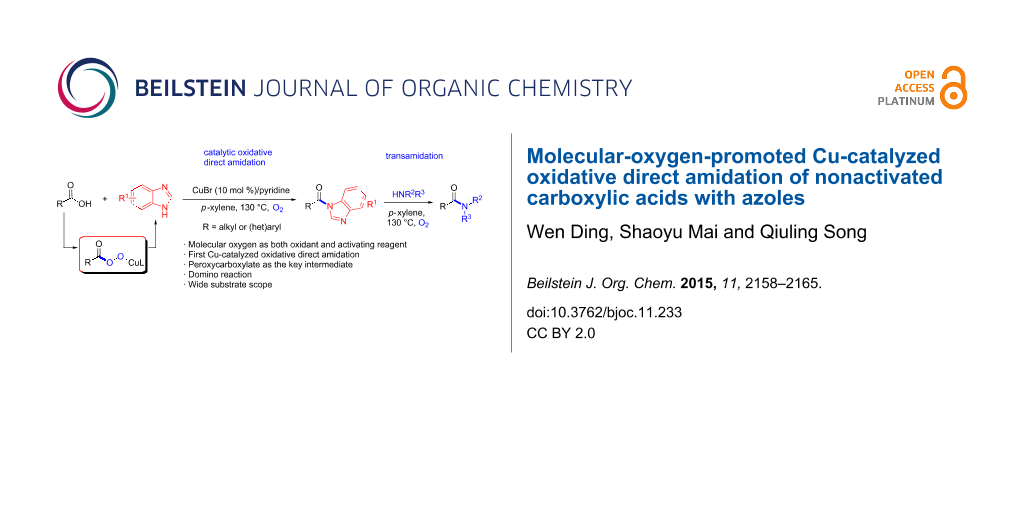
Abstract
A copper-catalyzed oxidative direct formation of amides from nonactivated carboxylic acids and azoles with dioxygen as an activating reagent is reported. The azole amides were produced in good to excellent yields with a broad substrate scope. The mechanistic studies reveal that oxygen plays an essential role in the success of the amidation reactions with copper peroxycarboxylate as the key intermediate. Transamidation occurs smoothly between azole amide and a variety of amines.
Graphical Abstract
Introduction
Amides are prevalent scaffolds in numerous compounds ranging from biologically active natural products to pharmaceuticals [1]. As a result, numerous methods have been developed for the formation of amides [2-7]. Despite such advances [8-21], the acylation of amines with free carboxylic acids is still the most common method employed due to the availability of the starting materials and its relatively clean process feature, whereby water is the only byproduct in the transformation [22]. However, preactivation of the free carboxylic acids is always required, and stoichiometric activating reagents or coupling reagents are mandatory in the classic methods of amide formation (Scheme 1a) [23].
Scheme 1: Traditional activating mode and oxidative activation mode of free carboxylic acids in amide formation.
Scheme 1: Traditional activating mode and oxidative activation mode of free carboxylic acids in amide formati...
In some cases, corrosive byproducts (HCl) are unavoidable and the activating reagent is difficult to remove from the system. Given the growing demand for environmentally benign synthesis [24], it is highly desirable to bring forth a green, sustainable and simple new protocol for the activation of carboxylic acids. Molecular oxygen is an ideal oxidant owing to its negligible cost, availability and sustainability [25,26]. Currently, copper-catalyzed, aerobic, oxidative transformations with dioxygen as the sole oxidant have emerged as a useful and powerful strategy to construct carbon–carbon and carbon–heteroatom bonds [27-29].
It is known that Cu(II) superoxide species are usually reported to be formed in the system, and very recently McDonald and coworkers reported an unusual nucleophilic reactivity of a special Cu(II) superoxide complex, acting as a deformylating reagent in spite of the prevailing electrophilic property common for such a metal-bound superoxide intermediate [30]. Therefore, we envision that the newly discovered nucleophilic reactivity of Cu(II) superoxide species might lead to a novel, free carboxylic acid activation mode for amide formation: a Cu(II) superoxide species could attack the carbonyl group in an acid to afford a copper peroxycarboxylate, which might then serve as the key intermediate to make the subsequent amide formation feasible (Scheme 1b). In our work, azoles have been chosen as the amines due to their special bioactivity [31]. To the best of our knowledge, Cu salt has not yet been used for catalyzed, oxidative direct amide formation. We report the first amidation reaction from carboxylic acids with peroxycarboxylate as the key intermediate, which represents a novel activation mode with molecular oxygen as the activating reagent. Most remarkably, in sharp contrast to previous reports (which used complex N-containing ligands to form copper superoxide), inexpensive and readily available pyridine was employed as both the ligand and base in our case.
