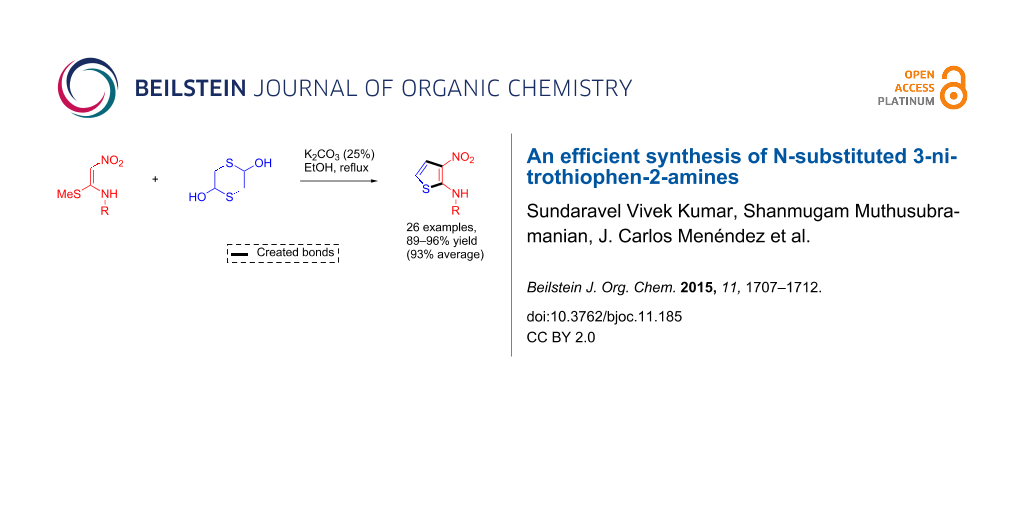
Abstract
A novel protocol for the synthesis of 3-nitro-N-aryl/alkylthiophen-2-amines in good yields from the reaction of α-nitroketene N,S-aryl/alkylaminoacetals and 1,4-dithiane-2,5-diol in the presence of K2CO3 in refluxing ethanol is described. This transformation generates two C–C bonds in a single operation and presumably proceeds through a reaction sequence comprising 2-mercaptoacetaldehyde generation, nucleophilic carbonyl addition, annelation and elimination steps.
Graphical Abstract
Introduction
Thiophenes constitute an important class of heterocyclic compounds, and are embedded in natural [1] and unnatural [2] organic molecules with diverse biological activities. Among them, many 2-aminothiophenes have reached the market or have undergone extensive pharmacological studies. Representative examples are the neuroleptic olanzapine [3], the 2A3BT family of allosteric modulators of the A1 adenosine receptor, derived from 2-amino-3-benzoylthiophene and compounds such as PD-81,723 and T-62 [4], the non-steroidal anti-inflammatory tinoridine [5] and ranelic acid, whose strontium salt is used as a medication for osteoporosis [6] (Figure 1). Other interesting properties identified in 2-aminothiophene derivatives include GluR6 antagonism [7], antiviral and antitumor activities [8-10], inhibition of p53-MDM2 interactions [11] and protein-tyrosine phosphatase 1B inhibition [12]. Furthermore, 2-aminothiophenes have potential applications in the field of functional materials such as liquid crystals [13], nonlinear optical materials (NLOs) [14], organic solar cells [15], photonic polymers [16] and electroluminescent materials [17]. Nitrothiophenes are also important as chemotherapeutic agents and in a few cases both functional groups are combined in the same molecule. For instance, compound I (Figure 1) is an antitubercular agent that acts by releasing nitric oxide following activation by F420-dependent nitroreductase DDN [18]. However, the pharmacological study of this class of compounds is hampered by limitations in existing synthetic methodologies, particularly in the direct preparation of N-substituted amino derivatives.
Figure 1: Selected examples of biologically active 2-aminothiophene derivatives.
Figure 1: Selected examples of biologically active 2-aminothiophene derivatives.
2-Aminothiophenes have traditionally been synthesized from mercapto- or halogen-substituted thiophenes through nucleophilic displacements [19]. More recently, these derivatives have been assembled from acyclic starting materials by methods such as i) reaction of lithiated 1-alkynes or allenes with substituted phenyl isothiocyanates [20], ii) treatment of N-allylbenzotriazole with substituted phenylisothiocyanates [21] and iii) reaction between phenyl isothiocyanate and electron-deficient allenes catalyzed by PPh3 [22] (Scheme 1a–c). On the other hand, studies on the synthesis of 3-nitrothiophenes are scarce. One of the traditional methods involves electrophilic aromatic substitution reactions of thiophenes, which introduces substituents at the 2- and 5-positions, but with some drawbacks associated with the lack of regioselectivity and difficult isomer separation [23]. Other methods for the synthesis of 2,3-disubsituted thiophenes include the reactions of (i) 1,4-dithiane-2,5-diol with nitroalkenes [24], activated nitriles [25] and cyanoacetamide [26]; (ii) 3-nitrothiophene with Grignard reagents [27] and (iii) 3-bromo-2-silylthiophene with aldehydes [28]. The major drawback of these methods is that none of them allow the simultaneous introduction of 2-amino and 3-nitro substituents (see Scheme 1d for a representative example).
