) 2.32 (s, 3H), 3.72 (s, 2H), 6.93 (d, J = 8.4 Hz, 2H), 7.10 (d, J = 8.1 Hz, 2H), 7.40–753 (m, 3H), 7.59 (d, J = 8.0 Hz, 1H), 7.68–7.74 (m, 1H), 7.81 (d, J = 7.5 Hz, 1H), 8.28–8.35 (m, 3H), 8.51 (s, 1H), 10.2 (d, J = 12.6 Hz, 1H); 13C NMR (75 MHz, CDCl3, δ, ppm) 20.1, 20.7, 113.0, 116.2, 118.4, 123.3, 123.6, 123.7, 124.4, 125.2, 125.9, 129.2, 130.2, 133.1, 133.9, 134.1, 138.4, 142.5, 147.3, 148.0, 156.1, 156.7, 179.9, 191.8; HRMS–ESI (m/z): [M − H]− calcd for C26H19N2O5, 439.12940; found, 439.12995; Anal. calcd for C26H20N2O5: C, 70.90; H, 4.58; N, 6.36; found: C, 70.84; H, 4.62; N, 6.29.
(E)-3-(3-(4-Chlorophenylamino)-2-(4-methylbenzoyl)allyl)-4H-chromen-4-one (5h): Isolated yield 0.361 g (84%); colorless solid; mp 219–220 °C; 1H NMR (300 MHz, CDCl3, δ, ppm) 2.40 (s, 3H), 3.70 (s, 2H), 6.95 (d, J = 8.7 Hz, 2H), 7.19–7.26 (m, 4H), 7.40–7.46 (m, 3H), 7.48–7.52 (m, 2H), 7.69 (t, J = 7.1 Hz, 1H), 8.30 (d, J = 7.2 Hz, 1H), 8.51 (s, 1H), 10.04 (d, J = 12.3 Hz, 1H); 13C NMR (75 MHz, CDCl3, δ, ppm) 20.4, 21.4, 114.4, 116.8, 118.4, 123.7, 125.1, 125.8, 127.4, 128.6, 128.8, 129.5, 133.8, 137.7, 140.1, 140.4, 145.3, 156.3, 156.7, 179.9, 195.2; HRMS–ESI (m/z): [M − H]− calcd for C26H19ClNO3, 428.10535; found, 428.10589; Anal. calcd for C26H20ClNO3: C, 72.64; H, 4.69; N, 3.26; found: C, 72.57; H, 4.75; N, 3.31.
(E)-3-(3-(4-Bromophenylamino)-2-(4-methylbenzoyl)allyl)-4H-chromen-4-one (5l): Isolated yield 0.384 g (81%); colorless solid; mp 223–224 °C; 1H NMR (300 MHz, CDCl3, δ, ppm) 2.40 (s, 3H), 3.70 (s, 2H), 6.90 (d, J = 8.7 Hz, 2H), 7.21 (d, J = 8.1 Hz, 2H), 7.36–7.41 (m, 3H), 7.43–7.52 (m, 4H), 7.69 (dd, J = 7.5 Hz, 1.5 Hz, 1H), 8.30 (dd, J = 8.1 Hz, 1.8 Hz, 1H), 8.51 (s, 1H), 10.04 (d, J = 12.0 Hz, 1H); 13C NMR (75 MHz, CDCl3, δ, ppm) 20.4, 21.4, 114.5, 114.7, 117.2, 118.4, 123.6, 125.1, 125.8, 128.6, 128.8, 132.5, 133.8, 137.7, 140.4, 140.6, 145.1, 156.4, 156.7, 179.9, 195.2; HRMS–ESI (m/z): [M − H]− calcd for C26H19BrNO3, 472.05483; found, 472.05538; Anal. calcd for C26H20BrNO3: C, 65.83; H, 4.25; N, 2.95; found: C, 65.70; H, 4.34; N, 2.91.
