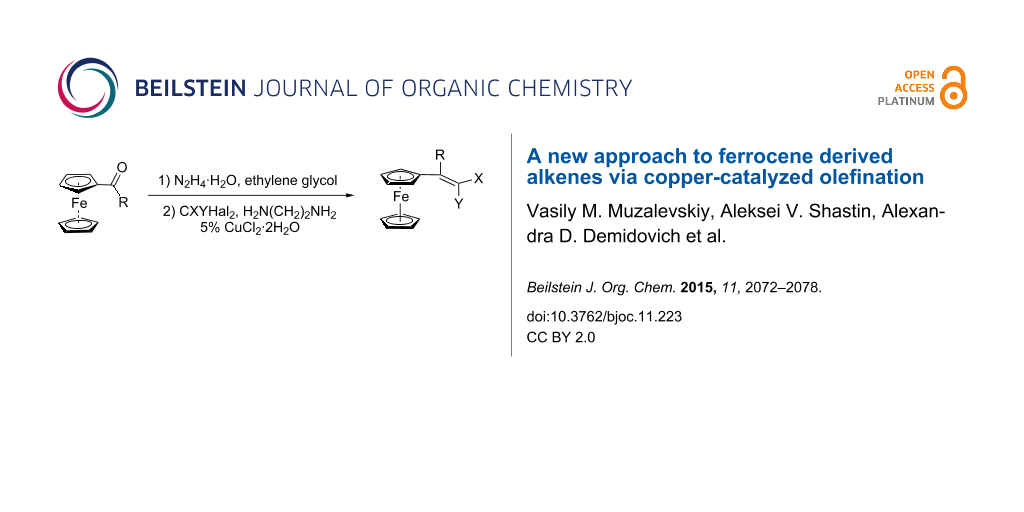
Abstract
A new approach to ferrocenyl haloalkenes and bis-alkenes was elaborated. The key procedure involves copper catalyzed olefination of N-unsubstituted hydrazones, obtained from ferrocene-containing carbonyl compounds and hydrazine, with polyhaloalkanes. The procedure is simple, cheap and could be applied for the utilization of environmentally harmful polyhalocarbons. The cyclic voltammetry study of the representative examples of the synthesized ferrocenyl alkenes shows the strong dependence of the cathodic behavior on the amount of vinyl groups: while for the monoalkene containing molecules no reduction is seen, the divinyl products are reduced in several steps.
Graphical Abstract
Introduction
The introduction of complex functional fragments into the specified place of the target molecule is of current interest in modern synthetic chemistry [1]. From this point of view, the development of ferrocene-based molecules as crucial fragments of new materials and novel pharmacological entities is of great importance. Indeed, since the discovery of ferrocene [2] and the synthesis of the first polymer based on vinylferrocene [3], the chemistry of ferrocene and its derivatives is developing very rapidly. Ferrocene derivatives are widely applied in industry, for example, diethylferrocene is a combustion accelerator used as additive to gasoline [4]. There are many drugs containing a ferrocene fragment in their structures (Scheme 1) [5]. The ferrocene core is also a very popular scaffold for ligand design, particularly, in asymmetric catalysis (Scheme 1).
Scheme 1: Examples of ferrocene derived drugs and ligands.
Scheme 1: Examples of ferrocene derived drugs and ligands.
Probably, the most challenging application of ferrocenes is the production of ferrocene-containing polymers [6-8] (Scheme 2). The polymers have a set of unique properties and are used in various fields of science, technology and medicine (conducting and semiconducting materials [9], drugs [10], biosensors [11-13], liquid crystal materials [14,15], coordination polymers [16], and more [10]).
Scheme 2: Structural types of ferrocene-based polymers.
Scheme 2: Structural types of ferrocene-based polymers.
Ethynylferrocenes are one of the most popular and extremely effective starting compounds in the creation of ferrocenyl polymers. They are widely used for making both polyferrocenylvinylenes (double bond linkers) [17-21] and polyethynylferrocenes (triple bond linkers) [22-25]. There are several methods for the preparation of ethynylferrocenes [24-29]. In most cases, the key reagents for the synthesis of ethynylferrocene are the corresponding mono- and dihalogenvinylferrocene derivatives. These compounds are generally prepared using a variation of the Wittig reaction (the Corey–Fuchs reaction) using a 2–4-fold molar excess of triphenylphosphine [27-31].