Scheme 1: Some strategies for the synthesis of 2-aryl/alkylaminothiophenes and 3-nitrothiophenes.
Scheme 1: Some strategies for the synthesis of 2-aryl/alkylaminothiophenes and 3-nitrothiophenes.
Multiple bond-forming transformations [29-31] are very attractive because of the high synthetic efficiency associated to the formation of several bonds in a single operation. The corresponding reduction in the number of isolation and purification steps and hence in the use of organic solvents and chromatographic stationary phases helps to achieve one of the main goals of green chemistry [32]. In this context, we wish to report herein a new and efficient synthesis of 3-nitro-N-aryl/alkylthiophen-2-amines based on domino reactions between α-nitroketene N,S-aryl/alkylaminoacetals and 1,4-dithiane-2,5-diol, the dimer of 2-mercaptoacetaldehyde (Scheme 2). This work emerges as a continuation of our recent efforts directed towards the construction of biologically relevant heterocycles employing multiple bond-forming transformations [33-40].
Scheme 2: Our plan for the synthesis of N-substituted 3-nitrothiophen-2-amines.
Scheme 2: Our plan for the synthesis of N-substituted 3-nitrothiophen-2-amines.
Results and Discussion
In a preparatory study, the optimization of the reaction between (E)-4-methyl-N-(1-(methylthio)-2-nitrovinyl)aniline (1e, 1 mmol) and 1,4-dithiane-2,5-diol (2, 0.5 mmol, 1 equiv) was performed. Initially, this model reaction was attempted in ethanol and in the absence of base, with negative results (Table 1, entry 1). In the presence of TEA (1 equiv), it gave 62% yield (Table 1, entry 2), which was increased to 88% when the reaction was performed in refluxing ethanol for 25 min (Table 1, entry 3). In both cases, work-up had the advantage of being very simple. After completion of the reaction, the mixture was poured into water and the precipitated solid was filtered and washed with ethanol to give the pure 3-nitro-N-(p-tolyl)thiophen-2-amine (3e) without the need for chromatography. Then the model reaction was further investigated by employing alternative bases such as 1,4-diazabicyclo[2.2.2]octane (DABCO, Table 1, entry 4), 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU, Table 1, entry 5), pyridine (Table 1, entry 6), N,N-dimethylaminopyridine (DMAP, Table 1, entry 7), piperidine (Table 1, entry 8), L-proline (Table 1, entry 9), potassium carbonate (Table 1, entry 10), sodium carbonate (Table 1, entry 11) and caesium carbonate (Table 1, entry 12). After identifying potassium carbonate as the optimal base in refluxing ethanol (93% yield), alternative solvents such as methanol (Table 1, entry 13), N,N-dimethylformamide (Table 1, entry 14), acetonitrile (Table 1, entry 15), tetrahydrofuran (Table 1, entry 16), 1,4-dioxane (Table 1, entry 17) and water (Table 1, entry 18) were examined, without affording additional improvements. Finally, the use of substoichiometric amounts of base was examined (Table 1, entries 19–21), leading to the conclusion that 0.25 equivalents of potassium carbonate were sufficient to promote the desired transformation, but lower amounts led to longer reaction times and diminished yields (Table 1, entry 22). All reactions included in Table 1 were performed under heating at reflux temperature of the respective solvents used, except reactions corresponding to entries 1 and 2, which were performed at room temperature.
Table 1: Optimization of the synthesis of compound 3ea.
