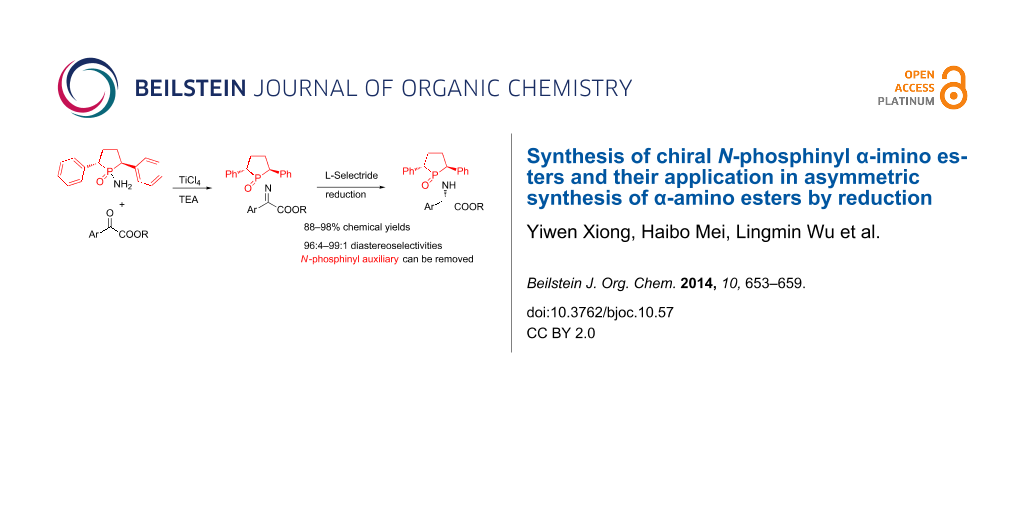
Abstract
A variety of chiral N-phosphinyl α-imino esters have been synthesized for the first time from ketoesters and phosphinylamide, which were then reduced by L-selectride to give the corresponding N-phosphinyl-protected α-amino esters. The reduction proceeded very well with excellent chemical yields (88–98%) as well as high diastereoselectivities (96:4 to 99:1). Some of these products could be obtained without column chromatography and recrystallization. The chiral phosphinyl auxiliary could be easily cleaved under acidic conditions.
Graphical Abstract
Introduction
α-Imino esters play a very important role in the field of imine chemistry [1-3], because the ester group can serve as an activator to enhance the reactivity of the C=N double bond, thus making the following nucleophilic addition easier [4,5]. More attractively, the transformation of α-imino esters can provide an easy access to various natural and unnatural α-amino acid esters [6-10]. Up to now, a series of nucleophilic substrates have been reported to react with α-imino esters, such as enamine [11-14], carbamate ammonium ylide [15], 1,3-dipolar cycle [16], boronic acid [17], acetylide [18], proparygylic anion [19] and ketene silyl acetal [20]. These resulted α-amino acid derivatives are useful building blocks in modern organic synthetic and medicinal chemistry [21,22]. For example, we can easily find the active participation of α-imino esters in the total synthesis of the bioactive molecule fumimycin [23]. Moreover, α-amino acids are the most general fragments constituting peptides and proteins, especially for human insulin [24-28].
One of the most straightforward methods to prepare α-amino esters is the direct reduction of α-imino esters [29,30]. Several heterocyle-based reduction reagents have been developed for this transformation in the past years [31-35]. They usually served as mild hydrogen donors, but the use of them also made the purification difficult at the same time. Hydrogen gas is a traditional reduction source [36] and silanes and boranes are also well documented as the choices of reduction reagents [37-39].
Recently, our groups have developed a new type of chiral phosphonyl auxiliaries for imines [40-44]. Compared with the reported sulfinyl auxiliaries (Figure 1a) [45-48], the phosphonyl ones exhibited advantages in the concise preparation method from readily available starting materials, an easy modification of the auxiliary and a highly diastereoselective control. Furthermore, the phosphonyl auxiliaries can do favor in the final purification, and it did not need any column chromatography or recrystallization, which was summarized as GAP (group assisted purification) [49-51]. So far, a number of N-phosphonyl aldimines (Figure 1b) have been synthesized and successfully used in many asymmetric additions [52-54]. In our continuous efforts on the chiral N-phosphonyl imine chemistry, we tried to develop novel N-phosphinyl protected α-imino esters and to use them for the asymmetric synthesis of α-amino esters. Herein, we report for the first time the synthesis of N-phosphinyl-protected α-imino esters (Figure 1c), followed by the reduction of these α-imino esters by L-selectride, to give the corresponding α-amino esters with excellent yields (88–98%) and virtually complete diastereoselectivities.
Figure 1: Typical sulfinyl (a), phosphonyl aldimines (b) and phosphinyl imino esters (c).
Figure 1: Typical sulfinyl (a), phosphonyl aldimines (b) and phosphinyl imino esters (c).
Results and Discussion
Preparation of the N-phosphinyl-protected α-imino esters
After screening the classical procedures for the synthesis of α-imino esters [3,55,56], we initially tried an approach with chlorophosphine and ester oxime as starting materials, which proceeded through rearrangement at low temperature (Scheme 1). 31P NMR and TLC of the crude product disclosed that several byproducts formed in this system. The isolation of the N-phosphinyl α-imino esters was very difficult and only 26% isolated yield of this product was obtained.