Results and Discussion
Synthesis of halovinylferrocenes
A few years ago we discovered a new reaction for a double carbon–carbon bond formation – the reaction of catalytic olefination. It was shown that the copper-catalyzed reaction of unsubstituted hydrazones of aromatic (aliphatic) aldehydes and ketones with a wide range of polyhalogenalkanes leads to the corresponding substituted ethylenes with one or two geminal halogen atoms [32-36]. In the present study, we investigated the possibility of using a catalytic olefination reaction for the synthesis of ferrocene derivatives. As a result, several well-known and previously unknown ferrocene-containing alkene compounds were obtained.
First, ferrocene carbaldehyde was investigated. It was found, that under usual conditions of the reaction (equal amount of N2H4·H2O, DMSO or EtOH as a solvent) the yields of the desired alkenes were not good enough. Better results were obtained in ethylene glycol with a 4-fold excess of N2H4·H2O (novel media just reported for COR [37]). In this case, target alkenes were isolated in up to 62% yield. Using C2-freons fluorinated alkenes 3–5 were synthesized (Scheme 3). The reaction proceeds stereoselectively to give a mixture of isomers in which the less hindered Z-isomer dominates. The assignment of the isomers was easily performed by comparison of NMR spectral data with those previously reported for the Z,E-isomers of similar alkenes [38-40]. The trifluoromethyl group of the Z-isomers of compounds 3–5 resonates in higher field in 19F NMR (3: −72.8 ppm, 4: −67.1 ppm, 5: −69.0 ppm) than that of the E-isomers (3: −67.9 ppm, 4: −60.2 ppm, 5: −62.4 ppm). In the case of compound 3 an additional conformation can be seen in the 1H NMR spectrum. It is well-known, that 2JHF coupling constants in alkenes with relative trans-configuration of hydrogen and fluorine atoms are usually approximately twice bigger than the corresponding coupling constants in alkenes with cis-configuration. The values found in 3 are 36.5 Hz (Z-isomer by systematic nomenclature, but trans-configuration of H and F) and 16.2 Hz (E-isomer).
Scheme 3: Synthesis of ferrocene-derived alkenes from ferrocene carbaldehyde.
Scheme 3: Synthesis of ferrocene-derived alkenes from ferrocene carbaldehyde.
Acetylferrocene and 1,1’-diacetylferrocene were also involved successfully into this transformation (Scheme 4). Our preliminary study of the catalytic olefination of acetylferrocene by CF3CBr3 (alkene 8) and CBr4 (alkene 7) indicates, that in contrast to ferrocene carbaldehyde, better results were achieved in DMSO using previously prepared hydrazones [37]. A series of ferrocene derivatives including fluorinated ones was prepared in good yields. Unsymmetrical alkenes 8 and 9 were obtained as a mixture of isomers in approximately equal amounts. Identification of the structures of the E- and Z-isomers of alkene 8 was accurately performed by an X-ray crystallographic study using crystals of 8 obtained from ethanol solution (see Supporting Information File 1 and [41]). Due to the presence of two double bonds in alkene 12 the formation of three isomers (Z,Z, Z,E and E,E) is possible. Similar to alkenes 8 and 9 no stereoselectivity is observed to give equal numbers of cis- and trans-double bonds in the obtained molecules. As a result a 25:50:25 mixture of Z,Z, Z,E and E,E was formed. The higher quantity of the Z,E-isomer is a result of statistical doubling (in fact, the reaction gives Z,E and E,Z-alkenes which are identical).
Scheme 4: Synthesis of ferrocene-derived alkenes from acetylferrocene and 1,1’-diacetylferrocene.
Scheme 4: Synthesis of ferrocene-derived alkenes from acetylferrocene and 1,1’-diacetylferrocene.
Di- and tetrahalovinylferrocenes obtained in our work are of interest for the synthesis of ethynylferrocenes, as monomers for making ferrocene-containing polymers and as intermediates in the synthesis of ferrocene analogs of tamoxifen and other medicinally relevant molecules.
Electrochemical properties of halovinylferrocenes
The ferrocene unit possesses several exciting electrochemical characteristics, such as fast electron-transfer rate, low oxidation potential, and stability of two redox states. The combination of a ferrocene and an alkene moiety in one molecule might be of interest for the synthesis of functional devises that can be exploited in electrocatalysis, electroanalysis, and biosensing applications, since the alkene fragment could be used to graft the molecule to the polymer support or to copolymerize it with the appropriate monomers. Therefore, we have also examined the electrochemical properties of the compounds obtained. It was shown that the oxidation of the ferrocene unit proceeds in accordance to the data found in literature, whereas the reduction was found only in the case of bis(halovinyl)ferrocenes. Our results clearly indicate that the anodic and cathodic electrochemical processes proceed on different parts of the molecules. While in the anodic region the changes are localized on the iron atom, the electron transfer from the cathode occurs at a double bond.