Results and Discussion
Our initial exploration commenced with benzoic acid (1) and benzimidazole (2) as the model substrates to investigate the copper-catalyzed oxidative direct amidation reaction (Table 1).
Table 1: Optimization of the reaction conditions.a
|
|
||||||
| entry | catalyst (mol %) | atmosphere | ligand (equiv) | temperature | solvent | yield (%)b |
|---|---|---|---|---|---|---|
| 1 | CuCl (10) | O2 | pyridine (3) | 130 °C | p-xylene | 33 |
| 2 | CuI (10) | O2 | pyridine (3) | 130 °C | p-xylene | 79 |
| 3 | Cu(OAc)2 (10) | O2 | pyridine (3) | 130 °C | p-xylene | 0 |
| 4 | CuBr (10) | O2 | pyridine (3) | 130 °C | p-xylene | 97 (85)c |
| 5 | CuBr2 (10) | O2 | pyridine (3) | 130 °C | p-xylene | 33 |
| 6 | CuBr (10) | O2 | pyridine (3) | 130 °C | o-xylene | 48 |
| 7 | CuBr (10) | O2 | pyridine (3) | 130 °C | DMSO | no product |
| 8 | CuBr (10) | O2 | pyridine (3) | 130 °C | DMF | no product |
| 9 | CuBr (10) | O2 | pyridine (3) | 130 °C | toluene | 4 |
| 10 | CuBr (10) | O2 | 1, 10-Phen (3) | 130 °C | p-xylene | no product |
| 11 | CuBr (10) | O2 | 2, 2'-bipy (3) | 130 °C | p-xylene | no product |
| 12 | CuBr (10) | O2 | 2-aminopyridine (3) | 130 °C | p-xylene | no product |
| 13 | CuBr (10) | O2 | Et3N (3) | 130 °C | p-xylene | no product |
| 14 | CuBr (10) | O2 | pyridine (3) | 120 °C | p-xylene | 31 |
| 15 | CuBr (10) | O2 | pyridine (0.5) | 130 °C | p-xylene | 39 |
| 16 | CuBr (10) | O2 | pyridine (2) | 130 °C | p-xylene | 77 |
| 17d | CuBr (10) | O2 | pyridine (3) | 130 °C | p-xylene | 7 |
| 18 | CuBr (10) | N2 | pyridine (3) | 130 °C | p-xylene | no product |
| 19 | CuBr (10) | air | pyridine (3) | 130 °C | p-xylene | 53 |
| 20 | – | O2 | pyridine (3) | 130 °C | p-xylene | no product |
| 21 | CuBr (10) | O2 | – | 130 °C | p-xylene | 46 |
aReaction conditions: all reactions were performed with a mixture of 1 (0.25 mmol), 2 (0.5 mmol), Cu salt (10 mol %), ligand, solvent (1 mL), 16 h, temp., corresponding atmosphere. bGC yield. cIsolated yield. d1 (0.25 mmol), 2 (0.30 mmol).
The copper salts demonstrated good activity for this novel transformation (Table 1, entries 1–5) and 85% of the desired product was obtained using 10 mol % of CuBr with pyridine (3 equiv) at 130 °C in p-xylene (1 mL) under O2 in a sealed tube (Table 1, entry 4). Notably, CuBr2 only gave 33% of the desired product under similar conditions (Table 1, entry 5), inferring that molecular oxygen was not just acting as an oxidant to convert Cu(I) into Cu(II), but that it might be involved in the reaction. Further screening of solvents and ligands revealed that p-xylene and pyridine are the optimal choices. The reaction cannot go to completion and part of the carboxylic acid remains when the amount of benzimidazole is not twice that of the carboxylic acid. The temperature affected this reaction dramatically and when the temperature was decreased to 120 °C, the yield of the desired product 3 was reduced to 31% of the corresponding GC yield (Table 1, entry 14). Copper salt, pyridine and dioxygen were all found to be pivotal to this transformation (Table 1, entries 19–21): without CuBr, no desired product was detected; in the absence of pyridine, the yield of the desired product was reduced to 46% with some unknown compounds generated; when the reactions were conducted under N2 atmosphere or air, the desired products were either not detected or reduced to 53%.