|
|
||||
| Entry | Base (1 equiv) | Solvent | Time, min | Yield, %b |
|---|---|---|---|---|
| 1 | – | EtOH | 60 | 0b,c |
| 2 | TEA | EtOH | 180 | 62b |
| 3 | TEA | EtOH | 25 | 88 |
| 4 | DABCO | EtOH | 25 | 76 |
| 5 | DBU | EtOH | 25 | 64 |
| 6 | pyridine | EtOH | 30 | 0 |
| 7 | DMAP | EtOH | 25 | 44 |
| 8 | piperidine | EtOH | 30 | 0 |
| 9 | L-proline | EtOH | 30 | 0 |
| 10 | K2CO3 | EtOH | 25 | 93 |
| 11 | Na2CO3 | EtOH | 25 | 83 |
| 12 | Cs2CO3 | EtOH | 25 | 90 |
| 13 | K2CO3 | MeOH | 25 | 88 |
| 14 | K2CO3 | DMF | 25 | 86 |
| 15 | K2CO3 | CH3CN | 30 | 53 |
| 16 | K2CO3 | THF | 30 | trace |
| 17 | K2CO3 | 1,4-dioxane | 30 | 22 |
| 18 | K2CO3 | water | 30 | 48 |
| 19 | K2CO3d | EtOH | 25 | 94 |
| 20 | K2CO3e | EtOH | 25 | 93 |
| 21 | K2CO3f | EtOH | 25 | 94 |
| 22 | K2CO3g | EtOH | 30 | 88 |
aAll reactions were performed with a mixture of 1e (1 mmol) and 2 (0.5 mmol). bIsolated yield after washing with cold ethanol. cReaction performed at room temperature. d75 mol %. e50 mol %. f25 mol %. g20 mol %.
The scope and generality of this transformation was investigated by examining the reaction between a variety of α-nitroketene N,S-anilinoacetals 1 with 1,4-dithiane-2,5-diol (2, Scheme 3 and Table 2). The starting materials 1 were readily available by refluxing in ethanol the suitable primary amine and 1,1-bis(methylthio)-2-nitroethylene. Regarding aryl substituents, the thiophene synthesis was compatible with the presence of unsubstituted phenyl rings (compound 3e) and phenyl substituents bearing moderately electron-withdrawing groups such as m- and p-halogens and m-trifluoroalkyl substituents (compounds 3a–d and 3l–n), although substrates 1 with aryl rings bearing strongly electron-withdrawing groups such as p-nitro, p-cyano and p-trifluoromethyl failed to afford the final products. Furthermore, it was gratifying to observe that our method was compatible with the presence of electron-releasing substituents at the aromatic ring, including alkyl (compounds 3f–h, 3j, 3o and 3q) and alkoxy (compounds 3i, 3k and 3p) substituents, even at the more hindered ortho-positions (3j, 3k, 3q). The preparation of a compound bearing a 1-naphthylamino substituent (compound 3r) also proceeded uneventfully. We also investigated the reactions of α-nitroketene N,S-alkylaminoacetals and 1,4-dithiane-2,5-diol (2). These reactions provided the desired 2-(alkylamino)-3-nitrothiophenes in excellent yields, albeit with the need for longer reaction times. The method allowed the preparation of compounds bearing linear (compounds 3s–u) and α-branched alkyl substituents (compounds 3v, 3x). Benzyl substituents could also be accommodated, as shown by the efficient preparation of 3y and 3z.
Scheme 3: Synthesis of N-substituted 3-nitrothiophen-2-amines.
Scheme 3: Synthesis of N-substituted 3-nitrothiophen-2-amines.
Table 2: Scope of the 2-amino-3-nitrothiophene synthesis.
| Compound | R | Time, min | Yield, % |
|---|---|---|---|
| 3a | 4-FC6H4 | 25 | 90 |
| 3b | 4-ClC6H4 | 25 | 92 |
| 3c | 4-BrC6H4 | 25 | 91 |
| 3d | 4-IC6H4 | 25 | 93 |
| 3e | C6H5 | 24 | 94 |
| 3f | 4-MeC6H4 | 23 | 94 |
| 3g | 4-EtC6H4 | 23 | 95 |
| 3h | 4-iPrC6H4 | 22 | 93 |
| 3i | 4-MeOC6H4 | 20 | 96 |
| 3j | 2-MeC6H4 | 22 | 93 |
| 3k | 2-MeOC6H4 | 25 | 91 |
| 3l | 3-FC6H4 | 24 | 94 |
| 3m | 3-BrC6H4 | 24 | 90 |
| 3n | 3-F3CC6H4 | 25 | 89 |
| 3o | 3-MeC6H4 | 24 | 94 |
| 3p | 3-MeOC6H4 | 22 | 95 |
| 3q | 2,4-Me2C6H3 | 23 | 82 |
| 3r | 1-naphthyl | 25 | 91 |
| 3s | Me | 205 | 93 |
| 3t | n-Pr | 190 | 95 |
| 3u | n-Bu | 190 | 98 |
| 3v | iPr | 205 | 93 |
| 3w | cyclopropyl | 190 | 94 |
| 3x | cyclohexyl | 190 | 95 |
| 3y | Bn | 180 | 92 |
| 3z | (R)-α-MeBn | 180 | 96 |
The structure of the 3-nitro-N-arylthiophen-2-amines 3 was deduced from one- and two-dimensional NMR spectroscopic, microanalytical and mass spectral data, as detailed for 3c as a representative example (see Supporting Information File 1) and confirmed unambiguously by a single-crystal X-ray crystallographic study [41] of 3p (Figure 2).