Scheme 1: Synthesis of α-imino ester by rearrangement.
Scheme 1: Synthesis of α-imino ester by rearrangement.
This unacceptable result shifted our attention to the previously reported methods for the preparation of N-phosphonyl aldimines, which was the condensation between aldehyde and phosphonyl amide [40,41]. Then, we carried out the optimization of the reaction conditions for the condensation of keto-ester 2a and phosphinyl amide 1 (Table 1). Common dehydrating reagents, like 4 Å molecular sieves and magnesium sulfate, resulted in no desired product neither at room temperature nor under reflux (Table 1, entries 1–4). Very poor chemical yields of α-imino ester 3a were detected when tetraethoxytitanium or tetraisopropoxytitanium was used as condensing reagent (Table 1, entries 5–8). Further treatment with titanium tetrachloride/triethyl amine gave a positive result and 46% chemical yield was obtained (Table 1, entry 9). Then, further investigation revealed that the best yield (47%) was observed when the reaction was conducted in the presence of TiCl4/Et3N at room temperature for 12 h (Table 1, entries 9–16). 31P NMR of the crude product predicted almost complete consumption of phosphinyl amide 1 but yields were very low. After careful study, we found that the obtained α-imino ester was not stable and decomposed slowly along with column chromatography purification and storage. For this reason, this α-imino ester was directly used in the following reaction after column chromatography to avoid decomposition.
Table 1: Optimization of the synthesis of α-imino ester 3a by the condensation method.a
|
|
|||||
| entry | reagent | T (°C) | solvent | time (h) | yield (%)b |
|---|---|---|---|---|---|
| 1 | 4 Å MS | rt | CH2Cl2 | 24 | NR |
| 2 | 4 Å MS | reflux | CH2Cl2 | 24 | NR |
| 3 | MgSO4 | rt | CH2Cl2 | 24 | NR |
| 4 | MgSO4 | reflux | CH2Cl2 | 24 | NR |
| 5 | Ti(OEt)4/Et3N | rt | CH2Cl2 | 24 | trace |
| 6 | Ti(OEt)4/Et3N | reflux | CH2Cl2 | 24 | trace |
| 7 | Ti(OEt)4/Et3N | reflux | toluene | 24 | 10 |
| 8 | Ti(OiPr)4/Et3N | reflux | CH2Cl2 | 24 | trace |
| 9 | TiCl4/Et3N | rt | CH2Cl2 | 24 | 46 |
| 10 | TiCl4/Et3N | rt | CH2Cl2 | 12 | 47 |
| 11 | TiCl4/Et3N | rt | CH2Cl2 | 6 | 33 |
| 12 | TiCl4/Et3N | reflux | CH2Cl2 | 12 | 21 |
| 13 | TiCl4/Et3N | rt | Et2O | 12 | 20 |
| 14 | TiCl4/Et3N | rt | THF | 12 | 29 |
| 15 | TiCl4/Et3N | rt | toluene | 12 | 11 |
| 16 | TiCl4/DIPEA | rt | CH2Cl2 | 12 | 31 |
aRearrangement conditions: Phosphinyl amide 1 (0.5 mmol), ketoester 2a (1.0 mmol), solvent (3.0 mL); bisolated yields.
With the optimized conditions in hand, we then used varieties of keto-esters 2 as starting materials for the preparation of chiral α-imino esters 3 (Table 2). As shown in Table 2, modest yields were obtained (29–59%) for all the cases. In general, electron-deficient substrates performed better and the best yield was found for the substrate with para-fluoro substituent (Table 2, entry 4). The substrate with ortho-substituent led to lower yield due to steric effects (Table 2, entry 7). Besides, the reaction with ethyl ester also worked well and resulted in a slightly lower yield (Table 2, entry 11).
Table 2: Synthesis of N-phosphinyl-protected α-imino estersa.
|
|
||||
| entry | Ar | R | product | yield (%)b |
|---|---|---|---|---|
| 1 | C6H5 | Me | 3a | 47 |
| 2 | 4-BrC6H4 | Me | 3b | 50 |
| 3 | 4-ClC6H4 | Me | 3c | 49 |
| 4 | 4-FC6H4 | Me | 3d | 59 |
| 5 | 3-FC6H4 | Me | 3e | 53 |
| 6 | 3-CH3C6H4 | Me | 3f | 40 |
| 7 | 2-CH3C6H4 | Me | 3g | 38 |
| 8 | 3,5-Cl2C6H3 | Me | 3h | 29 |
| 9 | 2-Naphthyl | Me | 3i | 26 |
| 10 | 4-Ph-C6H4 | Me | 3j | 35 |
| 11 | C6H5 | Et | 3k | 43 |
aReaction conditions: Phosphinyl amide 1 (0.5 mmol), ketoester 2 (1.0 mmol), titanium(IV) chloride (0.5 mmol), triethylamine (2.0 mmol), dichloromethane (4.0 mL), rt for 12 h; bisolated yields.