With the optimized conditions in hand, we studied the scope and limitations of the Cu-catalyzed oxidative direct amidation reaction. First, we surveyed different carboxylic acids. To our delight, both aromatic and aliphatic carboxylic acids were competent candidates in this transformation (Scheme 2).
Scheme 2: Substrate scope for catalytic, direct amide formation from carboxylic acids and azoles. Reaction conditions: carboxylic acid (0.25 mmol), azole derivative (0.5 mmol), CuBr (10 mol %), pyridine (0.75 mmol), p-xylene (1 mL), 130 °C, 20 h, under O2 atmosphere in a sealed tube. Isolated yield based on carboxylic acids.
Scheme 2: Substrate scope for catalytic, direct amide formation from carboxylic acids and azoles. Reaction co...
For aromatic carboxylic acids, various substituents on the aromatic ring were tolerable under the standard conditions, which suggests that the reaction has a broad substrate scope. Electron-donating (4–9, 13, and 15–17) and electron-withdrawing groups (10–12, 14, 18, and 19) are also compatible in this reaction. Meanwhile, other aromatic acids such as 2-naphthylcarboxylic acid and pyrene-4-carboxylic acid also worked well under the reaction conditions, providing the desired products 20 and 21 in 59% and 88% yield, respectively. Additionally, heterocyclic acids, such as thiophene- and furan-3-carboxylic acid were well tolerated, providing the amides 22 and 23 in acceptable yields. Gratifyingly, aliphatic carboxylic acids, such as cyclohexanecarboxylic acid, hexanoic acid and acetic acid were all proven to be good substrates in this transformation and the desired products 24, 25 and 26 were obtained in reasonable yields. Moreover, bis-substituted benzimidazole also worked very well under the standard conditions to give the desired product 27 in 89% yield.
The scope of amine was further investigated and it seems that only benzimidazole and its derivatives were good candidates in the above direct amide formation (see Scheme S1 in Supporting Information File 1 for details). However, remarkably, when 2-aminopyridine (28) and benzimidazole (2) were mixed with benzoic acid under the standard conditions, compound 29 was obtained in 45% yield instead of the predicted amide 3 (Scheme 3a). Compound 3 was not detected in the reaction. Similarly, when 2-naphthoic acid (30) was used instead of benzoic acid (1), compound 31 was afforded in 56% yield without formation of compound 20 (Scheme 3b). However, when a control experiment was conducted without benzimidazole (2) (Scheme 3c), compound 29 was not detected and the reaction resulted in a complex mixture of undesired compounds.
Scheme 3: Further investigation into the scope of amine.
Scheme 3: Further investigation into the scope of amine.
In order to determine the possibility of transamidation between compound 3 and 2-aminopyridine (28), azole amide 3 was mixed with 2-aminopyridine (28) under the standard conditions. Surprisingly, the azole amide 3 was transformed into 29 in 92% yield, which clearly demonstrated that transamidation occurred in this process (Scheme 4).
Scheme 4: Possible transamidation process.
Scheme 4: Possible transamidation process.
Further exploration into a variety of amines suggested that transamidation could be efficiently accomplished in the absence of the copper catalyst, pyridine and the versatile amines, including aromatic (29, 31–34, 36 and 37) and aliphatic (35, 38–40), primary (29, 31, 34, 37 and 40) and secondary (32, 33, 35, 36, 38 and 39). Even the amino group as a natural product (methyl L-leucinate hydrochloride, 40) was compatible under the conditions, affording corresponding amides in good to excellent yield (Scheme 5), suggesting the potential utility in complex molecule manipulation. In addition, for the carboxylic acid part, both aromatic and aliphatic (37) compounds were competent as well.
Scheme 5: Scope of the amine transamidation from benzimidazole amides. Reaction conditions: benzimidazole amide (0.25 mmol), amine (0.375 mmol), p-xylene (1 mL), 130 °C, sealed tube. Isolated yields. aMethyl L-leucinate hydrochloride as the ammonia source and Et3N (1.5 equiv) were added, the mixture was stirred at room temperature for 3 min, then the mixture was heated to 130 °C.
Scheme 5: Scope of the amine transamidation from benzimidazole amides. Reaction conditions: benzimidazole ami...
The amidation reaction could be easily scaled up without significant decrease in the yield (Scheme 6).
Scheme 6: Preparative scale of the reaction.