Three representative groups of halovinylferrocenes were studied using cyclic voltammetry (Table 1). The first group was formed from three omega-dichloro derivatives 1, 6, and 10. The second one was made from three omega-dibromo products 2, 7, and 11, and the last group consisted of bromotrifluoromethyl molecules 4, 8, and 12. Such selection allowed us to estimate the effect of the nature of substituent on the electrochemical behavior of the molecule.
Table 1: Cyclic voltammetry data for selected halovinylferrocenes. Pt electrode, DMF, Ag/AgCl/KCl (sat.), Bu4NBF4.
| Compound | EpOx, V | −EpRed1, V | −EpRed2, V | −EpRed3, V |
|---|---|---|---|---|
|
1 |
0.646 (0.580)a | – | – | – |
|
2 |
0.651 (0.584) | – | – | – |
|
4 |
0.735 (0.669) | – | – | – |
|
6 |
0.655 (0.576) | – | – | |
|
7 |
0.641 (0.579) | – | – | – |
|
8 |
0.726 (0.607) | – | – | – |
|
10 |
0.741 (0.675) | 0.371 IRb | 0.983 (0.763) | – |
|
11 |
0.740 (0.670) | 0.68 IR | 1.022 IR | 1.66 IR |
|
12 |
0.836 (0.867) | 0.343 IR | 1.232 (0.71) | – |
aIn the case of a reversible peak the reverse potentials are shown in paranthesis. bIR means irreversible.
All compounds are characterized by reversible oxidation waves in the range of 0.64–0.84 V that corresponds to the Fe2+/Fe3+ redox transformation. The values of the respective oxidation potential depend mainly on the amount of the vinyl groups attached to the ferrocene core. There is also the effect of the halogen atom, though to a lesser extent compared to the effect of the amount of vinyl groups. For example, all dichloro and dibromo compounds (1, 2, 6, and 7, one vinyl fragment) are oxidized at potentials of 0.64–0.65 V, whereas for the corresponding tetrahalo compounds (10 and 11, two vinyl fragments) these values are shifted to more anodic potentials, namely, 0.74 V. The same is true for trifluoromethyl ferrocenes 4, 8, and 12 (Figure 1).
![[1860-5397-11-223-1]](/bjoc/content/figures/1860-5397-11-223-1.png?scale=2.5&max-width=1024&background=FFFFFF)
Figure 1: Typical voltammogramms of vinylferrocenes 7 (blue), 11 (green), 12 (red), anodic region.
Figure 1: Typical voltammogramms of vinylferrocenes 7 (blue), 11 (green), 12 (red), anodic region.
In the cathodic region, monovinylic compounds 1, 2, 4, and 6–8 show no reduction up to 1.80 V, whilst the corresponding divinylic molecules 10–12 exhibit pronounced reduction waves in the range of 0.34–1.66 V (Figure 2).
![[1860-5397-11-223-2]](/bjoc/content/figures/1860-5397-11-223-2.png?scale=2.5&max-width=1024&background=FFFFFF)
Figure 2: Typical voltammogramms of divinylferrocenes 10 (black), 11 (red), 12 (blue), cathodic region.
Figure 2: Typical voltammogramms of divinylferrocenes 10 (black), 11 (red), 12 (blue), cathodic region.
The processes behind the cathodic waves are not totally clear at the moment, but we assume that the radical-ions formed after the first electron transfer would enter the intramolecular cyclization reaction involving the second adjacent double bond with subsequent electropolymerization. The latter is confirmed by a pronounced decrease in current values (3–4 times) as compared to current values of oxidation at the iron atom. These findings allow us to state that the synthesized molecules are promising starting materials for the electrochemical synthesis of ferrocene-containing conjugated polymers.
Conclusion
In conclusion, a novel stereoselective route to ferrocenyl haloalkenes and bis-alkenes was elaborated on the basis of a catalytic olefination reaction of N-unsubstituted hydrazones obtained from ferrocene-containing aldehydes and ketones. Electrochemical properties of synthesized alkenes were investigated and promising electrochemical characteristics were demonstrated.