Optimization of the asymmetric reduction reaction conditions
Then, the obtained chiral N-phosphinyl α-imino esters were used for the asymmetric synthesis of α-amino esters through reduction. The reduction condition scan was firstly focused on the examination of reductants. A number of reductants, including Hantzsch ester, silanes, organoaluminum and boranes were tested in the system. Unfortunately, silanes failed to trigger the reduction (Table 3, entries 2 and 3). Hantzsch ester and DIBAL were also not suitable for this reduction, and almost no desired product was obtained (Table 3, entries 1 and 5). Lithium triethylborohydride (Table 3, entry 4) and sodium borohydride (Table 3, entry 6) were decent choices, giving modest yields and very poor diastereoselectivity. Further screening of reductants found that L-Selectride and N-Selectride were good candidates, especially for L-Selectride, 92% yield and virtually complete controlled diastereoselectivity (99:1) were found (Table 3, entry 7). It did not matter at all when the reaction time was shortened to 8 h. However, the yield decreased when the time was further shortened to 6 h (Table 3, entries 9 and 10). The examination of solvents showed that polar solvents (Table 3, entries 11 and 13) were superior to a non-polar one such as toluene (Table 3, entry 12). The temperature influenced the reaction obviously, and raising the temperature to −40 °C reduces the yield to 89% and the diastereoselectivity to 87:13 (Table 3, entry 14).
Table 3: Optimization of asymmetric reduction of α-imino estersa.
|
|
||||||
| entry | reagent | T (°C) | solvent | time (h) | Yield (%)b | drc |
|---|---|---|---|---|---|---|
| 1 | Hantzsch ester | −78 | THF | 12 | Trace | — |
| 2 | HSiCl3 | −78 | THF | 12 | NR | — |
| 3 | HSiEt3 | −78 | THF | 12 | NR | — |
| 4 | LiBHEt3 | −78 | THF | 12 | 71 | 75:25 |
| 5 | DIBAL | −78 | THF | 12 | Trace | — |
| 6 | NaBH4 | −78 | THF | 12 | 48 | 66:34 |
| 7 | L-Selectride | −78 | THF | 12 | 92 | 99:1 |
| 8 | N-Selectride | −78 | THF | 12 | 90 | 90:10 |
| 9 | L-Selectride | −78 | THF | 8 | 92 | 99:1 |
| 10 | L-Selectride | −78 | THF | 6 | 85 | 99:1 |
| 11 | L-Selectride | −78 | 4-MeTHF | 8 | 90 | 99:1 |
| 12 | L-Selectride | −78 | toluene | 8 | 47 | 96:4 |
| 13 | L-Selectride | −78 | diethyl ether | 8 | 82 | 99:1 |
| 14 | L-Selectride | −40 | THF | 8 | 89 | 87:13 |
aReaction conditions: α-imino ester 3a (0.15 mmol), reduction reagent (0.30 mmol), solvent (5.0 mL); bisolated yields; cdetermined by 31P NMR of the crude reaction mixture.
Scope of the asymmetric reduction reaction
After getting the optimized reduction conditions, several chiral N-phosphinyl α-imino esters prepared above were subjected to this system to examine the reaction scope. As shown in Table 4, reduction of these chiral α-imino esters provided excellent yields (up to 98%) and diastereoselectivities (up to 99:1). Almost all the reductions could achieve more than 90% yield, only for the case of para-phenyl-substituted substrate, a slightly lower yield was found (88%, Table 4, entry 10). The diastereoselectivities of the reactions were almost completely controlled, and most of them were 99:1. Except for the 4-bromo-substituted case, a dr of 96:4 was observed (Table 4, entry 2). It is interesting that GAP (group-assisted purification) was introduced into the reaction [49-51]. In five cases (Table 4, entries 1, 3–5 and 11), the final purified products were obtained by just washing with hexane, and no column chromatography or recrystallization was needed.
Table 4: Scope of novel phophinyl substituted α-amino esters.a
|
|
||||||
| entry | Ar | R | α-amino ester | purification method | yield (%)b | drc |
|---|---|---|---|---|---|---|
| 1 | C6H5 | Me | 4a | GAPd | 92 | 99:1 |
| 2 | 4-BrC6H4 | Me | 4b | Column | 90 | 96:4 |
| 3 | 4-ClC6H4 | Me | 4c | GAPd | 96 | 99:1 |
| 4 | 4-FC6H4 | Me | 4d | GAPd | 98 | 99:1 |
| 5 | 3-FC6H4 | Me | 4e | GAPd | 95 | 99:1 |
| 6 | 3-CH3C6H4 | Me | 4f | Column | 95 | 99:1 |
| 7 | 2-CH3C6H4 | Me | 4g | Column | 94 | 99:1 |
| 8 | 3,5-Cl2C6H3 | Me | 4h | Column | 92 | 99:1 |
| 9 | 2-Naphthyl | Me | 4i | Column | 93 | 99:1 |
| 10 | 4-Ph-C6H4 | Me | 4j | Column | 88 | 99:1 |
| 11 | C6H5 | Et | 4k | GAPd | 94 | 99:1 |
aReaction conditions: α-imino esters 3 (0.15 mmol), L-Selectride (0.30 mmol), THF (5.0 mL); bisolated yields; c determined by 31P NMR; dgroup-assisted purification.
Determination of the absolute configuration
The phosphinyl auxiliary could be easily cleaved by treating with concentrated hydrochloric acid in methanol. After stirring of 4a with acid overnight, the conversion was complete and the corresponding amino ester hydrochloride was obtained. Then, the obtained free amino ester was directly converted into its N-Cbz-protected derivative 5a by treating with CbzCl/Et3N (Scheme 2). The absolute configuration of the newly formed chiral center was assigned as S by comparing the optical rotation with that of known sample [57]. The stereochemical assignments of other products were made by analogy correspondingly.