(E)-3-(2-(4-Chlorobenzoyl)-3-(phenylamino)allyl)-4H-chromen-4-one (5o): Isolated yield 0.357 g (86%); colorless solid; mp 245–246 °C; 1H NMR (300 MHz, CDCl3, δ, ppm) 3.71 (s, 2H), 7.00–7.05 (m, 3H), 7.26–7.33 (m, 2H), 7.37–7.40 (m, 2H), 7.44–7.55 (m, 5H), 7.67–7.72 (m, 1H), 8.31 (d, J = 7.8 Hz, 1H), 8.50 (s, 1H), 10.08 (d, J = 12.3 Hz, 1H); 13C NMR (75 MHz, CDCl3, δ, ppm) 20.2, 113.7, 115.9, 118.4, 122.9, 123.7, 125.1, 125.9, 128.4, 129.7, 129.9, 133.8, 136.1, 139.2, 141.1, 146.2, 156.2, 156.7, 179.8, 193.8; HRMS–ESI (m/z): [M − H]− calcd for C25H17ClNO3, 414.08970; found, 414.09024; Anal. calcd for C25H18ClNO3: C, 72.20; H, 4.36; N, 3.37; found: C, 72.09; H, 4.24; N, 3.41.
General procedure for the isolation of intermediates 4. A mixture of the suitable enaminone 2 (1 mmol) and aniline 3 (1 mmol) in DMF (5 mL) was heated at 130 °C for 3 h. The reaction progress was monitored by TLC. After completion of the reaction, the solvent was removed and the product was purified by column chromatography with a petroleum ether–ethyl acetate mixture (4:1 v/v) as an eluent. Characterization data for compounds 4 can be found in Supporting Information File 1.
Supporting Information
| Supporting Information File 1: Experimental details, full characterization data, detailed structural characterization of compound 5h and copies of the spectra of all compounds. | ||
| Format: PDF | Size: 4.2 MB | Download |
Acknowledgements
SP and JCM thank the Department of Science and Technology (India) and the Ministerio de Ciencia e Innovación (MICINN, Spain) for the Indo-Spanish collaborative research project, which has made this joint work possible (DST/INT/SPAIN/P-11/09 and ACI2009-0956, respectively). The authors acknowledge (i) the DST for granting a major research project to SP (No. SR/S1/OC-50/2011), (ii) the University Grants Commission for the award of a BSR Faculty Fellowship to SP, and (iii) the Ministerio de Economía y Competitividad (MINECO) for the grant CTQ-2012-33272-BQU to JCM. We also thank an anonymous reviewer for bringing reference [62] to our attention.
References
-
Kumar, R.; Yusuf, M. ARKIVOC 2006, No. ix, 239–264. doi:10.3998/ark.5550190.0007.908
Return to citation in text: [1] -
Khadem, S.; Marles, R. J. Molecules 2012, 17, 191–206. doi:10.3390/molecules17010191
Return to citation in text: [1] -
Dyrager, C.; Nilsson Möllers, L.; Kjäll, L. K.; Alao, J. P.; Dinér, P.; Wallner, F. K.; Sunnerhagen, P.; Grøtli, M. J. Med. Chem. 2011, 54, 7427–7431. doi:10.1021/jm200818j
Return to citation in text: [1] -
Mori, K.; Audran, G.; Monti, H. Synlett 1998, 259–260. doi:10.1055/s-1998-1628
Return to citation in text: [1] -
Ghani, S. B. A.; Mugisha, P. J.; Wilcox, J. C.; Gado, E. A. M.; Medu, E. O.; Lamb, A. J.; Brown, R. C. D. Synth. Commun. 2013, 43, 1549–1556. doi:10.1080/00397911.2011.647222
Return to citation in text: [1] -
China Raju, B.; Rao, R. N.; Suman, P.; Yogeeswari, P.; Sriram, D.; Shaik, T. B.; Kalivendi, S. V. Bioorg. Med. Chem. Lett. 2011, 21, 2855–2859. doi:10.1016/j.bmcl.2011.03.079
Return to citation in text: [1] -
Bhat, A. S.; Whetstone, J. L.; Brueggemeier, R. W. Tetrahedron Lett. 1999, 40, 2469–2472. doi:10.1016/S0040-4039(99)00279-8
Return to citation in text: [1] -
Wu, E. S. C.; Cole, T. E.; Davidson, T. A.; Blosser, J. C.; Borrelli, A. R.; Kinsolving, C. R.; Milgate, T. E.; Parker, R. B. J. Med. Chem. 1987, 30, 788–792. doi:10.1021/jm00388a007
Return to citation in text: [1] -
Pietta, P.-G. J. Nat. Prod. 2000, 63, 1035–1042. doi:10.1021/np9904509
Return to citation in text: [1] -
Bennett, C. J.; Caldwell, S. T.; McPhail, D. B.; Morrice, P. C.; Duthie, G. G.; Hartley, R. C. Bioorg. Med. Chem. 2004, 12, 2079–2098. doi:10.1016/j.bmc.2004.02.031
Return to citation in text: [1] -
Krishnamachari, V.; Levine, L. H.; Zhou, C.; Pare, P. W. Chem. Res. Toxicol. 2004, 17, 795–804. doi:10.1021/tx034242z
Return to citation in text: [1] -
Kumar, M.; Kumar, S.; Kaur, S. Afr. J. Pharm. Pharmacol. 2011, 5, 421–427.
