Abstract
Thioxanthones – being readily available in one step from thiosalicylic acid and arenes – were used in ruthenium-catalyzed C–H-activation reaction to produce 1-mono- or 1,8-disubstituted thioxanthones in good to excellent yields. Scope and limitation of this reaction are presented.
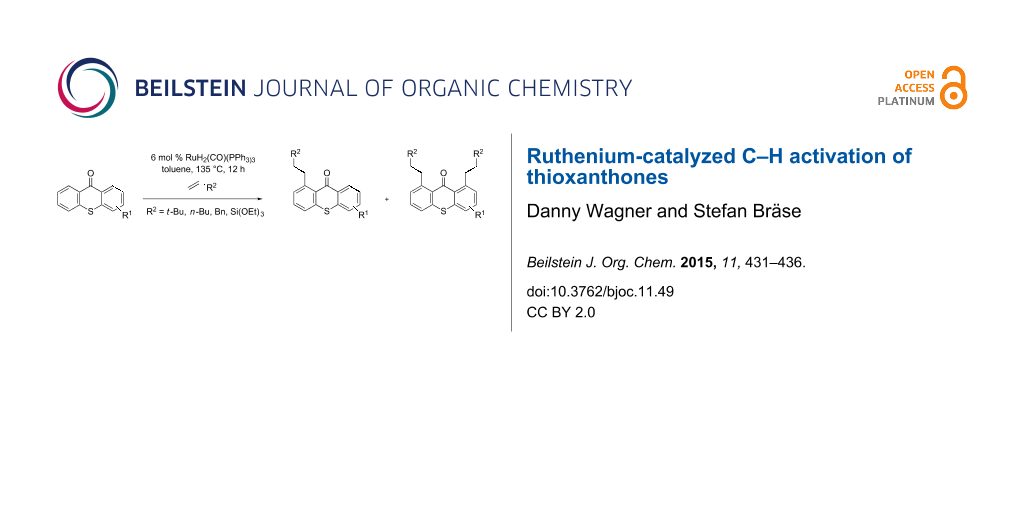
Graphical Abstract
Introduction
Thioxanthones (Figure 1) belong as a unique member to the large group of benzoannelated heterocycles [1]. They have found extensive use in biomedical applications (drugs and other bioactive compounds [2-5]) and material sciences, e.g., as photosensitizers (e.g., isopropylthioxanthone or diethylthioxanthone) [6-8] or as ligands [9,10]. Despite the widespread occurrences, there are only few modular syntheses reported so far and photosensitizing materials are often used as undefined mixtures. In addition, functionalization reactions for thioxanthones, such as C–C-bond formations [11,12], are underdeveloped [13]. For example, there are only a handful of 1,8-dialkyl/aryl-functionalized thioxanthones known [14-16], while more than 500 1-substituted thioxanthones are reported according to Scifinder. In contrast, xanthone chemistry aiming at a high degree of substitution seems to be well explored [17,18].
This fact motivated us to extend existing methods [14,15] for the synthesis of substituted thioxanthones. We were intrigued by the fact that carbonyl-substituted arenes can undergo a smooth C–H activation and alkylation in the presence of metal catalysts [19] (for general reviews see [20,21]). However, there are only few examples [14,15] with sulfur-containing heterocycles as in general sulfur inhibits the catalytic activity of many transition metal catalysts [22].
Results and Discussion
Synthesis of functionalized thioxanthones
The required thioxanthones 1 were prepared using standard procedures [1,23]. For certain examples, optimizations of the standard protocol were required (Table 1 and Supporting Information File 1).
Table 1: Synthesis of substituted thioxanthones from thiosalicylic acid.a
|
|
|||
| Entry | Arene 3 | Thioxanthone 1 | Yieldb |
|---|---|---|---|
| 1 |
3b |
1b |
57 |
| 2 |
3c |
1c |
9 |
| 3 |
3e |
1e |
87 |
| 4 |
3h |
1g |
25 |
aFor conditions see Supporting Information File 1; bisolated yields.
In case of methoxyarenes this method was not successful due to a partial ether cleavage catalyzed by hot sulfuric acid. In this case, methylation (Me2SO4, K2CO3) of the hydroxythioxanthones 1c, 1e and 1g provided the required methyl ethers 1d, 1f and 1h, respectively in good yields (80, 85 and 95%), Scheme 1.
Scheme 1: Route to methoxyarenes 1d, 1f, and 1h.