Scheme 2: Cleavage of the chiral auxiliary.
Scheme 2: Cleavage of the chiral auxiliary.
Conclusion
In conclusion, we have developed a method for the synthesis of chiral N-phophinyl α-imino esters for the first time, which have been used as precursors for asymmetric reductions with L-Selectride as reductant. Varieties of α-amino esters were obtained in excellent chemical yields and almost completely controlled diastereoselectivities. Furthermore, a part of the products could be purified without use of any column chromatography or recrystallization, which provides an alternative way for the synthesis of α-amino esters. The auxiliary was easily removed by treatment with acid to result the free α-amino esters.
Experimental
General procedure for the asymmetric reduction of N-phophinyl α-imino esters: A reaction vial under argon was charged with L-Selectride (0.3 mmol) with THF (2.5 mL). The reaction mixture was then cooled to −78 °C for 10 min. Meanwhile, α-imino ester 3 (0.15 mmol, dissolved in 2.5 mL of THF) was cooled to −78 °C for 10 min. Then the α-imino ester 3 solution was transferred dropwise via a cannula at −78 °C and the reaction was kept at the same temperature for 8 h. The reaction mixture was quenched with saturated aqueous ammonium chloride (4.0 mL) and the organic layer was extracted with dichloromethane. The organic layers were dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure. The crude product was purified using the GAP method or flash column chromatography on silica gel using EtOAc/hexanes (2:1, v/v) as the eluent to afford protected α-amino ester 4.
Supporting Information
| Supporting Information File 1: Experimental details and spectral data. | ||
| Format: PDF | Size: 1.5 MB | Download |
References
-
Skarpos, H.; Vorob’eva, D. V.; Osipov, S. N.; Odinets, I. L.; Breuer, E.; Röschenthaler, G. V. Org. Biomol. Chem. 2006, 4, 3669–3674. doi:10.1039/b607060d
Return to citation in text: [1] -
Gu, C.-L.; Liu, L.; Wang, D.; Chen, Y.-J. J. Org. Chem. 2009, 74, 5754–5757. doi:10.1021/jo900977y
Return to citation in text: [1] -
Collados, J. F.; Toledano, E.; Guijarro, D.; Yus, M. J. Org. Chem. 2012, 77, 5744–5750. doi:10.1021/jo300919x
Return to citation in text: [1] [2] -
Dickstein, J. S.; Kozlowski, M. C. Chem. Soc. Rev. 2008, 37, 1166–1173. doi:10.1039/b709139g
Return to citation in text: [1] -
Taggi, A. E.; Hafez, A. M.; Lectka, T. Acc. Chem. Res. 2003, 36, 10–19. doi:10.1021/ar020137p
Return to citation in text: [1] -
Basra, S.; Fennie, M. W.; Kozlowski, M. C. Org. Lett. 2006, 8, 2659–2662. doi:10.1021/ol0602093
Return to citation in text: [1] -
Kang, Q.; Zhao, Z.-A.; You, S.-L. Org. Lett. 2008, 10, 2031–2034. doi:10.1021/ol800494r
Return to citation in text: [1] -
Yang, C.-F.; Shen, C.; Wang, J.-Y.; Tian, S.-K. Org. Lett. 2012, 14, 3092–3095. doi:10.1021/ol301180z
Return to citation in text: [1] -
Li, Y.; Ji, D.; Xu, M. Org. Biomol. Chem. 2011, 9, 8452–8458. doi:10.1039/c1ob06450a
Return to citation in text: [1] -
Dickstein, J. S.; Fennie, M. W.; Norman, A. L.; Paulose, B. J.; Kozlowski, M. C. J. Am. Chem. Soc. 2008, 130, 15794–15795. doi:10.1021/ja8073006
Return to citation in text: [1] -
Zhang, H.; Mitsumori, S.; Utsumi, N.; Imai, M.; Garcia-Delgado, N.; Mifsud, M.; Albertshofer, K.; Cheong, P. H.; Houk, K. N.; Tanaka, F.; Barbas, C. F. J. Am. Chem. Soc. 2008, 130, 875–886. doi:10.1021/ja074907+
Return to citation in text: [1] -