Scheme 6: Preparative scale of the reaction.
Radical trapping experiments were performed in order to gain further insight into the mechanism of the catalytic direct amide formation from nonactivated carboxylic acids. The reactions were totally inhibited by radical scavengers 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO) and BHT (2,6-di-tert-butyl-4-methylphenol), suggesting that a radical pathway might be involved in these transformations (Scheme 7).
A careful look at the literature reveals that Cu(II) superoxide species were reported to be formed under our standard conditions and might act as a nucleophile to attack carbonyl groups in certain cases [30], although it was always reported as an electrophile [32]. Thus, a new intermediate, peroxycarboxylate, will be formed in our case. In order to figure out whether peroxycarboxylate acts as our key intermediate, the readily available m-CPBA (41) was used as substrate under the standard conditions and the corresponding product 19 was obtained in 54% yield (Scheme 8a).
Compared to the isolated yield from m-chlorophenylacetic acid (Scheme 2, 19, 51%), we have reason to believe that the above proposed mechanism is plausible. In addition, pyridine N-oxide was applied to the benzoic acid standard conditions in the presence/absence of dioxygen. However, only a trace amount of the desired product 3 was ever detected, thus it was ruled out as a possible pathway (Scheme 8b).
Based on the above control experiments, we postulated a tentative mechanism (Scheme 9). The copper catalyst was postulated to play dual roles in the initial activation of benzoic acid: (1) it acts as a Lewis acid to activate benzoic acid (1), making it vulnerable to nucleophilic attack and (2) it forms Cu(II) superoxide species [30,32-34] to attack activated benzoic acid A to afford intermediate B. Under the standard conditions, intermediate B was switched to intermediate C, once again, with the nucleophilic attack from benzimidazole (2) and eventually intermediate C was converted into the desired 3 via intermediate D along with the recycling of the Cu catalyst.
Conclusion
In conclusion, Cu-catalyzed oxidative direct amidation from nonactivated carboxylic acid with benzimidazoles under dioxygen atmosphere with molecular oxygen as an activating reagent has been described. Azole amides were obtained in good yield in an oxidative protocol with a very broad range of substrates. Subsequent transamidation could be performed using the prepared azole amides and a variety of amines. This reaction has many advantages, in particular the use of an inexpensive copper salt as the catalyst, the use of oxygen as both the sole terminal oxidant and activating reagent, and inexpensive and readily available starting materials. This is the first Cu-catalyzed, direct, amide formation between nonactivated carboxylic acids and benzimidazoles in coupling reagent, traditional activating, reagent-free conditions. A mechanistic study demonstrated that a peroxycarboxylate was the key intermediate. This was the first reported example in such a simple system which could be employed in amide formation. Notably, the transamidation occurred smoothly between the azole amide and a variety of amines, thus providing versatile amides with our new strategy. Therefore, this is a very general method for azole amide formation from versatile carboxylic acids with potential application in organic synthesis.