![[1860-5397-11-185-2]](/bjoc/content/figures/1860-5397-11-185-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: X-ray diffraction study of compound 3p.
Figure 2: X-ray diffraction study of compound 3p.
The transformation leading to compounds 3 presumably proceeds through the initial formation of 2-mercaptoacetaldehyde (4) from the base-promoted decomposition of 1,4-dithiane-2,5-diol (2). This would be followed by a formal [3 + 2] annexation via nucleophilic addition of compounds 1 to the carbonyl of intermediate 4 followed by addition of the thiolate anion onto the iminium functionality thus generated to give 5. Subsequent elimination of methylmercaptan and water furnishes the product 3 (Scheme 4).
Scheme 4: Proposed reaction sequence leading to the formation of 3.
Scheme 4: Proposed reaction sequence leading to the formation of 3.
Conclusion
In conclusion, we have developed a very efficient synthesis of a synthetically and biologically relevant class of push–pull heterocyclic compounds under mild conditions and starting from simple acyclic substrates and catalysts. Previously, these compounds were available only through multistep sequences, most notably one involving as the final step an SNAr reaction with the amine and having the serious limitation of requiring the presence of an additional strong electron-withdrawing group at C-5 [42-44]. Our protocol requires only two steps (including the preparation of starting materials 1) and generates two C–C bonds through a sequence of reactions that comprise up to five individual steps and proceeds in excellent yields (93% in average for 26 examples) and atom economies.
Supporting Information
| Supporting Information File 1: Experimental procedures, characterization data, details of the NMR structural determination of 3c and copies of 1H NMR, 13C NMR and ESI mass spectra of all new compounds 1 and 3. | ||
| Format: PDF | Size: 2.6 MB | Download |
References
-
Koike, K.; Jia, Z.; Nikaib, T.; Liu, Y.; Zhao, Y.; Guo, D. Org. Lett. 1999, 1, 197–198. doi:10.1021/ol9905295
Return to citation in text: [1] -
Fevig, T. L.; Phillips, W. G.; Lau, P. H. J. Org. Chem. 2001, 66, 2493–2497. doi:10.1021/jo001376y
Return to citation in text: [1] -
Meltzer, H. Y.; Fibiger, H. C. Eur. Neuropsychopharmacol. 1996, 14, 83–85. doi:10.1016/0893-133X(95)00197-L
Return to citation in text: [1] -
Valant, C.; Aurelio, L.; Devine, S. M.; Ashton, T. D.; White, J. M.; Sexton, P. M.; Christopoulos, A.; Scammells, P. J. J. Med. Chem. 2012, 55, 2367–2375. doi:10.1021/jm201600e
Return to citation in text: [1] -
Shimada, O.; Yasuda, H. Agents Actions 1986, 19, 208–214. doi:10.1007/BF01966208
Return to citation in text: [1] -
Meunier, P. J.; Roux, C.; Seeman, E.; Ortolani, S.; Badurski, J. E.; Spector, T. D.; Cannata, J.; Balogh, A.; Lemmel, E.-M.; Pors-Nielsen, S.; Rizzoli, R.; Genant, H. K.; Reginster, J.-Y. N. Engl. J. Med. 2004, 350, 459–468. doi:10.1056/NEJMoa022436
Return to citation in text: [1] -
Briel, D.; Rybak, A.; Kronbach, C.; Unverferth, K. Eur. J. Med. Chem. 2010, 45, 69–77. doi:10.1016/j.ejmech.2009.09.025
Return to citation in text: [1] -