Return to citation in text: [1] -
Khan, I. A.; Avery, M. A.; Burandt, C. L.; Goins, D. K.; Mikell, J. R.; Nash, T. E.; Azadegan, A.; Walker, L. A. J. Nat. Prod. 2000, 63, 1414–1416. doi:10.1021/np000010d
Return to citation in text: [1] -
Kim, H. P.; Son, K. H.; Chang, H. W.; Kang, S. S. J. Pharm. Sci. 2004, 96, 229–245. doi:10.1254/jphs.CRJ04003X
Return to citation in text: [1] -
Middleton, E.; Kandaswami, C.; Theoharides, T. C. Pharmacol. Rev. 2000, 52, 673–751.
Return to citation in text: [1] -
Wang, Y.-B.; Huang, R.; Zhang, H.-B.; Li, L. J. Asian Nat. Prod. Res. 2006, 8, 663–670. doi:10.1080/10286020500246303
Return to citation in text: [1] -
Lin, C.-N.; Kuo, S.-H.; Chung, M.-I.; Ko, F.-N.; Teng, C.-M. J. Nat. Prod. 1997, 60, 851–853. doi:10.1021/np970011e
Return to citation in text: [1] -
Bauvois, B.; Puiffe, M.-L.; Bongui, J.-B.; Paillat, S.; Monneret, C.; Dauzonne, D. J. Med. Chem. 2003, 46, 3900–3913. doi:10.1021/jm021109f
Return to citation in text: [1] -
Quintin, J.; Roullier, C.; Thoret, S.; Lewin, G. Tetrahedron 2006, 62, 4038–4051. doi:10.1016/j.tet.2006.02.024
Return to citation in text: [1] -
Maicheen, C.; Jittikoon, J.; Vajragupta, O.; Ungwitayatorn, J. Med. Chem. 2013, 9, 329–339. doi:10.2174/1573406411309030003
Return to citation in text: [1] -
Dyrager, C.; Friberg, A.; Dahlén, K.; Fridén-Saxin, M.; Börjesson, K.; Wilhelmsson, L. M.; Smedh, L.; Grøtli, M.; Luthman, K. Chem.–Eur. J. 2009, 15, 9417–9423. doi:10.1002/chem.200900279
Return to citation in text: [1] -
Ellis, G. P. Chromenes, Chromanones and Chromones; John Wiley and Sons: New York, 1977.
Return to citation in text: [1] -
Ibrahim, M. A.; Ali, T. E.; Alnamer, Y. A.; Gabr, Y. A. ARKIVOC 2010, No. i, 98–135. doi:10.3998/ark.5550190.0011.103
Return to citation in text: [1] -
Pinto, D. C. G. A.; Silva, A. M. S. Curr. Org. Chem. 2012, 9, 561–572. doi:10.2174/157017912802651429
Return to citation in text: [1] -
Lue, P.; Greenhill, J. V. Adv. Heterocycl. Chem. 1996, 67, 207–343. doi:10.1016/S0065-2725(08)60072-0
Return to citation in text: [1] -
Elassar, A.-Z. A.; El-Khair, A. A. Tetrahedron 2003, 59, 8463–8480. doi:10.1016/S0040-4020(03)01201-8
Return to citation in text: [1] -
Govindh, B.; Diwakar, B. S.; Murthy, Y. L. N. Org. Commun. 2012, 5, 105–119.