Scheme 1: Route to methoxyarenes 1d, 1f, and 1h.
Ru-catalyzed C–H activation
Following the precedence for other carbonyl compounds, we used the protocol of Murai et al. [19] to investigate the use of thioxanthones in this C–H-alkylation reaction (Scheme 2). For recent examples and reviews, also for related rhodium-catalyzed systems, see [24-38].
Scheme 2: Ru-catalyzed C–H activation of thioxanthones. Conditions: 6 mol % RuH2(CO)(PPh3)3, toluene, 135 °C, 12 h.
Scheme 2: Ru-catalyzed C–H activation of thioxanthones. Conditions: 6 mol % RuH2(CO)(PPh3)3, toluene, 135 °C,...
It should be noted that already in the pioneering work there are also examples using sulfur heterocycles such as thiophene derivatives [19]. Gratifyingly, the reaction of dimethyl-substituted thioxanthone 1b with the model olefin neohexene (3,3-dimethyl-1-butene, 4a) was successful and the product 1ba was obtained in 65% yield (Table 2, entry 4). This and all the products obtained exhibit exclusively n-alkylation – branched alkyl chains originating from addition at the 2-position were not found. Other olefins like 1-hexene (4b) or 3-phenylpropene (4c) also worked smoothly (Table 2, entries 5 and 6). In addition, the silyl-substituted olefin 4d was also successfully used in this reaction (Table 2, entry 7). The product 1bd is formed in good yield, however, it is prone to hydrolysis thus the isolated yield of the pure product was rather low. In contrast to literature precedence for other carbonyl compounds [19], other olefins (styrene, vinyl/allyl ethers, perfluoroalkylethenes) failed since they polymerize during the reaction.
Table 2: C–H activation.
|
|
||||
| Entry | Thioxanthone | Alkene (equiv) | Product | Yield [%]a |
|---|---|---|---|---|
| 1 |
1a |
4a (1.2 equiv) |
1aa |
47 |
|
1aabis |
20 | |||
| 2 |
1a |
4b (6 equiv) |
1abbis |
43 |
| 3 |
1a |
4c (1.2 equiv) |
1ac |
7 |
| 4 |
1b |
4a (3 equiv) |
1ba |
65 |
| 5 |
1b |
4b (3 equiv) |
1bb |
66 |
| 6 |
1b |
4c (3 equiv) |
1bc |
27 |
| 7 |
1b |
4d (3 equiv) |
1bd |
55b |
| 8 |
1h |
4a (3 equiv) |
1ha |
83 |
| 9 |
1h |
4b (3 equiv) |
1hb |
65 |
aIsolated yields; bcrude yield close to quantitative, but product prone to hydrolysis.
Extension of the unsymmetrical heterocycle system 1b to the unsubstituted thioxanthone (1a) was also successful: Depending on the amount of alkene, mono- (Table 2, entry 3) or 1,8-disubstituted thioxanthones such as 1aabis or 1abbis were isolated (see Table 2, entries 1 and 2). In addition, other thioxanthones such as 1h are also suitable substrates (Table 2, entries 8 and 9).
However, other thioxanthones such as a phenanthrene-annelated thioxanthone (not shown) failed to give the desired products due to insolubility of the starting materials.
Conclusion
We have presented a C–H-activation route towards the preparation of functionalized thioxanthones. Despite the fact that mono- and disubstituted thioxanthones can be found starting from the parent system, the selectivity can be controlled using different stoichiometries. It should be noted that alkoxy and silyl functionalities are tolerated in the reaction.
Experimental
The catalyst RuH2(CO)(PPh3)3 was prepared according to literature [19] and stored under Argon with exclusion of water.
General procedure for C–H activation
In a sealed Schlenk pressure tube, 1.00 mmol of the thioxanthone, 0.060 mmol (55 mg) RuH2(CO)(PPh3)3, 1.2 to 6 mmol of the olefin and 2 mL toluene were stirred and heated to 135 °C for 12 h. After cooling, the solvent was evaporated under reduced pressure and the residue submitted to column chromatography on silica gel using cyclohexane/ethyl acetate as eluent.
Supporting Information
| Supporting Information File 1: Characterization data and spectra for compounds 2 and 3. | ||
| Format: PDF | Size: 2.0 MB | Download |
References
-
Nelson, A. Sci. Synth. 2003, 14, 787.