Supporting Information
| Supporting Information File 1: Experimental details, analytical data and copies of NMR spectra of all synthesized compounds, X-ray data of compound 8. | ||
| Format: PDF | Size: 1.7 MB | Download |
Acknowledgements
This work was supported by the Russian Foundation for the Basic Research (Grants no 13-03-01129-a and 14-03-91160-GFEN, investigation of electrochemical properties of halovinylferrocenes) and the Russian Science Foundation (grant no. 14-13-00083, synthesis of starting materials and halovinylferrocenes). The authors acknowledge partial support in measuring of NMR from the M.V. Lomonosov Moscow State University Program of Development.
References
-
Ananikov, V. P.; Khemchyan, L. L.; Ivanova, Yu. V.; Bukhtiyarov, V. I.; Sorokin, A. M.; Prosvirin, I. P.; Vatsadze, S. Z.; Medved'ko, A. V.; Nuriev, V. N.; Dilman, A. D.; Levin, V. V.; Koptyug, I. V.; Kovtunov, K. V.; Zhivonitko, V. V.; Likholobov, V. A.; Romanenko, A. V.; Simonov, P. A.; Nenajdenko, V. G.; Shmatova, O. I.; Muzalevskiy, V. M.; Nechaev, M. S.; Asachenko, A. F.; Morozov, O. S.; Dzhevakov, P. B.; Osipov, S. N.; Vorobyeva, D. V.; Topchiy, M. A.; Zotova, M. A.; Ponomarenko, S. A.; Borshchev, O. V.; Luponosov, Yu. N.; Rempel, A. A.; Valeeva, A. A.; Stakheev, A. Yu.; Turova, O. V.; Mashkovsky, I. S.; Sysolyatin, S. V.; Malykhin, V. V.; Bukhtiyarova, G. A.; Terent'ev, A. O.; Krylov, I. B. Russ. Chem. Rev. 2014, 83, 885–985. doi:10.1070/RC2014v83n10ABEH004471
Return to citation in text: [1] -
Kealy, T. J.; Pauson, P. L. Nature 1951, 168, 1039–1040. doi:10.1038/1681039b0
Return to citation in text: [1] -
Arimoto, F. S.; Haven, A. C., Jr. J. Am. Chem. Soc. 1955, 77, 6295–6297. doi:10.1021/ja01628a068
Return to citation in text: [1] -
Tong, R.; Zhao, Y.; Wang, L.; Yu, H.; Ren, F.; Saleem, M.; Amer, W. A. J. Organomet. Chem. 2014, 755, 16–32. doi:10.1016/j.jorganchem.2013.12.052
Return to citation in text: [1] -
Braga, S. S.; Silva, A. M. S. Organometallics 2013, 32, 5626–5639. doi:10.1021/om400446y
Return to citation in text: [1] -
Wang, L.; Haogie, Y. Synthesis, Properties and Application of Ferrocenyl Polymers; Zheijang University Press, 2013.
Return to citation in text: [1] -
Hardy, C. G.; Ren, L.; Zhang, J.; Tang, C. Isr. J. Chem. 2012, 52, 230–245. doi:10.1002/ijch.201100110
Return to citation in text: [1] -
Heo, R. W.; Lee, T. R. J. Organomet. Chem. 1999, 578, 31–42. doi:10.1016/S0022-328X(98)01126-7
Return to citation in text: [1] -
Deng, W.; Yamaguchi, H.; Takashima, Y.; Harada, A. Angew. Chem. 2007, 119, 5236–5239. doi:10.1002/ange.200701272
Return to citation in text: [1] -
Neuse, E. W. J. Inorg. Organomet. Polym. Mater. 2005, 15, 3–31. doi:10.1007/s10904-004-2371-9
Return to citation in text: [1] [2] -
Amer, W. A.; Wang, L.; Amin, A. M.; Ma, L.; Yu, H. J. Inorg. Organomet. Polym. Mater. 2010, 20, 605–615. doi:10.1007/s10904-010-9373-6
Return to citation in text: [1] -
Takahashi, S.; Anzai, J. Materials 2013, 6, 5742–5762. doi:10.3390/ma6125742