Return to citation in text: [1] -
Bernini, R.; Fabrizi, G.; Sferrazza, A.; Cacchi, S. Angew. Chem., Int. Ed. 2009, 48, 8078–8081. doi:10.1002/anie.200902440
Return to citation in text: [1] -
Yang, J.; Wang, C.; Xie, X.; Li, H.; Li, Y. Eur. J. Org. Chem. 2010, 4189–4193. doi:10.1002/ejoc.201000607
Return to citation in text: [1] -
Mahata, P. K.; Venkatesh, C.; Syam Kumar, U. K.; Ila, H.; Junjappa, H. J. Org. Chem. 2003, 68, 3966–3975. doi:10.1021/jo034053l
Return to citation in text: [1] -
Yan, R.-L.; Luo, J.; Wang, C.-X.; Ma, C.-W.; Huang, G.-S.; Liang, Y.-M. J. Org. Chem. 2010, 75, 5395–5397. doi:10.1021/jo101022k
Return to citation in text: [1] -
Schirok, H.; Alonso-Alija, C.; Benet-Buchholz, J.; Göller, A. H.; Grosser, R.; Michels, M.; Paulsen, H. J. Org. Chem. 2005, 70, 9463–9469. doi:10.1021/jo0515428
Return to citation in text: [1] -
Tenti, G.; Egea, J.; Villarroya, M.; León, R.; Fernández, J. C.; Padín, J. F.; Sridharan, V.; Ramos, M. T.; Menéndez, J. C. Med. Chem. Commun. 2013, 4, 590–594. doi:10.1039/c3md20345j
Return to citation in text: [1] -
Rocchi, D.; González, J. F.; Menéndez, J. C. Green Chem. 2013, 15, 511–517. doi:10.1039/c2gc36221j
Return to citation in text: [1] -
Estévez, V.; Villacampa, M.; Menéndez, J. C. Chem. Commun. 2013, 49, 591–593. doi:10.1039/c2cc38099d
Return to citation in text: [1] -
Tenti, G.; Ramos, M. T.; Menéndez, J. C. ACS Comb. Sci. 2012, 14, 551–557. doi:10.1021/co300081k
Return to citation in text: [1] -
Maiti, S.; Menéndez, J. C. Chem. Commun. 2011, 47, 10554–10556. doi:10.1039/c1cc11246e
Return to citation in text: [1] -
Maiti, S.; Sridharan, V.; Menéndez, J. C. J. Comb. Chem. 2010, 12, 713–722. doi:10.1021/cc100084b
Return to citation in text: [1] -
Suryavanshi, P. A.; Sridharan, V.; Menéndez, J. C. Org. Biomol. Chem. 2010, 8, 3426–3436. doi:10.1039/c004703a
Return to citation in text: [1] -
Sridharan, V.; Maiti, S.; Menéndez, J. C. J. Org. Chem. 2009, 74, 9365–9371. doi:10.1021/jo9021309
Return to citation in text: [1] -
Sridharan, V.; Maiti, S.; Menéndez, J. C. Chem.–Eur. J. 2009, 15, 4565–4572. doi:10.1002/chem.200900044
Return to citation in text: [1] -
Sridharan, V.; Menéndez, J. C. Org. Lett. 2008, 10, 4303–4306. doi:10.1021/ol801738d
Return to citation in text: [1] -
Prasanna, P.; Balamurugan, K.; Perumal, S.; Menéndez, J. C. Green Chem. 2011, 13, 2123–2129. doi:10.1039/c0gc00952k
Return to citation in text: [1] -
Rajesh, S. M.; Bala, B. D.; Perumal, S.; Menéndez, J. C. Green Chem. 2011, 13, 3248–3254. doi:10.1039/c1gc15794a
Return to citation in text: [1] -
Indumathi, S.; Perumal, S.; Menéndez, J. C. Tetrahedron 2011, 67, 7101–7105. doi:10.1016/j.tet.2011.06.107