Stephens, C. E.; Felder, T. M.; Sowell, J. W., Sr.; Andrei, G.; Balzarini, J.; Snoeck, R.; De Clercq, E. Bioorg. Med. Chem. 2001, 9, 1123–1132. doi:10.1016/S0968-0896(00)00333-3
Return to citation in text: [1] -
Romagnoli, R.; Baraldi, P. G.; Pavani, M. G.; Tabrizi, M. A.; Preti, D.; Fruttarolo, F.; Piccagli, L.; Jung, M. K.; Hamel, E.; Borgatti, M.; Gambari, R. J. Med. Chem. 2006, 49, 3906–3915. doi:10.1021/jm060355e
Return to citation in text: [1] -
Romagnoli, R.; Baraldi, P. G.; Carrion, M. D.; Cara, C. L.; Preti, D.; Fruttarolo, F.; Pavani, M. G.; Tabrizi, M. A.; Tolomeo, M.; Grimaudo, S.; Di Cristina, A.; Balzarini, J.; Hadfield, J. A.; Brancale, A.; Hamel, E. J. Med. Chem. 2007, 50, 2273–2277. doi:10.1021/jm070050f
Return to citation in text: [1] -
Wang, W.; Lv, D.; Qiu, N.; Zhang, L.; Hu, C.; Hu, Y. Bioorg. Med. Chem. 2013, 21, 2886–2894. doi:10.1016/j.bmc.2013.03.070
Return to citation in text: [1] -
Andersen, H. S.; Olsen, O. H.; Iversen, L. F.; Sørensen, A. L. P.; Mortensen, S. B.; Christensen, M. S.; Branner, S.; Hansen, T. K.; Lau, J. F.; Jeppesen, L.; Moran, E. J.; Su, J.; Bakir, F.; Judge, L.; Shahbaz, M.; Collins, T.; Vo, T.; Newman, M. J.; Ripka, W. C.; Møller, N. P. H. J. Med. Chem. 2002, 45, 4443–4459. doi:10.1021/jm0209026
Return to citation in text: [1] -
Romiszewski, J.; Puterová-Tokarová, Z.; Mieczkowski, J.; Gorecka, E. New J. Chem. 2014, 38, 2927–2934. doi:10.1039/c4nj00298a
Return to citation in text: [1] -
Borbone, F.; Caruso, U.; Diana, R.; Panunzi, B.; Roviello, A.; Tingoli, M.; Tuzi, A. Org. Electron. 2009, 10, 53–60. doi:10.1016/j.orgel.2008.10.004
Return to citation in text: [1] -
Stylianakis, M. M.; Mikroyannidis, J. A.; Kymakis, E. Sol. Energy Mater. Sol. Cells 2010, 94, 267–274. doi:10.1016/j.solmat.2009.09.013
Return to citation in text: [1] -
Yuquan, S.; Yuxia, Z.; Zao, L.; Jianghong, W.; Ling, Q.; Shixiong, L.; Jianfeng, Z.; Jiayun, Z. J. Chem. Soc., Perkin Trans. 1 1999, 3691–3695. doi:10.1039/A904945B
Return to citation in text: [1] -
Su, Y. Z.; Lin, J. T.; Tao, Y.-T.; Ko, C.-W.; Lin, S.-C.; Sun, S.-S. Chem. Mater. 2002, 14, 1884–1890. doi:10.1021/cm011671e
Return to citation in text: [1] -
Hartkoorn, R. C.; Ryabova, O. B.; Chiarelli, L. R.; Riccardi, G.; Makarov, V.; Cole, S. T. Antimicrob. Agents Chemother. 2014, 58, 2944–2947. doi:10.1128/AAC.02693-13
Return to citation in text: [1] -
King, F. D.; Walton, D. R. M. J. Chem. Soc., Chem. Commun. 1974, 256–257. doi:10.1039/c39740000256
Return to citation in text: [1] -
Tarasova, O. A.; Klyba, L. V.; Vvedensky, V. Yu.; Nedolya, N. A.; Trofimov, B. A.; Brandsma, L.; Verkruijsse, H. D. Eur. J. Org. Chem. 1998, 253–256. doi:10.1002/(SICI)1099-0690(199802)1998:2<253::AID-EJOC253>3.0.CO;2-E
Return to citation in text: [1] -
Katritzky, A. R.; Wang, X.; Denisenko, A. J. Org. Chem. 2001, 66, 2850–2853. doi:10.1021/jo001159x
Return to citation in text: [1] -
Virieux, D.; Guillouzic, A.-F.; Cristau, H.-J. Heteroat. Chem. 2007, 18, 312–315. doi:10.1002/hc.20300
Return to citation in text: [1] -
Gronowitz, S.; Hörnfeldt, A.-B. Thiophenes; Elsevier Academic Press: San Diego, CA, 2004.