Córdova, A.; Watanabe, S.; Tanaka, F.; Notz, W.; Barbas, C. F., III. J. Am. Chem. Soc. 2002, 124, 1866–1867. doi:10.1021/ja017833p
Return to citation in text: [1] -
Córdova, A.; Barbas, C. F., III. Tetrahedron Lett. 2003, 44, 1923–1926. doi:10.1016/S0040-4039(03)00019-4
Return to citation in text: [1] -
Chowdari, N. S.; Suri, J. T.; Barbas, C. F., III. Org. Lett. 2004, 6, 2507–2510. doi:10.1021/ol049248+
Return to citation in text: [1] -
Jiang, J.; Ma, X.; Liu, S.; Qian, Y.; Lv, F.; Qiu, L.; Wu, X.; Hu, W. Chem. Commun. 2013, 49, 4238–4240. doi:10.1039/c3cc36972b
Return to citation in text: [1] -
Hernández-Toribio, J.; Padilla, S.; Adrio, J.; Carretero, J. C. Angew. Chem., Int. Ed. 2012, 51, 8854–8858. doi:10.1002/anie.201203828
Return to citation in text: [1] -
Chen, J.; Lu, X.; Lou, W.; Ye, Y.; Jiang, H.; Zeng, W. J. Org. Chem. 2012, 77, 8541–8548. doi:10.1021/jo301423e
Return to citation in text: [1] -
Rueping, M.; Antonchick, A. P.; Brinkmann, C. Angew. Chem., Int. Ed. 2007, 46, 6903–6906. doi:10.1002/anie.200702439
Return to citation in text: [1] -
Jin, S.-S.; Xu, M.-H. Adv. Synth. Catal. 2010, 352, 3136–3140. doi:10.1002/adsc.201000688
Return to citation in text: [1] -
Saaby, S.; Nakama, K.; Lie, M. A.; Hazell, R. G.; Jørgensen, K. A. Chem.–Eur. J. 2003, 9, 6145–6154. doi:10.1002/chem.200305302
Return to citation in text: [1] -
Williams, R. M.; Hendrix, J. A. Chem. Rev. 1992, 92, 889–917. doi:10.1021/cr00013a007
Return to citation in text: [1] -
Mortensen, M.; Husmann, R.; Veri, E.; Bolm, C. Chem. Soc. Rev. 2009, 38, 1002–1010. doi:10.1039/b816769a
Return to citation in text: [1] -
Gross, P. J.; Bräse, S. Chem.–Eur. J. 2010, 16, 12660–12667. doi:10.1002/chem.201001036
Return to citation in text: [1] -
Kricheldorf, H. R. Angew. Chem., Int. Ed. 2006, 45, 5752–5784. doi:10.1002/anie.200600693
Return to citation in text: [1] -
Darbre, T.; Reymond, J.-L. Acc. Chem. Res. 2006, 39, 925–934. doi:10.1021/ar050203y
Return to citation in text: [1] -
Bell, D. S. H. Drugs 2007, 67, 1813–1827. doi:10.2165/00003495-200767130-00002
Return to citation in text: [1] -
Chapman, T. M.; Perry, C. M. Drugs 2004, 64, 2577–2595. doi:10.2165/00003495-200464220-00008
Return to citation in text: [1] -
Simpson, D.; McCormack, P. L.; Keating, G. M.; Lyseng-Williamson, K. A. Drugs 2007, 67, 407–434. doi:10.2165/00003495-200767030-00006
Return to citation in text: [1] -
Li, G.; Liang, Y.; Antilla, J. C. J. Am. Chem. Soc. 2007, 129, 5830–5831. doi:10.1021/ja070519w
Return to citation in text: [1] -
Xue, Z.-Y.; Jiang, Y.; Yuan, W.-C.; Zhang, X.-M. Eur. J. Org. Chem. 2010, 616–619. doi:10.1002/ejoc.200901312
Return to citation in text: [1] -
Bachu, P.; Zhu, C.; Akiyama, T. Tetrahedron Lett. 2013, 54, 3977–3981. doi:10.1016/j.tetlet.2013.05.071
Return to citation in text: [1] -
Kang, Q.; Zhao, Z.-A.; You, S.-L. Adv. Synth. Catal. 2007, 349, 1657–1660. doi:10.1002/adsc.200700235
Return to citation in text: [1] -
Zhu, C.; Akiyama, T. Adv. Synth. Catal. 2010, 352, 1846–1850. doi:10.1002/adsc.201000328
Return to citation in text: [1] -
Zheng, C.; You, S. L. Chem. Soc. Rev. 2012, 41, 2498–2518. doi:10.1039/c1cs15268h
Return to citation in text: [1] -
Reeping, M.; Dufour, J.; Schoepke, F. R. Green Chem. 2011, 13, 1084–1105. doi:10.1039/c1gc15027h
Return to citation in text: [1] -
Shang, G.; Yang, Q.; Zhang, X. Angew. Chem., Int. Ed. 2006, 45, 6360–6362. doi:10.1002/anie.200601540
Return to citation in text: [1] -
Colyer, J. T.; Andersen, N. G.; Tedrow, J. S.; Soukup, T. S.; Faul, M. M. J. Org. Chem. 2006, 71, 6859–6862. doi:10.1021/jo0609834