Return to citation in text: [1] -
Bala, B. D.; Balamurugan, K.; Perumal, S. Tetrahedron Lett. 2011, 52, 4562–4566. doi:10.1016/j.tetlet.2011.06.102
Return to citation in text: [1] -
Balamurugan, K.; Jeyachandran, V.; Perumal, S.; Menéndez, J. C. Tetrahedron 2011, 67, 1432–1437. doi:10.1016/j.tet.2010.12.052
Return to citation in text: [1] -
Kumar, R. S.; Osman, H.; Perumal, S.; Menéndez, J. C.; Ali, M. A.; Ismail, R.; Choon, T. S. Tetrahedron 2011, 67, 3132–3139. doi:10.1016/j.tet.2011.02.058
Return to citation in text: [1] -
Balamurugan, K.; Perumal, S.; Menéndez, J. C. Tetrahedron 2011, 67, 3201–3208. doi:10.1016/j.tet.2011.03.020
Return to citation in text: [1] -
Indumathi, S.; Perumal, S.; Menéndez, J. C. J. Org. Chem. 2010, 75, 472–475. doi:10.1021/jo9021327
Return to citation in text: [1] -
Balamurugan, K.; Perumal, S.; Reddy, A. S. K.; Yogeeswari, P.; Sriram, D. Tetrahedron Lett. 2009, 50, 6191–6195. doi:10.1016/j.tetlet.2009.08.085
Return to citation in text: [1] -
Indumathi, S.; Kumar, R. R.; Perumal, S. Tetrahedron 2007, 63, 1411–1416. doi:10.1016/j.tet.2006.11.093
Return to citation in text: [1] -
Srinivasan, M.; Perumal, S. Tetrahedron 2007, 63, 2865–2874. doi:10.1016/j.tet.2007.01.038
Return to citation in text: [1] -
Karthikeyan, S. V.; Perumal, S. Tetrahedron Lett. 2007, 48, 2261–2265. doi:10.1016/j.tetlet.2007.01.168
Return to citation in text: [1] -
Muthusaravanan, S.; Perumal, S.; Almansour, A. I. Tetrahedron Lett. 2012, 53, 1144–1148. doi:10.1016/j.tetlet.2011.12.097
Return to citation in text: [1] [2] -
Cambridge Crystallographic Database, CCDC number 894829.
Return to citation in text: [1] -
Pletnev, A. A.; Tian, Q.; Larock, R. C. J. Org. Chem. 2002, 67, 9276–9287. doi:10.1021/jo026178g
And references cited therein.
Return to citation in text: [1] -
Marx, G. S. J. Org. Chem. 1971, 36, 1725–1726. doi:10.1021/jo00811a044
Return to citation in text: [1] -
Zawisza, A. M.; Muzart, J. Tetrahedron Lett. 2007, 48, 6738–6742. doi:10.1016/j.tetlet.2007.07.077
Return to citation in text: [1] -
Kim, H. S.; Gowrisankar, S.; Kim, S. H.; Kim, J. N. Tetrahedron Lett. 2008, 49, 3858–3861. doi:10.1016/j.tetlet.2008.04.080
Return to citation in text: [1] -
Liu, B.; Zheng, G.; Liu, X.; Xu, C.; Liu, J.; Wang, M. Chem. Commun. 2013, 49, 2201–2203. doi:10.1039/c3cc37571d
Return to citation in text: [1] -
Ai, K.; Liu, Y.; Lu, L.; Cheng, X.; Huo, L. J. Mater. Chem. 2011, 21, 3365–3370. doi:10.1039/c0jm02865g
Return to citation in text: [1] [2]
| 62. | Ai, K.; Liu, Y.; Lu, L.; Cheng, X.; Huo, L. J. Mater. Chem. 2011, 21, 3365–3370. doi:10.1039/c0jm02865g |