Return to citation in text: [1] -
O’ Connor, C. J.; Roydhouse, M. D.; Przybył, A. M.; Wall, M. D.; Southern, J. M. J. Org. Chem. 2010, 75, 2534–2538. doi:10.1021/jo902656y
Return to citation in text: [1] -
Ma, L.; Yuan, L.; Xu, C.; Li, G.; Tao, M.; Zhang, W. Synthesis 2013, 45, 45–52. doi:10.1055/s-0032-1316821
Return to citation in text: [1] -
Wang, K.; Nguyen, K.; Huang, Y.; Dömling, A. J. Comb. Chem. 2009, 11, 920–927. doi:10.1021/cc9000778
Return to citation in text: [1] -
Blatt, A.; Bach, S.; Kresch, L. J. Org. Chem. 1957, 22, 1693–1695. doi:10.1021/jo01363a604
Return to citation in text: [1] -
Devarie-Baez, N. O.; Shuhler, B. J.; Wang, H.; Xian, M. Org. Lett. 2007, 9, 4655–4658. doi:10.1021/ol702149c
Return to citation in text: [1] -
Coquerel, Y.; Boddaert, T.; Presset, M.; Mailhol, D.; Rodriguez, J. Multiple Bond-Forming Transformations: The Key Concept toward Eco-Compatible Synthetic Organic Chemistry. In Ideas in Chemistry and Molecular Sciences: Advances in synthetic chemistry; Pignataro, B., Ed.; Wiley-VCH: Weinheim, 2010; Vol. 1, pp 187–202. doi:10.1002/9783527630554.ch9
Return to citation in text: [1] -
Menéndez, J. C. Curr. Org. Chem. 2013, 17, 1919–2064. doi:10.2174/1385272811317180002
Return to citation in text: [1] -
Rodriguez, J.; Bonne, D., Eds. Stereoselective multiple bond-forming transformations in organic synthesis; John Wiley and Sons, Inc., 2015. doi:10.1002/9781119006220
Return to citation in text: [1] -
Cioc, R. C.; Ruijter, E.; Orru, R. V. A. Green Chem. 2014, 16, 2958–2975. doi:10.1039/c4gc00013g
Return to citation in text: [1] -
Indumathi, S.; Perumal, S.; Menéndez, J. C. J. Org. Chem. 2010, 75, 472–475. doi:10.1021/jo9021327
Return to citation in text: [1] -
Prasanna, P.; Balamurugan, K.; Perumal, S.; Menéndez, J. C. Green Chem. 2011, 13, 2123–2129. doi:10.1039/c0gc00952k
Return to citation in text: [1] -
Gunasekaran, P.; Balamurugan, K.; Sivakumar, S.; Perumal, S.; Menéndez, J. C.; Almansour, A. I. Green Chem. 2012, 14, 750–757. doi:10.1039/c2gc16517a
Return to citation in text: [1] -
Gunasekaran, P.; Indumathi, S.; Perumal, S. RSC Adv. 2013, 3, 8318–8325. doi:10.1039/c3ra00136a
Return to citation in text: [1] -
Muthusaravanan, S.; Sasikumar, C.; Bala, B. D.; Perumal, S. Green Chem. 2014, 16, 1297–1304. doi:10.1039/C3GC42150C
Return to citation in text: [1] -
Vivek Kumar, S.; Prasanna, P.; Perumal, S. Tetrahedron Lett. 2013, 54, 6651–6655. doi:10.1016/j.tetlet.2013.09.123
Return to citation in text: [1] -
Muthusaravanan, S.; Bala, B. D.; Perumal, S. Tetrahedron Lett. 2013, 54, 5302–5306. doi:10.1016/j.tetlet.2013.07.091
Return to citation in text: [1] -
Gunasekaran, P.; Perumal, S.; Menéndez, J. C.; Mancinelli, M.; Ranieri, S.; Mazzanti, A. J. Org. Chem. 2014, 79, 11039–11050. doi:10.1021/jo502047n
Return to citation in text: [1] -
Cambridge Crystallographic Data Centre (CCDC) reference for compound 3p: 1000701.
Return to citation in text: [1] -
Consiglio, G.; Frenna, V.; Guernelli, S.; Macaluso, G. ARKIVOC 2002, No. xi, 104–117.
Return to citation in text: [1] -
Morley, J. O.; Matthews, T. P. Org. Biomol. Chem. 2006, 4, 359–366. doi:10.1039/B514441H
Return to citation in text: [1] -
Sarkar, P.; Maiti, S.; Ghosh, K.; Sengupta (Bandyopadhyay), S.; Butcher, R. J.; Mukhopadhyay, C. Tetrahedron Lett. 2014, 55, 996–1001. doi:10.1016/j.tetlet.2013.12.068