Return to citation in text: [1] [2] -
Woo, S.-W.; Kang, D.-H.; Kim, J.-S.; Lee, C.-S.; Lee, E.-S.; Jahng, Y.-D.; Kwon, Y.-J.; Na, Y.-H. Bull. Korean Chem. Soc. 2008, 29, 471. doi:10.5012/bkcs.2008.29.2.471
Return to citation in text: [1] -
Lima, R. T.; Sousa, D.; Choosang, K.; Pakkong, P.; Palmeira, A.; Paiva, A. M.; Seca, H.; Cerqueira, F.; Pedro, M.; Pinto, M. M.; Sousa, E.; Vasconcelos, M. H. Ann. Oncol. 2013, 24 (Suppl. 1), i26. doi:10.1093/annonc/mdt045.14
Return to citation in text: [1] -
Lockhart, A. C.; Calvo, E.; Tolcher, A. W.; Rowinsky, E. K.; Shackleton, G.; Morrison, J. G.; Rafi, R.; VerMeulen, W.; Rothenberg, M. L. Am. J. Clin. Oncol. 2009, 32, 9. doi:10.1097/COC.0b013e318178331b
Return to citation in text: [1] -
Paiva, A. M.; Pinto, M. M.; Sousa, E. Curr. Med. Chem. 2013, 20, 2438. doi:10.2174/0929867311320190004
Return to citation in text: [1] -
Karasu, F.; Arsu, N.; Jockusch, S.; Turro, N. J. J. Org. Chem. 2013, 78, 9161. doi:10.1021/jo401386t
Return to citation in text: [1] -
Balta, D. K.; Temel, G.; Goksu, G.; Ocal, N.; Arsu, N. Macromolecules 2012, 45, 119. doi:10.1021/ma202168m
Return to citation in text: [1] -
Malval, J.-P.; Jin, M.; Morlet-Savary, F.; Chaumeil, H.; Defoin, A.; Soppera, O.; Scheul, T.; Bouriau, M.; Baldeck, P. L. Chem. Mater. 2011, 23, 3411. doi:10.1021/cm200595y
Return to citation in text: [1] -
Breslow, R.; Mehta, M. P. J. Am. Chem. Soc. 1986, 108, 6417. doi:10.1021/ja00280a065
Return to citation in text: [1] -
Breslow, R.; Guo, T. Tetrahedron Lett. 1987, 28, 3187. doi:10.1016/S0040-4039(00)95467-4
Return to citation in text: [1] -
Zinad, D. S.; Feist, H.; Villinger, A.; Langer, P. Tetrahedron 2012, 68, 711. doi:10.1016/j.tet.2011.10.095
Return to citation in text: [1] -
Zinad, D. S.; Hussain, M.; Akrawi, O. A.; Villinger, A.; Langer, P. Tetrahedron Lett. 2011, 52, 3451. doi:10.1016/j.tetlet.2011.04.102
Return to citation in text: [1] -
Karasu, F.; Arsu, N.; Yagci, Y. J. Appl. Polym. Sci. 2007, 103, 3766. doi:10.1002/app.25467
Return to citation in text: [1] -
Gupta, S. K.; Weber, W. P. Polym. Prepr. (Am. Chem. Soc., Div. Polym. Chem.) 1999, 40, 62.
Return to citation in text: [1] [2] [3] -
Niesert, C.-P.; Pawlowski, G.; Gries, W.-K.; Przybilla, K.-J. Mit Silikonen kompatible Photoinitiatoren und diese enthaltende lichtempfindliche Gemische. Eur. Pat. Appl. 0705865 A1, April 1, 1996.
Return to citation in text: [1] [2] [3] -
Schaarschmidt, A. Justus Liebigs Ann. Chem. 1915, 409, 59. doi:10.1002/jlac.19154090106
Return to citation in text: [1] -
Gérard, E. M. C.; Bräse, S. Chem. – Eur. J. 2008, 14, 8086. doi:10.1002/chem.200801507
Return to citation in text: [1] -
Masters, K.-S.; Bräse, S. Chem. Rev. 2012, 112, 3717. doi:10.1021/cr100446h
Return to citation in text: [1] -
Murai, S.; Kakiuchi, F.; Sekine, S.; Tanaka, Y.; Kamatani, A.; Sonoda, M.; Chatani, N. Nature 1993, 366, 529. doi:10.1038/366529a0
Return to citation in text: [1] [2] [3] [4] [5] -
Jean-Gerard, L.; Jazzar, R.; Baudoin, O. In Metal-Cross Coupling Reactions and more; de Meijere, A.; Bräse, S.; Oestreich, M., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, 2014; Vol. 3, p 1427.