| 55. | Muthusaravanan, S.; Perumal, S.; Almansour, A. I. Tetrahedron Lett. 2012, 53, 1144–1148. doi:10.1016/j.tetlet.2011.12.097 |
| 62. | Ai, K.; Liu, Y.; Lu, L.; Cheng, X.; Huo, L. J. Mater. Chem. 2011, 21, 3365–3370. doi:10.1039/c0jm02865g |
| 1. | Kumar, R.; Yusuf, M. ARKIVOC 2006, No. ix, 239–264. doi:10.3998/ark.5550190.0007.908 |
| 2. | Khadem, S.; Marles, R. J. Molecules 2012, 17, 191–206. doi:10.3390/molecules17010191 |
| 7. | Bhat, A. S.; Whetstone, J. L.; Brueggemeier, R. W. Tetrahedron Lett. 1999, 40, 2469–2472. doi:10.1016/S0040-4039(99)00279-8 |
| 25. | Lue, P.; Greenhill, J. V. Adv. Heterocycl. Chem. 1996, 67, 207–343. doi:10.1016/S0065-2725(08)60072-0 |
| 26. | Elassar, A.-Z. A.; El-Khair, A. A. Tetrahedron 2003, 59, 8463–8480. doi:10.1016/S0040-4020(03)01201-8 |
| 27. | Govindh, B.; Diwakar, B. S.; Murthy, Y. L. N. Org. Commun. 2012, 5, 105–119. |
| 6. | China Raju, B.; Rao, R. N.; Suman, P.; Yogeeswari, P.; Sriram, D.; Shaik, T. B.; Kalivendi, S. V. Bioorg. Med. Chem. Lett. 2011, 21, 2855–2859. doi:10.1016/j.bmcl.2011.03.079 |
| 28. | Bernini, R.; Fabrizi, G.; Sferrazza, A.; Cacchi, S. Angew. Chem., Int. Ed. 2009, 48, 8078–8081. doi:10.1002/anie.200902440 |
| 4. | Mori, K.; Audran, G.; Monti, H. Synlett 1998, 259–260. doi:10.1055/s-1998-1628 |
| 5. | Ghani, S. B. A.; Mugisha, P. J.; Wilcox, J. C.; Gado, E. A. M.; Medu, E. O.; Lamb, A. J.; Brown, R. C. D. Synth. Commun. 2013, 43, 1549–1556. doi:10.1080/00397911.2011.647222 |
| 21. | Dyrager, C.; Friberg, A.; Dahlén, K.; Fridén-Saxin, M.; Börjesson, K.; Wilhelmsson, L. M.; Smedh, L.; Grøtli, M.; Luthman, K. Chem.–Eur. J. 2009, 15, 9417–9423. doi:10.1002/chem.200900279 |
| 3. | Dyrager, C.; Nilsson Möllers, L.; Kjäll, L. K.; Alao, J. P.; Dinér, P.; Wallner, F. K.; Sunnerhagen, P.; Grøtli, M. J. Med. Chem. 2011, 54, 7427–7431. doi:10.1021/jm200818j |
| 22. | Ellis, G. P. Chromenes, Chromanones and Chromones; John Wiley and Sons: New York, 1977. |
| 23. | Ibrahim, M. A.; Ali, T. E.; Alnamer, Y. A.; Gabr, Y. A. ARKIVOC 2010, No. i, 98–135. doi:10.3998/ark.5550190.0011.103 |
| 24. | Pinto, D. C. G. A.; Silva, A. M. S. Curr. Org. Chem. 2012, 9, 561–572. doi:10.2174/157017912802651429 |
| 14. | Kim, H. P.; Son, K. H.; Chang, H. W.; Kang, S. S. J. Pharm. Sci. 2004, 96, 229–245. doi:10.1254/jphs.CRJ04003X |
| 15. | Middleton, E.; Kandaswami, C.; Theoharides, T. C. Pharmacol. Rev. 2000, 52, 673–751. |
| 17. | Lin, C.-N.; Kuo, S.-H.; Chung, M.-I.; Ko, F.-N.; Teng, C.-M. J. Nat. Prod. 1997, 60, 851–853. doi:10.1021/np970011e |