Return to citation in text: [1]
| 32. | Cioc, R. C.; Ruijter, E.; Orru, R. V. A. Green Chem. 2014, 16, 2958–2975. doi:10.1039/c4gc00013g |
| 33. | Indumathi, S.; Perumal, S.; Menéndez, J. C. J. Org. Chem. 2010, 75, 472–475. doi:10.1021/jo9021327 |
| 34. | Prasanna, P.; Balamurugan, K.; Perumal, S.; Menéndez, J. C. Green Chem. 2011, 13, 2123–2129. doi:10.1039/c0gc00952k |
| 35. | Gunasekaran, P.; Balamurugan, K.; Sivakumar, S.; Perumal, S.; Menéndez, J. C.; Almansour, A. I. Green Chem. 2012, 14, 750–757. doi:10.1039/c2gc16517a |
| 36. | Gunasekaran, P.; Indumathi, S.; Perumal, S. RSC Adv. 2013, 3, 8318–8325. doi:10.1039/c3ra00136a |
| 37. | Muthusaravanan, S.; Sasikumar, C.; Bala, B. D.; Perumal, S. Green Chem. 2014, 16, 1297–1304. doi:10.1039/C3GC42150C |
| 38. | Vivek Kumar, S.; Prasanna, P.; Perumal, S. Tetrahedron Lett. 2013, 54, 6651–6655. doi:10.1016/j.tetlet.2013.09.123 |
| 39. | Muthusaravanan, S.; Bala, B. D.; Perumal, S. Tetrahedron Lett. 2013, 54, 5302–5306. doi:10.1016/j.tetlet.2013.07.091 |
| 40. | Gunasekaran, P.; Perumal, S.; Menéndez, J. C.; Mancinelli, M.; Ranieri, S.; Mazzanti, A. J. Org. Chem. 2014, 79, 11039–11050. doi:10.1021/jo502047n |
| 41. | Cambridge Crystallographic Data Centre (CCDC) reference for compound 3p: 1000701. |
| 1. | Koike, K.; Jia, Z.; Nikaib, T.; Liu, Y.; Zhao, Y.; Guo, D. Org. Lett. 1999, 1, 197–198. doi:10.1021/ol9905295 |
| 5. | Shimada, O.; Yasuda, H. Agents Actions 1986, 19, 208–214. doi:10.1007/BF01966208 |
| 17. | Su, Y. Z.; Lin, J. T.; Tao, Y.-T.; Ko, C.-W.; Lin, S.-C.; Sun, S.-S. Chem. Mater. 2002, 14, 1884–1890. doi:10.1021/cm011671e |
| 4. | Valant, C.; Aurelio, L.; Devine, S. M.; Ashton, T. D.; White, J. M.; Sexton, P. M.; Christopoulos, A.; Scammells, P. J. J. Med. Chem. 2012, 55, 2367–2375. doi:10.1021/jm201600e |
| 18. | Hartkoorn, R. C.; Ryabova, O. B.; Chiarelli, L. R.; Riccardi, G.; Makarov, V.; Cole, S. T. Antimicrob. Agents Chemother. 2014, 58, 2944–2947. doi:10.1128/AAC.02693-13 |
| 3. | Meltzer, H. Y.; Fibiger, H. C. Eur. Neuropsychopharmacol. 1996, 14, 83–85. doi:10.1016/0893-133X(95)00197-L |
| 15. | Stylianakis, M. M.; Mikroyannidis, J. A.; Kymakis, E. Sol. Energy Mater. Sol. Cells 2010, 94, 267–274. doi:10.1016/j.solmat.2009.09.013 |
| 2. | Fevig, T. L.; Phillips, W. G.; Lau, P. H. J. Org. Chem. 2001, 66, 2493–2497. doi:10.1021/jo001376y |
| 16. | Yuquan, S.; Yuxia, Z.; Zao, L.; Jianghong, W.; Ling, Q.; Shixiong, L.; Jianfeng, Z.; Jiayun, Z. J. Chem. Soc., Perkin Trans. 1 1999, 3691–3695. doi:10.1039/A904945B |
| 11. | Wang, W.; Lv, D.; Qiu, N.; Zhang, L.; Hu, C.; Hu, Y. Bioorg. Med. Chem. 2013, 21, 2886–2894. doi:10.1016/j.bmc.2013.03.070 |
| 13. | Romiszewski, J.; Puterová-Tokarová, Z.; Mieczkowski, J.; Gorecka, E. New J. Chem. 2014, 38, 2927–2934. doi:10.1039/c4nj00298a |
| 8. | Stephens, C. E.; Felder, T. M.; Sowell, J. W., Sr.; Andrei, G.; Balzarini, J.; Snoeck, R.; De Clercq, E. Bioorg. Med. Chem. 2001, 9, 1123–1132. doi:10.1016/S0968-0896(00)00333-3 |
| 9. | Romagnoli, R.; Baraldi, P. G.; Pavani, M. G.; Tabrizi, M. A.; Preti, D.; Fruttarolo, F.; Piccagli, L.; Jung, M. K.; Hamel, E.; Borgatti, M.; Gambari, R. J. Med. Chem. 2006, 49, 3906–3915. doi:10.1021/jm060355e |
| 10. | Romagnoli, R.; Baraldi, P. G.; Carrion, M. D.; Cara, C. L.; Preti, D.; Fruttarolo, F.; Pavani, M. G.; Tabrizi, M. A.; Tolomeo, M.; Grimaudo, S.; Di Cristina, A.; Balzarini, J.; Hadfield, J. A.; Brancale, A.; Hamel, E. J. Med. Chem. 2007, 50, 2273–2277. doi:10.1021/jm070050f |