Return to citation in text: [1] -
Gracia, R.; Mecerreyes, D. Polym. Chem. 2013, 4, 2206–2214. doi:10.1039/c3py21118e
Return to citation in text: [1] -
Gao, Y.; Shreeve, J. M. J. Inorg. Organomet. Polym. Mater. 2007, 17, 19–36. doi:10.1007/s10904-006-9095-y
Return to citation in text: [1] -
Kadkin, O. N.; Galyametdinov, Yu. G. Russ. Chem. Rev. 2012, 81, 675–699. doi:10.1070/RC2012v081n08ABEH004270
Return to citation in text: [1] -
Horikoshi, R.; Mochida, T. Eur. J. Inorg. Chem. 2010, 5355–5371. doi:10.1002/ejic.201000525
Return to citation in text: [1] -
Dragutan, I.; Dragutan, V.; Fischer, H. J. Inorg. Organomet. Polym. Mater. 2008, 18, 311–324. doi:10.1007/s10904-008-9213-0
Return to citation in text: [1] -
Buchmeiser, M.; Schrock, R. R. Macromolecules 1995, 28, 6642–6649. doi:10.1021/ma00123a034
Return to citation in text: [1] -
Buchmeiser, M. R. Macromolecules 1997, 30, 2274–2277. doi:10.1021/ma961317f
Return to citation in text: [1] -
Buchmeiser, M. R.; Schuler, N.; Kaltenhauser, G.; Ongania, K.-H.; Lagoja, I.; Wurst, K.; Schottenberger, H. Macromolecules 1998, 31, 3175–3183. doi:10.1021/ma9716948
Return to citation in text: [1] -
Camus, A.; Faruffini, V.; Furlani, A.; Marsich, N.; Ortaggi, G.; Paolesse, R.; Russo, M. V. Appl. Organomet. Chem. 1988, 2, 533–537. doi:10.1002/aoc.590020606
Return to citation in text: [1] -
Hudson, R. D. A. J. Organomet. Chem. 2001, 637–639, 47–69. doi:10.1016/S0022-328X(01)01142-1
Return to citation in text: [1] -
Abd-El-Aziz, A. S. Macromol. Rapid Commun. 2002, 23, 995–1031. doi:10.1002/marc.200290003
Return to citation in text: [1] -
Butler, I. R.; Boyes, A. L.; Kelly, G.; Quayle, S. C.; Herzig, T.; Szewczyk, J. Inorg. Chem. Commun. 1999, 2, 403–406. doi:10.1016/S1387-7003(99)00099-4
Return to citation in text: [1] [2] -
Plenio, H.; Hermann, J.; Sehring, A. Chem. – Eur. J. 2000, 6, 1820–1829. doi:10.1002/(SICI)1521-3765(20000515)6:10<1820::AID-CHEM1820>3.0.CO;2-A
Return to citation in text: [1] [2] -
Schloegl, K.; Egger, H. Monatsh. Chem. 1963, 94, 376–392.
Return to citation in text: [1] -
Luo, S.-J.; Liu, Y.-H.; Liu, C.-M.; Liang, Y.-M.; Ma, Y.-X. Synth. Commun. 2000, 30, 1569–1572. doi:10.1080/00397910008087190
Return to citation in text: [1] [2] -
Clément, S.; Guyard, L.; Knorr, M.; Dilsky, S.; Strohmann, C.; Arroyo, M. J. Organomet. Chem. 2007, 692, 839–850. doi:10.1016/j.jorganchem.2006.10.039
Return to citation in text: [1] [2] -
Pedersen, B.; Wagner, G.; Herrmann, R.; Scherer, W.; Meerholz, K.; Schmälzlin, E.; Bräuchle, C. J. Organomet. Chem. 1999, 590, 129–137. doi:10.1016/S0022-328X(99)00440-4
Return to citation in text: [1] [2] -
Tsuboya, N.; Hamasaki, R.; Ito, M.; Mitsuishi, M.; Miyashita, T.; Yamamoto, Y. J. Mater. Chem. 2003, 13, 511–513. doi:10.1039/b211019a
Return to citation in text: [1] -
Clément, S.; Guyard, L.; Knorr, M.; Gessner, V. H.; Strohmann, C. Acta Crystallogr., Sect. E 2009, 65, m334. doi:10.1107/S1600536809006102