Return to citation in text: [1] -
Dyker, G., Ed. Handbook of C-H Transformations: Applications in Organic Synthesis; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, 2005; Vol. 2.
Return to citation in text: [1] -
Badano, J. M.; Quiroga, M.; Betti, C.; Vera, C.; Canavese, S.; Coloma-Pascual, F. Catal. Lett. 2010, 137, 35. doi:10.1007/s10562-010-0336-x
Return to citation in text: [1] -
Okabayashi, I.; Kimura, M.; Fujiwara, H.; Kato, A. Chem. Pharm. Bull. 1987, 35, 2545. doi:10.1248/cpb.35.2545
Return to citation in text: [1] -
Jia, C.; Piao, D.; Oyamada, J.; Lu, W.; Kitamura, T.; Fujiwara, Y. Science 2000, 287, 1992. doi:10.1126/science.287.5460.1992
Return to citation in text: [1] -
Yamamoto, Y. Chem. Soc. Rev. 2014, 43, 1575. doi:10.1039/C3CS60369E
Return to citation in text: [1] -
Chinchilla, R.; Nájera, C. Chem. Rev. 2014, 114, 1783. doi:10.1021/cr400133p
Return to citation in text: [1] -
Trejos, A.; Odell, L. R. Sci. Synth. 2013, 3, 345.
Return to citation in text: [1] -
Hussain, H.; Green, I. R.; Ahmed, I. Chem. Rev. 2013, 113, 3329. doi:10.1021/cr3004373
Return to citation in text: [1] -
Engle, K. M.; Yu, J.-Q. J. Org. Chem. 2013, 78, 8927. doi:10.1021/jo400159y
Return to citation in text: [1] -
Yu, D.-G.; Li, B.-J.; Shi, Z.-J. Tetrahedron 2012, 68, 5130. doi:10.1016/j.tet.2012.05.040
Return to citation in text: [1] -
Patureau, F. W.; Wencel-Delord, J.; Glorius, F. Aldrichimica Acta 2012, 45, 31.
Return to citation in text: [1] -
Mei, T.-S.; Kou, L.; Ma, S.; Engle, K. M.; Yu, J.-Q. Synthesis 2012, 44, 1778. doi:10.1055/s-0031-1289766
Return to citation in text: [1] -
Leyva-Pérez, A.; Corma, A. Angew. Chem., Int. Ed. 2012, 51, 614. doi:10.1002/anie.201101726
Return to citation in text: [1] -
Kuhl, N.; Hopkinson, M. N.; Wencel-Delord, J.; Glorius, F. Angew. Chem., Int. Ed. 2012, 51, 10236. doi:10.1002/anie.201203269
Return to citation in text: [1] -
Fedorov, A. Yu.; Nyuchev, A. V.; Beletskaya, I. P. Chem. Heterocycl. Compd. 2012, 48, 166. doi:10.1007/s10593-012-0980-8
Return to citation in text: [1] -
Yeung, C. S.; Dong, V. M. Chem. Rev. 2011, 111, 1215. doi:10.1021/cr100280d
Return to citation in text: [1] -
Wencel-Delord, J.; Dröge, T.; Liu, F.; Glorius, F. Chem. Soc. Rev. 2011, 40, 4740. doi:10.1039/c1cs15083a
Return to citation in text: [1] -
Sun, C.-L.; Li, B.-J.; Shi, Z.-J. Chem. Rev. 2011, 111, 1293. doi:10.1021/cr100198w
Return to citation in text: [1]
| 19. | Murai, S.; Kakiuchi, F.; Sekine, S.; Tanaka, Y.; Kamatani, A.; Sonoda, M.; Chatani, N. Nature 1993, 366, 529. doi:10.1038/366529a0 |
| 19. | Murai, S.; Kakiuchi, F.; Sekine, S.; Tanaka, Y.; Kamatani, A.; Sonoda, M.; Chatani, N. Nature 1993, 366, 529. doi:10.1038/366529a0 |
| 19. | Murai, S.; Kakiuchi, F.; Sekine, S.; Tanaka, Y.; Kamatani, A.; Sonoda, M.; Chatani, N. Nature 1993, 366, 529. doi:10.1038/366529a0 |