| 13. | Khan, I. A.; Avery, M. A.; Burandt, C. L.; Goins, D. K.; Mikell, J. R.; Nash, T. E.; Azadegan, A.; Walker, L. A. J. Nat. Prod. 2000, 63, 1414–1416. doi:10.1021/np000010d |
| 18. | Bauvois, B.; Puiffe, M.-L.; Bongui, J.-B.; Paillat, S.; Monneret, C.; Dauzonne, D. J. Med. Chem. 2003, 46, 3900–3913. doi:10.1021/jm021109f |
| 19. | Quintin, J.; Roullier, C.; Thoret, S.; Lewin, G. Tetrahedron 2006, 62, 4038–4051. doi:10.1016/j.tet.2006.02.024 |
| 20. | Maicheen, C.; Jittikoon, J.; Vajragupta, O.; Ungwitayatorn, J. Med. Chem. 2013, 9, 329–339. doi:10.2174/1573406411309030003 |
| 9. | Pietta, P.-G. J. Nat. Prod. 2000, 63, 1035–1042. doi:10.1021/np9904509 |
| 10. | Bennett, C. J.; Caldwell, S. T.; McPhail, D. B.; Morrice, P. C.; Duthie, G. G.; Hartley, R. C. Bioorg. Med. Chem. 2004, 12, 2079–2098. doi:10.1016/j.bmc.2004.02.031 |
| 11. | Krishnamachari, V.; Levine, L. H.; Zhou, C.; Pare, P. W. Chem. Res. Toxicol. 2004, 17, 795–804. doi:10.1021/tx034242z |
| 12. | Kumar, M.; Kumar, S.; Kaur, S. Afr. J. Pharm. Pharmacol. 2011, 5, 421–427. |
| 8. | Wu, E. S. C.; Cole, T. E.; Davidson, T. A.; Blosser, J. C.; Borrelli, A. R.; Kinsolving, C. R.; Milgate, T. E.; Parker, R. B. J. Med. Chem. 1987, 30, 788–792. doi:10.1021/jm00388a007 |
| 16. | Wang, Y.-B.; Huang, R.; Zhang, H.-B.; Li, L. J. Asian Nat. Prod. Res. 2006, 8, 663–670. doi:10.1080/10286020500246303 |
| 31. | Yan, R.-L.; Luo, J.; Wang, C.-X.; Ma, C.-W.; Huang, G.-S.; Liang, Y.-M. J. Org. Chem. 2010, 75, 5395–5397. doi:10.1021/jo101022k |
| 29. | Yang, J.; Wang, C.; Xie, X.; Li, H.; Li, Y. Eur. J. Org. Chem. 2010, 4189–4193. doi:10.1002/ejoc.201000607 |
| 30. | Mahata, P. K.; Venkatesh, C.; Syam Kumar, U. K.; Ila, H.; Junjappa, H. J. Org. Chem. 2003, 68, 3966–3975. doi:10.1021/jo034053l |
| 59. | Zawisza, A. M.; Muzart, J. Tetrahedron Lett. 2007, 48, 6738–6742. doi:10.1016/j.tetlet.2007.07.077 |
| 60. | Kim, H. S.; Gowrisankar, S.; Kim, S. H.; Kim, J. N. Tetrahedron Lett. 2008, 49, 3858–3861. doi:10.1016/j.tetlet.2008.04.080 |
| 61. | Liu, B.; Zheng, G.; Liu, X.; Xu, C.; Liu, J.; Wang, M. Chem. Commun. 2013, 49, 2201–2203. doi:10.1039/c3cc37571d |
| 57. |
Pletnev, A. A.; Tian, Q.; Larock, R. C. J. Org. Chem. 2002, 67, 9276–9287. doi:10.1021/jo026178g
And references cited therein. |
| 43. | Prasanna, P.; Balamurugan, K.; Perumal, S.; Menéndez, J. C. Green Chem. 2011, 13, 2123–2129. doi:10.1039/c0gc00952k |
| 44. | Rajesh, S. M.; Bala, B. D.; Perumal, S.; Menéndez, J. C. Green Chem. 2011, 13, 3248–3254. doi:10.1039/c1gc15794a |
| 45. | Indumathi, S.; Perumal, S.; Menéndez, J. C. Tetrahedron 2011, 67, 7101–7105. doi:10.1016/j.tet.2011.06.107 |