Return to citation in text: [1] -
Shastin, A. V.; Korotchenko, V. N.; Nenaidenko, V. G.; Balenkova, E. S. Russ. Chem. Bull. 1999, 48, 2184–2185. doi:10.1007/BF02494876
Return to citation in text: [1] -
Shastin, A. V.; Korotchenko, V. N.; Nenajdenko, V. G.; Balenkova, E. S. Tetrahedron 2000, 56, 6557–6563. doi:10.1016/S0040-4020(00)00606-2
Return to citation in text: [1] -
Nenajdenko, V. G.; Varseev, G. N.; Korotchenko, V. N.; Shastin, A. V.; Balenkova, E. S. J. Fluorine Chem. 2003, 124, 115–118. doi:10.1016/S0022-1139(03)00199-4
Return to citation in text: [1] -
Shastin, A. V.; Muzalevsky, V. M.; Balenkova, E. S.; Nenajdenko, V. G. Mendeleev Commun. 2006, 16, 179–180. doi:10.1070/MC2006v016n03ABEH002282
Return to citation in text: [1] -
Muzalevskiy, V. M.; Shikhaliev, N. G.; Magerramov, A. M.; Gurbanova, N. V.; Geydarova, S. D.; Balenkova, E. S.; Shastin, A. V.; Nenajdenko, V. G. Russ. Chem. Bull. 2013, 62, 678–682. doi:10.1007/s11172-013-0091-4
Return to citation in text: [1] -
Hirotaki, K.; Kawazoe, G.; Hanamoto, T. J. Fluorine Chem. 2015, 171, 169–173. doi:10.1016/j.jfluchem.2014.07.018
Return to citation in text: [1] [2] -
Nenajdenko, V. G.; Varseev, G. N.; Korotchenko, V. N.; Shastin, A. V.; Balenkova, E. S. J. Fluorine Chem. 2004, 125, 1339–1345. doi:10.1016/j.jfluchem.2004.04.002
Return to citation in text: [1] -
Nenajdenko, V. G.; Varseev, G. N.; Shastin, A. V.; Balenkova, E. S. J. Fluorine Chem. 2005, 126, 907–913. doi:10.1016/j.jfluchem.2005.03.020
Return to citation in text: [1] -
Korotchenko, V. N.; Shastin, A. V.; Nenajdenko, V. G.; Balenkova, E. S. Tetrahedron 2001, 57, 7519–7527. doi:10.1016/S0040-4020(01)00701-3
Return to citation in text: [1] -
Shixaliyev, N. G.; Heydarova, S. J.; Muzalevskiy, V. M.; Nenajdenko, V. G.; Rahimova, A. G. Azerb. Khim. Zh. 2013, 78–83.
Return to citation in text: [1]
| 38. | Nenajdenko, V. G.; Varseev, G. N.; Korotchenko, V. N.; Shastin, A. V.; Balenkova, E. S. J. Fluorine Chem. 2004, 125, 1339–1345. doi:10.1016/j.jfluchem.2004.04.002 |
| 39. | Nenajdenko, V. G.; Varseev, G. N.; Shastin, A. V.; Balenkova, E. S. J. Fluorine Chem. 2005, 126, 907–913. doi:10.1016/j.jfluchem.2005.03.020 |
| 40. | Korotchenko, V. N.; Shastin, A. V.; Nenajdenko, V. G.; Balenkova, E. S. Tetrahedron 2001, 57, 7519–7527. doi:10.1016/S0040-4020(01)00701-3 |
| 32. | Shastin, A. V.; Korotchenko, V. N.; Nenaidenko, V. G.; Balenkova, E. S. Russ. Chem. Bull. 1999, 48, 2184–2185. doi:10.1007/BF02494876 |
| 33. | Shastin, A. V.; Korotchenko, V. N.; Nenajdenko, V. G.; Balenkova, E. S. Tetrahedron 2000, 56, 6557–6563. doi:10.1016/S0040-4020(00)00606-2 |
| 34. | Nenajdenko, V. G.; Varseev, G. N.; Korotchenko, V. N.; Shastin, A. V.; Balenkova, E. S. J. Fluorine Chem. 2003, 124, 115–118. doi:10.1016/S0022-1139(03)00199-4 |
| 35. | Shastin, A. V.; Muzalevsky, V. M.; Balenkova, E. S.; Nenajdenko, V. G. Mendeleev Commun. 2006, 16, 179–180. doi:10.1070/MC2006v016n03ABEH002282 |