| 11. | Zinad, D. S.; Feist, H.; Villinger, A.; Langer, P. Tetrahedron 2012, 68, 711. doi:10.1016/j.tet.2011.10.095 |
| 12. | Zinad, D. S.; Hussain, M.; Akrawi, O. A.; Villinger, A.; Langer, P. Tetrahedron Lett. 2011, 52, 3451. doi:10.1016/j.tetlet.2011.04.102 |
| 19. | Murai, S.; Kakiuchi, F.; Sekine, S.; Tanaka, Y.; Kamatani, A.; Sonoda, M.; Chatani, N. Nature 1993, 366, 529. doi:10.1038/366529a0 |
| 9. | Breslow, R.; Mehta, M. P. J. Am. Chem. Soc. 1986, 108, 6417. doi:10.1021/ja00280a065 |
| 10. | Breslow, R.; Guo, T. Tetrahedron Lett. 1987, 28, 3187. doi:10.1016/S0040-4039(00)95467-4 |
| 24. | Jia, C.; Piao, D.; Oyamada, J.; Lu, W.; Kitamura, T.; Fujiwara, Y. Science 2000, 287, 1992. doi:10.1126/science.287.5460.1992 |
| 25. | Yamamoto, Y. Chem. Soc. Rev. 2014, 43, 1575. doi:10.1039/C3CS60369E |
| 26. | Chinchilla, R.; Nájera, C. Chem. Rev. 2014, 114, 1783. doi:10.1021/cr400133p |
| 27. | Trejos, A.; Odell, L. R. Sci. Synth. 2013, 3, 345. |
| 28. | Hussain, H.; Green, I. R.; Ahmed, I. Chem. Rev. 2013, 113, 3329. doi:10.1021/cr3004373 |
| 29. | Engle, K. M.; Yu, J.-Q. J. Org. Chem. 2013, 78, 8927. doi:10.1021/jo400159y |
| 30. | Yu, D.-G.; Li, B.-J.; Shi, Z.-J. Tetrahedron 2012, 68, 5130. doi:10.1016/j.tet.2012.05.040 |
| 31. | Patureau, F. W.; Wencel-Delord, J.; Glorius, F. Aldrichimica Acta 2012, 45, 31. |
| 32. | Mei, T.-S.; Kou, L.; Ma, S.; Engle, K. M.; Yu, J.-Q. Synthesis 2012, 44, 1778. doi:10.1055/s-0031-1289766 |
| 33. | Leyva-Pérez, A.; Corma, A. Angew. Chem., Int. Ed. 2012, 51, 614. doi:10.1002/anie.201101726 |
| 34. | Kuhl, N.; Hopkinson, M. N.; Wencel-Delord, J.; Glorius, F. Angew. Chem., Int. Ed. 2012, 51, 10236. doi:10.1002/anie.201203269 |
| 35. | Fedorov, A. Yu.; Nyuchev, A. V.; Beletskaya, I. P. Chem. Heterocycl. Compd. 2012, 48, 166. doi:10.1007/s10593-012-0980-8 |
| 36. | Yeung, C. S.; Dong, V. M. Chem. Rev. 2011, 111, 1215. doi:10.1021/cr100280d |
| 37. | Wencel-Delord, J.; Dröge, T.; Liu, F.; Glorius, F. Chem. Soc. Rev. 2011, 40, 4740. doi:10.1039/c1cs15083a |
| 38. | Sun, C.-L.; Li, B.-J.; Shi, Z.-J. Chem. Rev. 2011, 111, 1293. doi:10.1021/cr100198w |
| 6. | Karasu, F.; Arsu, N.; Jockusch, S.; Turro, N. J. J. Org. Chem. 2013, 78, 9161. doi:10.1021/jo401386t |
| 7. | Balta, D. K.; Temel, G.; Goksu, G.; Ocal, N.; Arsu, N. Macromolecules 2012, 45, 119. doi:10.1021/ma202168m |
| 8. | Malval, J.-P.; Jin, M.; Morlet-Savary, F.; Chaumeil, H.; Defoin, A.; Soppera, O.; Scheul, T.; Bouriau, M.; Baldeck, P. L. Chem. Mater. 2011, 23, 3411. doi:10.1021/cm200595y |
| 22. | Badano, J. M.; Quiroga, M.; Betti, C.; Vera, C.; Canavese, S.; Coloma-Pascual, F. Catal. Lett. 2010, 137, 35. doi:10.1007/s10562-010-0336-x |