Supporting Information
| Supporting Information File 1: Experimental procedures, analytical data and NMR spectra. | ||
| Format: PDF | Size: 1.4 MB | Download |
References
-
Humphrey, M. J.; Chamberlin, R. A. Chem. Rev. 1997, 97, 2243–2266. doi:10.1021/cr950005s
Return to citation in text: [1] -
Valeur, E.; Bradley, M. Chem. Soc. Rev. 2009, 38, 606–631. doi:10.1039/B701677H
Return to citation in text: [1] -
Mitchell, J. A.; Reid, E. E. J. Am. Chem. Soc. 1931, 53, 1879–1883. doi:10.1021/ja01356a037
Return to citation in text: [1] -
Shepard, E. E.; Porter, H. D.; Noth, J. F.; Simmans, C. K. J. Org. Chem. 1952, 17, 568–576. doi:10.1021/jo01138a009
Return to citation in text: [1] -
Schmidt, U.; Dietsche, M. Angew. Chem., Int. Ed. Engl. 1982, 21, 143–144. doi:10.1002/anie.198201431
Return to citation in text: [1] -
Cheung, C. W.; Fung, H. S.; Lee, S. Y.; Qian, Y. Y.; Chan, Y. W.; Chan, K. S. Organometallics 2010, 29, 1343–1354. doi:10.1021/om9008668
Return to citation in text: [1] -
Das, S.; Biswas, R.; Mukherjee, B. J. Phys. Chem. B 2015, 119, 274–283. doi:10.1021/jp509869d
Return to citation in text: [1] -
Yoo, W.-J.; Li, C.-J. J. Am. Chem. Soc. 2006, 128, 13064–13065. doi:10.1021/ja064315b
Return to citation in text: [1] -
Ekoue-Kovi, K.; Wolf, C. Org. Lett. 2007, 9, 3429–3432. doi:10.1021/ol7014626
Return to citation in text: [1] -
Reddy, K. R.; Maheswari, C. U.; Venkateshwar, M.; Kantam, M. L. Eur. J. Org. Chem. 2008, 3619–3622. doi:10.1002/ejoc.200800454
Return to citation in text: [1] -
Ghosh, S. C.; Ngiam, J. S. Y.; Chai, C. L. L.; Seayad, A. M.; Dang, T. T.; Chen, A. Adv. Synth. Catal. 2012, 354, 1407–1412. doi:10.1002/adsc.201200020
Return to citation in text: [1] -
Prasad, V.; Kale, R. R.; Mishra, B. B.; Kumar, D.; Tiwari, V. K. Org. Lett. 2012, 14, 2936–2939. doi:10.1021/ol3012315
Return to citation in text: [1] -
Hosseini-Sarvari, M.; Sharghi, H. J. Org. Chem. 2006, 71, 6652–6654. doi:10.1021/jo060847z
Return to citation in text: [1] -
Ishihara, K.; Ohara, S.; Yamamoto, H. J. Org. Chem. 1996, 61, 4196–4197. doi:10.1021/jo9606564
Return to citation in text: [1] -
Allen, C. L.; Chhatwal, A. R.; Williams, J. M. J. Chem. Commun. 2012, 48, 666–668. doi:10.1039/C1CC15210F
Return to citation in text: [1] -
Goh, K. S.; Tan, C.-H. RSC Adv. 2012, 2, 5536–5538. doi:10.1039/C2RA20773G
Return to citation in text: [1] -
Subramanian, P.; Indu, S.; Kaliappan, K. P. Org. Lett. 2014, 16, 6212. doi:10.1021/ol5031266
Return to citation in text: [1] -
Starkov, P.; Sheppard, T. D. Org. Biomol. Chem. 2011, 9, 1320–1323. doi:10.1039/C0OB01069C
Return to citation in text: [1] -
Tan, B.; Toda, N.; Barbas, C. F., III. Angew. Chem., Int. Ed. 2012, 51, 12538–12541. doi:10.1002/anie.201205921
Return to citation in text: [1] -
Charville, H.; Jackson, D.; Hodges, G.; Whiting, A. Chem. Commun. 2010, 46, 1813–1823. doi:10.1039/B923093A
Return to citation in text: [1] -
Kosal, A. D.; Wilson, E. E.; Ashfeld, B. L. Chem. – Eur. J. 2012, 18, 14444–14453. doi:10.1002/chem.201201773
Return to citation in text: [1] -