| 36. | Muzalevskiy, V. M.; Shikhaliev, N. G.; Magerramov, A. M.; Gurbanova, N. V.; Geydarova, S. D.; Balenkova, E. S.; Shastin, A. V.; Nenajdenko, V. G. Russ. Chem. Bull. 2013, 62, 678–682. doi:10.1007/s11172-013-0091-4 |
| 37. | Hirotaki, K.; Kawazoe, G.; Hanamoto, T. J. Fluorine Chem. 2015, 171, 169–173. doi:10.1016/j.jfluchem.2014.07.018 |
| 1. | Ananikov, V. P.; Khemchyan, L. L.; Ivanova, Yu. V.; Bukhtiyarov, V. I.; Sorokin, A. M.; Prosvirin, I. P.; Vatsadze, S. Z.; Medved'ko, A. V.; Nuriev, V. N.; Dilman, A. D.; Levin, V. V.; Koptyug, I. V.; Kovtunov, K. V.; Zhivonitko, V. V.; Likholobov, V. A.; Romanenko, A. V.; Simonov, P. A.; Nenajdenko, V. G.; Shmatova, O. I.; Muzalevskiy, V. M.; Nechaev, M. S.; Asachenko, A. F.; Morozov, O. S.; Dzhevakov, P. B.; Osipov, S. N.; Vorobyeva, D. V.; Topchiy, M. A.; Zotova, M. A.; Ponomarenko, S. A.; Borshchev, O. V.; Luponosov, Yu. N.; Rempel, A. A.; Valeeva, A. A.; Stakheev, A. Yu.; Turova, O. V.; Mashkovsky, I. S.; Sysolyatin, S. V.; Malykhin, V. V.; Bukhtiyarova, G. A.; Terent'ev, A. O.; Krylov, I. B. Russ. Chem. Rev. 2014, 83, 885–985. doi:10.1070/RC2014v83n10ABEH004471 |
| 5. | Braga, S. S.; Silva, A. M. S. Organometallics 2013, 32, 5626–5639. doi:10.1021/om400446y |
| 24. | Butler, I. R.; Boyes, A. L.; Kelly, G.; Quayle, S. C.; Herzig, T.; Szewczyk, J. Inorg. Chem. Commun. 1999, 2, 403–406. doi:10.1016/S1387-7003(99)00099-4 |
| 25. | Plenio, H.; Hermann, J.; Sehring, A. Chem. – Eur. J. 2000, 6, 1820–1829. doi:10.1002/(SICI)1521-3765(20000515)6:10<1820::AID-CHEM1820>3.0.CO;2-A |
| 26. | Schloegl, K.; Egger, H. Monatsh. Chem. 1963, 94, 376–392. |
| 27. | Luo, S.-J.; Liu, Y.-H.; Liu, C.-M.; Liang, Y.-M.; Ma, Y.-X. Synth. Commun. 2000, 30, 1569–1572. doi:10.1080/00397910008087190 |
| 28. | Clément, S.; Guyard, L.; Knorr, M.; Dilsky, S.; Strohmann, C.; Arroyo, M. J. Organomet. Chem. 2007, 692, 839–850. doi:10.1016/j.jorganchem.2006.10.039 |
| 29. | Pedersen, B.; Wagner, G.; Herrmann, R.; Scherer, W.; Meerholz, K.; Schmälzlin, E.; Bräuchle, C. J. Organomet. Chem. 1999, 590, 129–137. doi:10.1016/S0022-328X(99)00440-4 |
| 4. | Tong, R.; Zhao, Y.; Wang, L.; Yu, H.; Ren, F.; Saleem, M.; Amer, W. A. J. Organomet. Chem. 2014, 755, 16–32. doi:10.1016/j.jorganchem.2013.12.052 |
| 27. | Luo, S.-J.; Liu, Y.-H.; Liu, C.-M.; Liang, Y.-M.; Ma, Y.-X. Synth. Commun. 2000, 30, 1569–1572. doi:10.1080/00397910008087190 |
| 28. | Clément, S.; Guyard, L.; Knorr, M.; Dilsky, S.; Strohmann, C.; Arroyo, M. J. Organomet. Chem. 2007, 692, 839–850. doi:10.1016/j.jorganchem.2006.10.039 |
| 29. | Pedersen, B.; Wagner, G.; Herrmann, R.; Scherer, W.; Meerholz, K.; Schmälzlin, E.; Bräuchle, C. J. Organomet. Chem. 1999, 590, 129–137. doi:10.1016/S0022-328X(99)00440-4 |
| 30. | Tsuboya, N.; Hamasaki, R.; Ito, M.; Mitsuishi, M.; Miyashita, T.; Yamamoto, Y. J. Mater. Chem. 2003, 13, 511–513. doi:10.1039/b211019a |
| 31. | Clément, S.; Guyard, L.; Knorr, M.; Gessner, V. H.; Strohmann, C. Acta Crystallogr., Sect. E 2009, 65, m334. doi:10.1107/S1600536809006102 |