Return to citation in text: [1] -
Reddy, L. R.; Gupta, A. P.; Liu, Y. J. Org. Chem. 2011, 76, 3409–3415. doi:10.1021/jo200401a
Return to citation in text: [1] -
Guizzetti, S.; Benaglia, M.; Rossi, S. Org. Lett. 2009, 11, 2928–2931. doi:10.1021/ol900945h
Return to citation in text: [1] -
Han, J.; Ai, T.; Li, G. Synthesis 2008, 2519–2526. doi:10.1055/s-2008-1067189
Return to citation in text: [1] [2] -
Han, J.; Ai, T.; Nguyen, T.; Li, G. Chem. Biol. Drug Des. 2008, 72, 120–126. doi:10.1111/j.1747-0285.2008.00682.x
Return to citation in text: [1] [2] -
Kattamuri, P. V.; Xiong, Y.; Pan, Y.; Li, G. Org. Biomol. Chem. 2013, 11, 3400–3408. doi:10.1039/c3ob40251g
Return to citation in text: [1] -
Pindi, S.; Kaur, P.; Shakya, G.; Li, G. Chem. Biol. Drug Des. 2011, 77, 20–29. doi:10.1111/j.1747-0285.2010.01047.x
Return to citation in text: [1] -
Sun, H.; Rajele, T.; Pan, Y.; Li, G. Tetrahedron Lett. 2010, 51, 4403–4407. doi:10.1016/j.tetlet.2010.06.072
Return to citation in text: [1] -
Robak, M. T.; Herbage, M. A.; Ellman, J. A. Chem. Rev. 2010, 110, 3600–3740. doi:10.1021/cr900382t
Return to citation in text: [1] -
Xie, C.; Mei, H.; Wu, L.; Soloshonok, V. A.; Han, J. L.; Pan, Y. RSC Adv. 2014, 4, 4763–4768. doi:10.1039/c3ra45773g
Return to citation in text: [1] -
Mei, H.; Xie, C.; Wu, L.; Soloshonok, V. A.; Han, J.; Pan, Y. Org. Biomol. Chem. 2013, 11, 8018–8021. doi:10.1039/c3ob41785a
Return to citation in text: [1] -
Mei, H.; Xiong, Y.; Han, J.; Pan, Y. Org. Biomol. Chem. 2011, 9, 1402–1406. doi:10.1039/c0ob00586j
Return to citation in text: [1] -
Pindi, S.; Wu, J.; Li, G. J. Org. Chem. 2013, 78, 4006–4012. doi:10.1021/jo400354r
Return to citation in text: [1] [2] -
Kattamuri, P. V.; Ai, T.; Pindi, S.; Sun, Y.; Gu, P.; Shi, M.; Li, G. J. Org. Chem. 2011, 76, 2792–2797. doi:10.1021/jo200070d
Return to citation in text: [1] [2] -
Kaur, P.; Wever, W.; Pindi, S.; Milles, R.; Gu, P.; Shi, M.; Li, G. G. Green Chem. 2011, 13, 1288–1292. doi:10.1039/c1gc15029d
Return to citation in text: [1] [2] -
Xiong, Y.; Mei, H.; Xie, C.; Han, J.; Li, G.; Pan, Y. RSC Adv. 2013, 3, 15820–15826. doi:10.1039/c3ra42927j
Return to citation in text: [1] -
Kaur, P.; Nguyen, T.; Li, G. Eur. J. Org. Chem. 2009, 912–916. doi:10.1002/ejoc.200801061
Return to citation in text: [1] -
Chen, Z. X.; Ai, T.; Kaur, P.; Li, G. G. Tetrahedron Lett. 2009, 50, 1079–1081. doi:10.1016/j.tetlet.2008.12.093
Return to citation in text: [1] -
Qian, Y.; Jing, C.; Zhai, C.; Hu, W.-h. Adv. Synth. Catal. 2012, 354, 301–307. doi:10.1002/adsc.201100615
Return to citation in text: [1] -
Masumoto, S.; Usuda, H.; Suzuki, M.; Kanai, M.; Shibasaki, M. J. Am. Chem. Soc. 2003, 125, 5634–5635. doi:10.1021/ja034980+
Return to citation in text: [1] -
Xu, B.; Zhu, S.-F.; Xie, X.-L.; Shen, J.-J.; Zhou, Q.-L. Angew. Chem., Int. Ed. 2011, 50, 11483–11486. doi:10.1002/anie.201105485
Return to citation in text: [1]
| 1. | Skarpos, H.; Vorob’eva, D. V.; Osipov, S. N.; Odinets, I. L.; Breuer, E.; Röschenthaler, G. V. Org. Biomol. Chem. 2006, 4, 3669–3674. doi:10.1039/b607060d |
| 2. | Gu, C.-L.; Liu, L.; Wang, D.; Chen, Y.-J. J. Org. Chem. 2009, 74, 5754–5757. doi:10.1021/jo900977y |
| 3. | Collados, J. F.; Toledano, E.; Guijarro, D.; Yus, M. J. Org. Chem. 2012, 77, 5744–5750. doi:10.1021/jo300919x |
| 15. | Jiang, J.; Ma, X.; Liu, S.; Qian, Y.; Lv, F.; Qiu, L.; Wu, X.; Hu, W. Chem. Commun. 2013, 49, 4238–4240. doi:10.1039/c3cc36972b |