| 2. | Woo, S.-W.; Kang, D.-H.; Kim, J.-S.; Lee, C.-S.; Lee, E.-S.; Jahng, Y.-D.; Kwon, Y.-J.; Na, Y.-H. Bull. Korean Chem. Soc. 2008, 29, 471. doi:10.5012/bkcs.2008.29.2.471 |
| 3. | Lima, R. T.; Sousa, D.; Choosang, K.; Pakkong, P.; Palmeira, A.; Paiva, A. M.; Seca, H.; Cerqueira, F.; Pedro, M.; Pinto, M. M.; Sousa, E.; Vasconcelos, M. H. Ann. Oncol. 2013, 24 (Suppl. 1), i26. doi:10.1093/annonc/mdt045.14 |
| 4. | Lockhart, A. C.; Calvo, E.; Tolcher, A. W.; Rowinsky, E. K.; Shackleton, G.; Morrison, J. G.; Rafi, R.; VerMeulen, W.; Rothenberg, M. L. Am. J. Clin. Oncol. 2009, 32, 9. doi:10.1097/COC.0b013e318178331b |
| 5. | Paiva, A. M.; Pinto, M. M.; Sousa, E. Curr. Med. Chem. 2013, 20, 2438. doi:10.2174/0929867311320190004 |
| 1. | Nelson, A. Sci. Synth. 2003, 14, 787. |
| 23. | Okabayashi, I.; Kimura, M.; Fujiwara, H.; Kato, A. Chem. Pharm. Bull. 1987, 35, 2545. doi:10.1248/cpb.35.2545 |
| 14. | Gupta, S. K.; Weber, W. P. Polym. Prepr. (Am. Chem. Soc., Div. Polym. Chem.) 1999, 40, 62. |
| 15. | Niesert, C.-P.; Pawlowski, G.; Gries, W.-K.; Przybilla, K.-J. Mit Silikonen kompatible Photoinitiatoren und diese enthaltende lichtempfindliche Gemische. Eur. Pat. Appl. 0705865 A1, April 1, 1996. |
| 20. | Jean-Gerard, L.; Jazzar, R.; Baudoin, O. In Metal-Cross Coupling Reactions and more; de Meijere, A.; Bräse, S.; Oestreich, M., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, 2014; Vol. 3, p 1427. |
| 21. | Dyker, G., Ed. Handbook of C-H Transformations: Applications in Organic Synthesis; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, 2005; Vol. 2. |
| 17. | Gérard, E. M. C.; Bräse, S. Chem. – Eur. J. 2008, 14, 8086. doi:10.1002/chem.200801507 |
| 18. | Masters, K.-S.; Bräse, S. Chem. Rev. 2012, 112, 3717. doi:10.1021/cr100446h |
| 14. | Gupta, S. K.; Weber, W. P. Polym. Prepr. (Am. Chem. Soc., Div. Polym. Chem.) 1999, 40, 62. |
| 15. | Niesert, C.-P.; Pawlowski, G.; Gries, W.-K.; Przybilla, K.-J. Mit Silikonen kompatible Photoinitiatoren und diese enthaltende lichtempfindliche Gemische. Eur. Pat. Appl. 0705865 A1, April 1, 1996. |
| 14. | Gupta, S. K.; Weber, W. P. Polym. Prepr. (Am. Chem. Soc., Div. Polym. Chem.) 1999, 40, 62. |
| 15. | Niesert, C.-P.; Pawlowski, G.; Gries, W.-K.; Przybilla, K.-J. Mit Silikonen kompatible Photoinitiatoren und diese enthaltende lichtempfindliche Gemische. Eur. Pat. Appl. 0705865 A1, April 1, 1996. |
| 16. | Schaarschmidt, A. Justus Liebigs Ann. Chem. 1915, 409, 59. doi:10.1002/jlac.19154090106 |
| 13. | Karasu, F.; Arsu, N.; Yagci, Y. J. Appl. Polym. Sci. 2007, 103, 3766. doi:10.1002/app.25467 |
| 19. | Murai, S.; Kakiuchi, F.; Sekine, S.; Tanaka, Y.; Kamatani, A.; Sonoda, M.; Chatani, N. Nature 1993, 366, 529. doi:10.1038/366529a0 |
© 2015 Wagner and Bräse; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)