| 3. | Arimoto, F. S.; Haven, A. C., Jr. J. Am. Chem. Soc. 1955, 77, 6295–6297. doi:10.1021/ja01628a068 |
| 17. | Dragutan, I.; Dragutan, V.; Fischer, H. J. Inorg. Organomet. Polym. Mater. 2008, 18, 311–324. doi:10.1007/s10904-008-9213-0 |
| 18. | Buchmeiser, M.; Schrock, R. R. Macromolecules 1995, 28, 6642–6649. doi:10.1021/ma00123a034 |
| 19. | Buchmeiser, M. R. Macromolecules 1997, 30, 2274–2277. doi:10.1021/ma961317f |
| 20. | Buchmeiser, M. R.; Schuler, N.; Kaltenhauser, G.; Ongania, K.-H.; Lagoja, I.; Wurst, K.; Schottenberger, H. Macromolecules 1998, 31, 3175–3183. doi:10.1021/ma9716948 |
| 21. | Camus, A.; Faruffini, V.; Furlani, A.; Marsich, N.; Ortaggi, G.; Paolesse, R.; Russo, M. V. Appl. Organomet. Chem. 1988, 2, 533–537. doi:10.1002/aoc.590020606 |
| 22. | Hudson, R. D. A. J. Organomet. Chem. 2001, 637–639, 47–69. doi:10.1016/S0022-328X(01)01142-1 |
| 23. | Abd-El-Aziz, A. S. Macromol. Rapid Commun. 2002, 23, 995–1031. doi:10.1002/marc.200290003 |
| 24. | Butler, I. R.; Boyes, A. L.; Kelly, G.; Quayle, S. C.; Herzig, T.; Szewczyk, J. Inorg. Chem. Commun. 1999, 2, 403–406. doi:10.1016/S1387-7003(99)00099-4 |
| 25. | Plenio, H.; Hermann, J.; Sehring, A. Chem. – Eur. J. 2000, 6, 1820–1829. doi:10.1002/(SICI)1521-3765(20000515)6:10<1820::AID-CHEM1820>3.0.CO;2-A |
| 11. | Amer, W. A.; Wang, L.; Amin, A. M.; Ma, L.; Yu, H. J. Inorg. Organomet. Polym. Mater. 2010, 20, 605–615. doi:10.1007/s10904-010-9373-6 |
| 12. | Takahashi, S.; Anzai, J. Materials 2013, 6, 5742–5762. doi:10.3390/ma6125742 |
| 13. | Gracia, R.; Mecerreyes, D. Polym. Chem. 2013, 4, 2206–2214. doi:10.1039/c3py21118e |
| 16. | Horikoshi, R.; Mochida, T. Eur. J. Inorg. Chem. 2010, 5355–5371. doi:10.1002/ejic.201000525 |
| 10. | Neuse, E. W. J. Inorg. Organomet. Polym. Mater. 2005, 15, 3–31. doi:10.1007/s10904-004-2371-9 |
| 10. | Neuse, E. W. J. Inorg. Organomet. Polym. Mater. 2005, 15, 3–31. doi:10.1007/s10904-004-2371-9 |
| 9. | Deng, W.; Yamaguchi, H.; Takashima, Y.; Harada, A. Angew. Chem. 2007, 119, 5236–5239. doi:10.1002/ange.200701272 |
| 37. | Hirotaki, K.; Kawazoe, G.; Hanamoto, T. J. Fluorine Chem. 2015, 171, 169–173. doi:10.1016/j.jfluchem.2014.07.018 |
| 6. | Wang, L.; Haogie, Y. Synthesis, Properties and Application of Ferrocenyl Polymers; Zheijang University Press, 2013. |
| 7. | Hardy, C. G.; Ren, L.; Zhang, J.; Tang, C. Isr. J. Chem. 2012, 52, 230–245. doi:10.1002/ijch.201100110 |
| 8. | Heo, R. W.; Lee, T. R. J. Organomet. Chem. 1999, 578, 31–42. doi:10.1016/S0022-328X(98)01126-7 |
| 14. | Gao, Y.; Shreeve, J. M. J. Inorg. Organomet. Polym. Mater. 2007, 17, 19–36. doi:10.1007/s10904-006-9095-y |
| 15. | Kadkin, O. N.; Galyametdinov, Yu. G. Russ. Chem. Rev. 2012, 81, 675–699. doi:10.1070/RC2012v081n08ABEH004270 |
| 41. | Shixaliyev, N. G.; Heydarova, S. J.; Muzalevskiy, V. M.; Nenajdenko, V. G.; Rahimova, A. G. Azerb. Khim. Zh. 2013, 78–83. |
© 2015 Muzalevskiy et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)