Dunlap, F. L. J. Am. Chem. Soc. 1902, 24, 758–763. doi:10.1021/ja02022a008
Return to citation in text: [1] -
Sheehan, J. C.; Hess, G. P. J. Am. Chem. Soc. 1955, 77, 1067–1068. doi:10.1021/ja01609a099
Return to citation in text: [1] -
Carey, J. S.; Laffan, D.; Thomson, C.; Williams, M. T. Org. Biomol. Chem. 2006, 4, 2337–2347. doi:10.1039/B602413K
Return to citation in text: [1] -
Punniyamurthy, T.; Velusamy, S.; Iqbal, J. Chem. Rev. 2005, 105, 2329–2364. doi:10.1021/cr050523v
Return to citation in text: [1] -
Wang, T.; Jiao, N. J. Am. Chem. Soc. 2013, 135, 11692–11695. doi:10.1021/ja403824y
Return to citation in text: [1] -
Ley, S. V.; Thomas, A. W. Angew. Chem., Int. Ed. 2003, 42, 5400–5449. doi:10.1002/anie.200300594
Return to citation in text: [1] -
Bi, H.-P.; Zhao, L.; Liang, Y.-M.; Li, C.-J. Angew. Chem., Int. Ed. 2009, 48, 792–795. doi:10.1002/anie.200805122
Return to citation in text: [1] -
Liu, J.; Liu, Q.; Yi, H.; Qin, C.; Bai, R.; Qi, X.; Lan, Y.; Lei, A. Angew. Chem., Int. Ed. 2014, 53, 502–506. doi:10.1002/anie.201308614
Return to citation in text: [1] -
Pirovano, P.; Magherusan, A. M.; McGlynn, C.; Ure, A.; Lynes, A.; McDonald, A. R. Angew. Chem., Int. Ed. 2014, 53, 5946–5950. doi:10.1002/anie.201311152
Return to citation in text: [1] [2] [3] -
Horton, D. A.; Bourne, G. T.; Smythe, M. L. Chem. Rev. 2003, 103, 893–930. doi:10.1021/cr020033s
Return to citation in text: [1] -
Yoon, J.; Solomon, E. I. J. Am. Chem. Soc. 2007, 129, 13127–13136. doi:10.1021/ja073947a
Return to citation in text: [1] [2] -
Kieber-Emmons, M. T.; Ginsbach, J. W.; Wick, P. K.; Lucas, H. R.; Helton, M. E.; Lucchese, B.; Suzuki, M.; Zuberbühler, A. D.; Karlin, K. D.; Solomon, E. I. Angew. Chem., Int. Ed. 2014, 53, 4935–4939. doi:10.1002/anie.201402166
Return to citation in text: [1] -
Bonnot, F.; Tremey, E.; von Stetten, D.; Rat, S.; Duval, S.; Carpentier, P.; Clemancey, M.; Desbois, A.; Nivière, V. Angew. Chem., Int. Ed. 2014, 53, 5926–5930. doi:10.1002/anie.201400356
Return to citation in text: [1]
| 1. | Humphrey, M. J.; Chamberlin, R. A. Chem. Rev. 1997, 97, 2243–2266. doi:10.1021/cr950005s |
| 23. | Sheehan, J. C.; Hess, G. P. J. Am. Chem. Soc. 1955, 77, 1067–1068. doi:10.1021/ja01609a099 |
| 8. | Yoo, W.-J.; Li, C.-J. J. Am. Chem. Soc. 2006, 128, 13064–13065. doi:10.1021/ja064315b |
| 9. | Ekoue-Kovi, K.; Wolf, C. Org. Lett. 2007, 9, 3429–3432. doi:10.1021/ol7014626 |
| 10. | Reddy, K. R.; Maheswari, C. U.; Venkateshwar, M.; Kantam, M. L. Eur. J. Org. Chem. 2008, 3619–3622. doi:10.1002/ejoc.200800454 |
| 11. | Ghosh, S. C.; Ngiam, J. S. Y.; Chai, C. L. L.; Seayad, A. M.; Dang, T. T.; Chen, A. Adv. Synth. Catal. 2012, 354, 1407–1412. doi:10.1002/adsc.201200020 |
| 12. | Prasad, V.; Kale, R. R.; Mishra, B. B.; Kumar, D.; Tiwari, V. K. Org. Lett. 2012, 14, 2936–2939. doi:10.1021/ol3012315 |
| 13. | Hosseini-Sarvari, M.; Sharghi, H. J. Org. Chem. 2006, 71, 6652–6654. doi:10.1021/jo060847z |
| 14. | Ishihara, K.; Ohara, S.; Yamamoto, H. J. Org. Chem. 1996, 61, 4196–4197. doi:10.1021/jo9606564 |
| 15. | Allen, C. L.; Chhatwal, A. R.; Williams, J. M. J. Chem. Commun. 2012, 48, 666–668. doi:10.1039/C1CC15210F |
| 16. | Goh, K. S.; Tan, C.-H. RSC Adv. 2012, 2, 5536–5538. doi:10.1039/C2RA20773G |