| 46. | Bala, B. D.; Balamurugan, K.; Perumal, S. Tetrahedron Lett. 2011, 52, 4562–4566. doi:10.1016/j.tetlet.2011.06.102 |
| 47. | Balamurugan, K.; Jeyachandran, V.; Perumal, S.; Menéndez, J. C. Tetrahedron 2011, 67, 1432–1437. doi:10.1016/j.tet.2010.12.052 |
| 48. | Kumar, R. S.; Osman, H.; Perumal, S.; Menéndez, J. C.; Ali, M. A.; Ismail, R.; Choon, T. S. Tetrahedron 2011, 67, 3132–3139. doi:10.1016/j.tet.2011.02.058 |
| 49. | Balamurugan, K.; Perumal, S.; Menéndez, J. C. Tetrahedron 2011, 67, 3201–3208. doi:10.1016/j.tet.2011.03.020 |
| 50. | Indumathi, S.; Perumal, S.; Menéndez, J. C. J. Org. Chem. 2010, 75, 472–475. doi:10.1021/jo9021327 |
| 51. | Balamurugan, K.; Perumal, S.; Reddy, A. S. K.; Yogeeswari, P.; Sriram, D. Tetrahedron Lett. 2009, 50, 6191–6195. doi:10.1016/j.tetlet.2009.08.085 |
| 52. | Indumathi, S.; Kumar, R. R.; Perumal, S. Tetrahedron 2007, 63, 1411–1416. doi:10.1016/j.tet.2006.11.093 |
| 53. | Srinivasan, M.; Perumal, S. Tetrahedron 2007, 63, 2865–2874. doi:10.1016/j.tet.2007.01.038 |
| 54. | Karthikeyan, S. V.; Perumal, S. Tetrahedron Lett. 2007, 48, 2261–2265. doi:10.1016/j.tetlet.2007.01.168 |
| 55. | Muthusaravanan, S.; Perumal, S.; Almansour, A. I. Tetrahedron Lett. 2012, 53, 1144–1148. doi:10.1016/j.tetlet.2011.12.097 |
| 32. | Schirok, H.; Alonso-Alija, C.; Benet-Buchholz, J.; Göller, A. H.; Grosser, R.; Michels, M.; Paulsen, H. J. Org. Chem. 2005, 70, 9463–9469. doi:10.1021/jo0515428 |
| 33. | Tenti, G.; Egea, J.; Villarroya, M.; León, R.; Fernández, J. C.; Padín, J. F.; Sridharan, V.; Ramos, M. T.; Menéndez, J. C. Med. Chem. Commun. 2013, 4, 590–594. doi:10.1039/c3md20345j |
| 34. | Rocchi, D.; González, J. F.; Menéndez, J. C. Green Chem. 2013, 15, 511–517. doi:10.1039/c2gc36221j |
| 35. | Estévez, V.; Villacampa, M.; Menéndez, J. C. Chem. Commun. 2013, 49, 591–593. doi:10.1039/c2cc38099d |
| 36. | Tenti, G.; Ramos, M. T.; Menéndez, J. C. ACS Comb. Sci. 2012, 14, 551–557. doi:10.1021/co300081k |
| 37. | Maiti, S.; Menéndez, J. C. Chem. Commun. 2011, 47, 10554–10556. doi:10.1039/c1cc11246e |
| 38. | Maiti, S.; Sridharan, V.; Menéndez, J. C. J. Comb. Chem. 2010, 12, 713–722. doi:10.1021/cc100084b |
| 39. | Suryavanshi, P. A.; Sridharan, V.; Menéndez, J. C. Org. Biomol. Chem. 2010, 8, 3426–3436. doi:10.1039/c004703a |
| 40. | Sridharan, V.; Maiti, S.; Menéndez, J. C. J. Org. Chem. 2009, 74, 9365–9371. doi:10.1021/jo9021309 |
| 41. | Sridharan, V.; Maiti, S.; Menéndez, J. C. Chem.–Eur. J. 2009, 15, 4565–4572. doi:10.1002/chem.200900044 |
| 42. | Sridharan, V.; Menéndez, J. C. Org. Lett. 2008, 10, 4303–4306. doi:10.1021/ol801738d |
© 2014 Prasanna et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)