| 31. | Bachu, P.; Zhu, C.; Akiyama, T. Tetrahedron Lett. 2013, 54, 3977–3981. doi:10.1016/j.tetlet.2013.05.071 |
| 32. | Kang, Q.; Zhao, Z.-A.; You, S.-L. Adv. Synth. Catal. 2007, 349, 1657–1660. doi:10.1002/adsc.200700235 |
| 33. | Zhu, C.; Akiyama, T. Adv. Synth. Catal. 2010, 352, 1846–1850. doi:10.1002/adsc.201000328 |
| 34. | Zheng, C.; You, S. L. Chem. Soc. Rev. 2012, 41, 2498–2518. doi:10.1039/c1cs15268h |
| 35. | Reeping, M.; Dufour, J.; Schoepke, F. R. Green Chem. 2011, 13, 1084–1105. doi:10.1039/c1gc15027h |
| 11. | Zhang, H.; Mitsumori, S.; Utsumi, N.; Imai, M.; Garcia-Delgado, N.; Mifsud, M.; Albertshofer, K.; Cheong, P. H.; Houk, K. N.; Tanaka, F.; Barbas, C. F. J. Am. Chem. Soc. 2008, 130, 875–886. doi:10.1021/ja074907+ |
| 12. | Córdova, A.; Watanabe, S.; Tanaka, F.; Notz, W.; Barbas, C. F., III. J. Am. Chem. Soc. 2002, 124, 1866–1867. doi:10.1021/ja017833p |
| 13. | Córdova, A.; Barbas, C. F., III. Tetrahedron Lett. 2003, 44, 1923–1926. doi:10.1016/S0040-4039(03)00019-4 |
| 14. | Chowdari, N. S.; Suri, J. T.; Barbas, C. F., III. Org. Lett. 2004, 6, 2507–2510. doi:10.1021/ol049248+ |
| 36. | Shang, G.; Yang, Q.; Zhang, X. Angew. Chem., Int. Ed. 2006, 45, 6360–6362. doi:10.1002/anie.200601540 |
| 6. | Basra, S.; Fennie, M. W.; Kozlowski, M. C. Org. Lett. 2006, 8, 2659–2662. doi:10.1021/ol0602093 |
| 7. | Kang, Q.; Zhao, Z.-A.; You, S.-L. Org. Lett. 2008, 10, 2031–2034. doi:10.1021/ol800494r |
| 8. | Yang, C.-F.; Shen, C.; Wang, J.-Y.; Tian, S.-K. Org. Lett. 2012, 14, 3092–3095. doi:10.1021/ol301180z |
| 9. | Li, Y.; Ji, D.; Xu, M. Org. Biomol. Chem. 2011, 9, 8452–8458. doi:10.1039/c1ob06450a |
| 10. | Dickstein, J. S.; Fennie, M. W.; Norman, A. L.; Paulose, B. J.; Kozlowski, M. C. J. Am. Chem. Soc. 2008, 130, 15794–15795. doi:10.1021/ja8073006 |
| 24. | Kricheldorf, H. R. Angew. Chem., Int. Ed. 2006, 45, 5752–5784. doi:10.1002/anie.200600693 |
| 25. | Darbre, T.; Reymond, J.-L. Acc. Chem. Res. 2006, 39, 925–934. doi:10.1021/ar050203y |
| 26. | Bell, D. S. H. Drugs 2007, 67, 1813–1827. doi:10.2165/00003495-200767130-00002 |
| 27. | Chapman, T. M.; Perry, C. M. Drugs 2004, 64, 2577–2595. doi:10.2165/00003495-200464220-00008 |
| 28. | Simpson, D.; McCormack, P. L.; Keating, G. M.; Lyseng-Williamson, K. A. Drugs 2007, 67, 407–434. doi:10.2165/00003495-200767030-00006 |
| 4. | Dickstein, J. S.; Kozlowski, M. C. Chem. Soc. Rev. 2008, 37, 1166–1173. doi:10.1039/b709139g |
| 5. | Taggi, A. E.; Hafez, A. M.; Lectka, T. Acc. Chem. Res. 2003, 36, 10–19. doi:10.1021/ar020137p |
| 29. | Li, G.; Liang, Y.; Antilla, J. C. J. Am. Chem. Soc. 2007, 129, 5830–5831. doi:10.1021/ja070519w |
| 30. | Xue, Z.-Y.; Jiang, Y.; Yuan, W.-C.; Zhang, X.-M. Eur. J. Org. Chem. 2010, 616–619. doi:10.1002/ejoc.200901312 |
| 19. | Jin, S.-S.; Xu, M.-H. Adv. Synth. Catal. 2010, 352, 3136–3140. doi:10.1002/adsc.201000688 |
| 21. | Williams, R. M.; Hendrix, J. A. Chem. Rev. 1992, 92, 889–917. doi:10.1021/cr00013a007 |
| 22. | Mortensen, M.; Husmann, R.; Veri, E.; Bolm, C. Chem. Soc. Rev. 2009, 38, 1002–1010. doi:10.1039/b816769a |
| 18. | Rueping, M.; Antonchick, A. P.; Brinkmann, C. Angew. Chem., Int. Ed. 2007, 46, 6903–6906. doi:10.1002/anie.200702439 |
| 23. | Gross, P. J.; Bräse, S. Chem.–Eur. J. 2010, 16, 12660–12667. doi:10.1002/chem.201001036 |
| 17. | Chen, J.; Lu, X.; Lou, W.; Ye, Y.; Jiang, H.; Zeng, W. J. Org. Chem. 2012, 77, 8541–8548. doi:10.1021/jo301423e |
| 16. | Hernández-Toribio, J.; Padilla, S.; Adrio, J.; Carretero, J. C. Angew. Chem., Int. Ed. 2012, 51, 8854–8858. doi:10.1002/anie.201203828 |