| 14. | Borbone, F.; Caruso, U.; Diana, R.; Panunzi, B.; Roviello, A.; Tingoli, M.; Tuzi, A. Org. Electron. 2009, 10, 53–60. doi:10.1016/j.orgel.2008.10.004 |
| 7. | Briel, D.; Rybak, A.; Kronbach, C.; Unverferth, K. Eur. J. Med. Chem. 2010, 45, 69–77. doi:10.1016/j.ejmech.2009.09.025 |
| 42. | Consiglio, G.; Frenna, V.; Guernelli, S.; Macaluso, G. ARKIVOC 2002, No. xi, 104–117. |
| 43. | Morley, J. O.; Matthews, T. P. Org. Biomol. Chem. 2006, 4, 359–366. doi:10.1039/B514441H |
| 44. | Sarkar, P.; Maiti, S.; Ghosh, K.; Sengupta (Bandyopadhyay), S.; Butcher, R. J.; Mukhopadhyay, C. Tetrahedron Lett. 2014, 55, 996–1001. doi:10.1016/j.tetlet.2013.12.068 |
| 6. | Meunier, P. J.; Roux, C.; Seeman, E.; Ortolani, S.; Badurski, J. E.; Spector, T. D.; Cannata, J.; Balogh, A.; Lemmel, E.-M.; Pors-Nielsen, S.; Rizzoli, R.; Genant, H. K.; Reginster, J.-Y. N. Engl. J. Med. 2004, 350, 459–468. doi:10.1056/NEJMoa022436 |
| 12. | Andersen, H. S.; Olsen, O. H.; Iversen, L. F.; Sørensen, A. L. P.; Mortensen, S. B.; Christensen, M. S.; Branner, S.; Hansen, T. K.; Lau, J. F.; Jeppesen, L.; Moran, E. J.; Su, J.; Bakir, F.; Judge, L.; Shahbaz, M.; Collins, T.; Vo, T.; Newman, M. J.; Ripka, W. C.; Møller, N. P. H. J. Med. Chem. 2002, 45, 4443–4459. doi:10.1021/jm0209026 |
| 21. | Katritzky, A. R.; Wang, X.; Denisenko, A. J. Org. Chem. 2001, 66, 2850–2853. doi:10.1021/jo001159x |
| 19. | King, F. D.; Walton, D. R. M. J. Chem. Soc., Chem. Commun. 1974, 256–257. doi:10.1039/c39740000256 |
| 20. | Tarasova, O. A.; Klyba, L. V.; Vvedensky, V. Yu.; Nedolya, N. A.; Trofimov, B. A.; Brandsma, L.; Verkruijsse, H. D. Eur. J. Org. Chem. 1998, 253–256. doi:10.1002/(SICI)1099-0690(199802)1998:2<253::AID-EJOC253>3.0.CO;2-E |
| 28. | Devarie-Baez, N. O.; Shuhler, B. J.; Wang, H.; Xian, M. Org. Lett. 2007, 9, 4655–4658. doi:10.1021/ol702149c |
| 29. | Coquerel, Y.; Boddaert, T.; Presset, M.; Mailhol, D.; Rodriguez, J. Multiple Bond-Forming Transformations: The Key Concept toward Eco-Compatible Synthetic Organic Chemistry. In Ideas in Chemistry and Molecular Sciences: Advances in synthetic chemistry; Pignataro, B., Ed.; Wiley-VCH: Weinheim, 2010; Vol. 1, pp 187–202. doi:10.1002/9783527630554.ch9 |
| 30. | Menéndez, J. C. Curr. Org. Chem. 2013, 17, 1919–2064. doi:10.2174/1385272811317180002 |
| 31. | Rodriguez, J.; Bonne, D., Eds. Stereoselective multiple bond-forming transformations in organic synthesis; John Wiley and Sons, Inc., 2015. doi:10.1002/9781119006220 |
| 26. | Wang, K.; Nguyen, K.; Huang, Y.; Dömling, A. J. Comb. Chem. 2009, 11, 920–927. doi:10.1021/cc9000778 |
| 27. | Blatt, A.; Bach, S.; Kresch, L. J. Org. Chem. 1957, 22, 1693–1695. doi:10.1021/jo01363a604 |
| 24. | O’ Connor, C. J.; Roydhouse, M. D.; Przybył, A. M.; Wall, M. D.; Southern, J. M. J. Org. Chem. 2010, 75, 2534–2538. doi:10.1021/jo902656y |
| 25. | Ma, L.; Yuan, L.; Xu, C.; Li, G.; Tao, M.; Zhang, W. Synthesis 2013, 45, 45–52. doi:10.1055/s-0032-1316821 |
| 22. | Virieux, D.; Guillouzic, A.-F.; Cristau, H.-J. Heteroat. Chem. 2007, 18, 312–315. doi:10.1002/hc.20300 |
| 23. | Gronowitz, S.; Hörnfeldt, A.-B. Thiophenes; Elsevier Academic Press: San Diego, CA, 2004. |
© 2015 Vivek Kumar et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)