| 17. | Subramanian, P.; Indu, S.; Kaliappan, K. P. Org. Lett. 2014, 16, 6212. doi:10.1021/ol5031266 |
| 18. | Starkov, P.; Sheppard, T. D. Org. Biomol. Chem. 2011, 9, 1320–1323. doi:10.1039/C0OB01069C |
| 19. | Tan, B.; Toda, N.; Barbas, C. F., III. Angew. Chem., Int. Ed. 2012, 51, 12538–12541. doi:10.1002/anie.201205921 |
| 20. | Charville, H.; Jackson, D.; Hodges, G.; Whiting, A. Chem. Commun. 2010, 46, 1813–1823. doi:10.1039/B923093A |
| 21. | Kosal, A. D.; Wilson, E. E.; Ashfeld, B. L. Chem. – Eur. J. 2012, 18, 14444–14453. doi:10.1002/chem.201201773 |
| 30. | Pirovano, P.; Magherusan, A. M.; McGlynn, C.; Ure, A.; Lynes, A.; McDonald, A. R. Angew. Chem., Int. Ed. 2014, 53, 5946–5950. doi:10.1002/anie.201311152 |
| 32. | Yoon, J.; Solomon, E. I. J. Am. Chem. Soc. 2007, 129, 13127–13136. doi:10.1021/ja073947a |
| 33. | Kieber-Emmons, M. T.; Ginsbach, J. W.; Wick, P. K.; Lucas, H. R.; Helton, M. E.; Lucchese, B.; Suzuki, M.; Zuberbühler, A. D.; Karlin, K. D.; Solomon, E. I. Angew. Chem., Int. Ed. 2014, 53, 4935–4939. doi:10.1002/anie.201402166 |
| 34. | Bonnot, F.; Tremey, E.; von Stetten, D.; Rat, S.; Duval, S.; Carpentier, P.; Clemancey, M.; Desbois, A.; Nivière, V. Angew. Chem., Int. Ed. 2014, 53, 5926–5930. doi:10.1002/anie.201400356 |
| 2. | Valeur, E.; Bradley, M. Chem. Soc. Rev. 2009, 38, 606–631. doi:10.1039/B701677H |
| 3. | Mitchell, J. A.; Reid, E. E. J. Am. Chem. Soc. 1931, 53, 1879–1883. doi:10.1021/ja01356a037 |
| 4. | Shepard, E. E.; Porter, H. D.; Noth, J. F.; Simmans, C. K. J. Org. Chem. 1952, 17, 568–576. doi:10.1021/jo01138a009 |
| 5. | Schmidt, U.; Dietsche, M. Angew. Chem., Int. Ed. Engl. 1982, 21, 143–144. doi:10.1002/anie.198201431 |
| 6. | Cheung, C. W.; Fung, H. S.; Lee, S. Y.; Qian, Y. Y.; Chan, Y. W.; Chan, K. S. Organometallics 2010, 29, 1343–1354. doi:10.1021/om9008668 |
| 7. | Das, S.; Biswas, R.; Mukherjee, B. J. Phys. Chem. B 2015, 119, 274–283. doi:10.1021/jp509869d |
| 30. | Pirovano, P.; Magherusan, A. M.; McGlynn, C.; Ure, A.; Lynes, A.; McDonald, A. R. Angew. Chem., Int. Ed. 2014, 53, 5946–5950. doi:10.1002/anie.201311152 |
| 30. | Pirovano, P.; Magherusan, A. M.; McGlynn, C.; Ure, A.; Lynes, A.; McDonald, A. R. Angew. Chem., Int. Ed. 2014, 53, 5946–5950. doi:10.1002/anie.201311152 |
| 27. | Ley, S. V.; Thomas, A. W. Angew. Chem., Int. Ed. 2003, 42, 5400–5449. doi:10.1002/anie.200300594 |
| 28. | Bi, H.-P.; Zhao, L.; Liang, Y.-M.; Li, C.-J. Angew. Chem., Int. Ed. 2009, 48, 792–795. doi:10.1002/anie.200805122 |
| 29. | Liu, J.; Liu, Q.; Yi, H.; Qin, C.; Bai, R.; Qi, X.; Lan, Y.; Lei, A. Angew. Chem., Int. Ed. 2014, 53, 502–506. doi:10.1002/anie.201308614 |
| 32. | Yoon, J.; Solomon, E. I. J. Am. Chem. Soc. 2007, 129, 13127–13136. doi:10.1021/ja073947a |
| 25. | Punniyamurthy, T.; Velusamy, S.; Iqbal, J. Chem. Rev. 2005, 105, 2329–2364. doi:10.1021/cr050523v |
| 26. | Wang, T.; Jiao, N. J. Am. Chem. Soc. 2013, 135, 11692–11695. doi:10.1021/ja403824y |
| 24. | Carey, J. S.; Laffan, D.; Thomson, C.; Williams, M. T. Org. Biomol. Chem. 2006, 4, 2337–2347. doi:10.1039/B602413K |
| 31. | Horton, D. A.; Bourne, G. T.; Smythe, M. L. Chem. Rev. 2003, 103, 893–930. doi:10.1021/cr020033s |
© 2015 Ding et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)