| 20. | Saaby, S.; Nakama, K.; Lie, M. A.; Hazell, R. G.; Jørgensen, K. A. Chem.–Eur. J. 2003, 9, 6145–6154. doi:10.1002/chem.200305302 |
| 45. | Robak, M. T.; Herbage, M. A.; Ellman, J. A. Chem. Rev. 2010, 110, 3600–3740. doi:10.1021/cr900382t |
| 46. | Xie, C.; Mei, H.; Wu, L.; Soloshonok, V. A.; Han, J. L.; Pan, Y. RSC Adv. 2014, 4, 4763–4768. doi:10.1039/c3ra45773g |
| 47. | Mei, H.; Xie, C.; Wu, L.; Soloshonok, V. A.; Han, J.; Pan, Y. Org. Biomol. Chem. 2013, 11, 8018–8021. doi:10.1039/c3ob41785a |
| 48. | Mei, H.; Xiong, Y.; Han, J.; Pan, Y. Org. Biomol. Chem. 2011, 9, 1402–1406. doi:10.1039/c0ob00586j |
| 37. | Colyer, J. T.; Andersen, N. G.; Tedrow, J. S.; Soukup, T. S.; Faul, M. M. J. Org. Chem. 2006, 71, 6859–6862. doi:10.1021/jo0609834 |
| 38. | Reddy, L. R.; Gupta, A. P.; Liu, Y. J. Org. Chem. 2011, 76, 3409–3415. doi:10.1021/jo200401a |
| 39. | Guizzetti, S.; Benaglia, M.; Rossi, S. Org. Lett. 2009, 11, 2928–2931. doi:10.1021/ol900945h |
| 40. | Han, J.; Ai, T.; Li, G. Synthesis 2008, 2519–2526. doi:10.1055/s-2008-1067189 |
| 41. | Han, J.; Ai, T.; Nguyen, T.; Li, G. Chem. Biol. Drug Des. 2008, 72, 120–126. doi:10.1111/j.1747-0285.2008.00682.x |
| 42. | Kattamuri, P. V.; Xiong, Y.; Pan, Y.; Li, G. Org. Biomol. Chem. 2013, 11, 3400–3408. doi:10.1039/c3ob40251g |
| 43. | Pindi, S.; Kaur, P.; Shakya, G.; Li, G. Chem. Biol. Drug Des. 2011, 77, 20–29. doi:10.1111/j.1747-0285.2010.01047.x |
| 44. | Sun, H.; Rajele, T.; Pan, Y.; Li, G. Tetrahedron Lett. 2010, 51, 4403–4407. doi:10.1016/j.tetlet.2010.06.072 |
| 49. | Pindi, S.; Wu, J.; Li, G. J. Org. Chem. 2013, 78, 4006–4012. doi:10.1021/jo400354r |
| 50. | Kattamuri, P. V.; Ai, T.; Pindi, S.; Sun, Y.; Gu, P.; Shi, M.; Li, G. J. Org. Chem. 2011, 76, 2792–2797. doi:10.1021/jo200070d |
| 51. | Kaur, P.; Wever, W.; Pindi, S.; Milles, R.; Gu, P.; Shi, M.; Li, G. G. Green Chem. 2011, 13, 1288–1292. doi:10.1039/c1gc15029d |
| 57. | Xu, B.; Zhu, S.-F.; Xie, X.-L.; Shen, J.-J.; Zhou, Q.-L. Angew. Chem., Int. Ed. 2011, 50, 11483–11486. doi:10.1002/anie.201105485 |
| 3. | Collados, J. F.; Toledano, E.; Guijarro, D.; Yus, M. J. Org. Chem. 2012, 77, 5744–5750. doi:10.1021/jo300919x |
| 55. | Qian, Y.; Jing, C.; Zhai, C.; Hu, W.-h. Adv. Synth. Catal. 2012, 354, 301–307. doi:10.1002/adsc.201100615 |
| 56. | Masumoto, S.; Usuda, H.; Suzuki, M.; Kanai, M.; Shibasaki, M. J. Am. Chem. Soc. 2003, 125, 5634–5635. doi:10.1021/ja034980+ |
| 40. | Han, J.; Ai, T.; Li, G. Synthesis 2008, 2519–2526. doi:10.1055/s-2008-1067189 |
| 41. | Han, J.; Ai, T.; Nguyen, T.; Li, G. Chem. Biol. Drug Des. 2008, 72, 120–126. doi:10.1111/j.1747-0285.2008.00682.x |
| 49. | Pindi, S.; Wu, J.; Li, G. J. Org. Chem. 2013, 78, 4006–4012. doi:10.1021/jo400354r |
| 50. | Kattamuri, P. V.; Ai, T.; Pindi, S.; Sun, Y.; Gu, P.; Shi, M.; Li, G. J. Org. Chem. 2011, 76, 2792–2797. doi:10.1021/jo200070d |
| 51. | Kaur, P.; Wever, W.; Pindi, S.; Milles, R.; Gu, P.; Shi, M.; Li, G. G. Green Chem. 2011, 13, 1288–1292. doi:10.1039/c1gc15029d |
| 52. | Xiong, Y.; Mei, H.; Xie, C.; Han, J.; Li, G.; Pan, Y. RSC Adv. 2013, 3, 15820–15826. doi:10.1039/c3ra42927j |
| 53. | Kaur, P.; Nguyen, T.; Li, G. Eur. J. Org. Chem. 2009, 912–916. doi:10.1002/ejoc.200801061 |
| 54. | Chen, Z. X.; Ai, T.; Kaur, P.; Li, G. G. Tetrahedron Lett. 2009, 50, 1079–1081. doi:10.1016/j.tetlet.2008.12.093 |
© 2014 Xiong et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)