Abstract
In this paper we present a synthetic approach to six new D–π–A–D conjugated chromophores containing the N-[ω-(4-methoxyphenoxy)alkyl]carbazole fragment. Such readily functionalizable heterocycle as carbazole was used as a main starting compound for their preparation. The investigation of the optical properties has shown that the positive solvatochromism is inherent to the chromophores containing an electron-withdrawing prop-2-en-1-one fragment, while the compounds containing a 2-aminopyrimidine moiety exhibit both positive and negative solvatochromism. The fluorescence quantum yields were experimentally determined for some of the synthesized chromophores; e.g., 1-(5-arylthiophen-2-yl)ethanones quantum yields were found to lie in an interval of 60–80%. Electrochemical oxidation of the synthesized chromophores has resulted in the formation of colored thin oligomeric films that became possible due to the presence of carbazole or pyrrole fragments with free electron-rich positions.
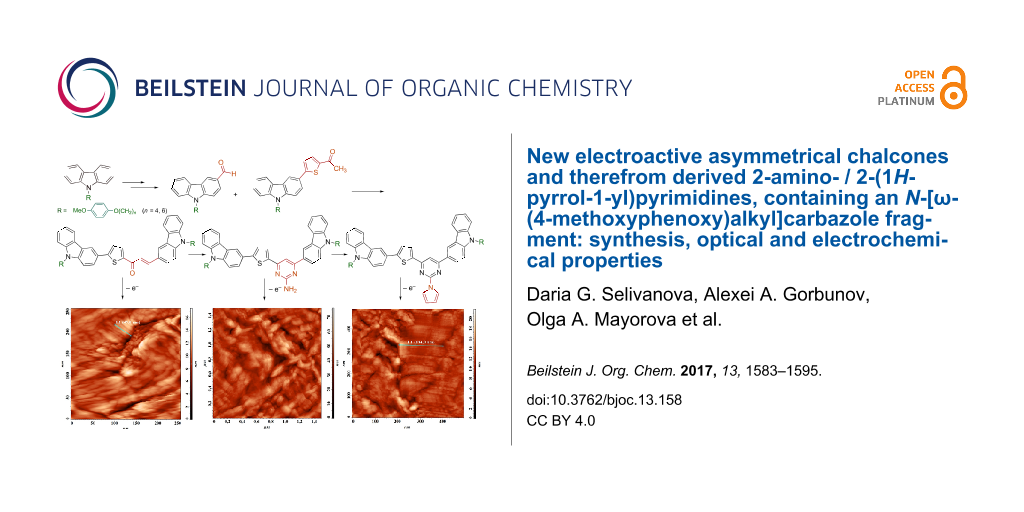
Graphical Abstract
Introduction
Nowadays a great number of research projects concerning the synthesis of organic conjugated systems with a wide scope of their applications, primarily as materials for organic electronic devices such as light-emitting diodes, field-effect transistors, and electrochromic devices exist. As it is said in a White Paper of Chemical Sciences and Society Summit (CS3) “these materials hold tremendous promise to expand our electronic landscape in ways that will radically change the way society interacts with technology” [1]. Analysis of the results obtained in this area of scientific and technical research confirms that at present time it is really possible to perform tailor-made syntheses of monomers to gain oligomers and polymers with desired properties and characteristics. The conjugated systems including carbazole units possess important advantages when compared with other conjugated aromatic systems [2-4].
First of all, 9H-carbazole is a relatively cheap starting material. At the same time, its fully aromatic system renders this heterocycle a good chemical and environmental stability. Nucleophilic substitution of a 9H atom proceeds quite smoothly; this feature gives the opportunity to improve solubility and to tune optical, electrochemical, and photovoltaic properties of carbazole-containing compounds. The carbazole moiety has a rigid planar conjugated structure and, as a result, its derivatives are characterized by a high charge mobility. Carbazole undergoes electrophilic substitution mainly at C3 or C6 positions to give 3(6)-substituted derivatives. Nevertheless, nowadays some new synthetic procedures [3,5-7] have given the opportunity to prepare conjugated systems which include 1,8-, 2,7- or 9(3)-carbazolylene units. The incorporation of such moieties changes the optical and electrochemical properties of the resulted conjugated systems [5]. Another effective way to change the properties of a conjugated system involves the modification of substituents attached to a nitrogen atom of the carbazole unit [8]. The long-chain alkyl groups terminated with an aromatic moiety (substituted or non-substituted benzenes, carbazoles, biphenyls, etc.) can be used for these purposes [8,9]. The incorporation of bulky side chains is applied in order to exclude the possible interaction of polymer chains with each other. Such interaction often causes the formation of excimers, which degrades the quality of the blue luminescence. On the other hand, the interaction of bulky side chains with each other can intensify an undesirable charge transfer in thin polymer films that can be overcome by introducing of terminal aromatic moieties into the structure of side chains [10].
In this paper we describe a synthetic approach to some new D–π–A–D conjugated systems which include 9-[ω-(4-methoxyphenoxy)alkyl]carbazole as a donor fragment (D) and prop-2-enone and pyrimidine units as electron acceptor moieties (A). The optical and electrochemical properties of the prepared compounds were investigated by UV–vis absorption spectroscopy and cyclic voltammetry. Electrochemical oxidation of these heterocycles resulted in the formation of thin films on the surface of an ITO working electrode. The morphology of grown films has been investigated by means of scanning tunneling microscopy.
Results and Discussion
Synthetic toolbox
The objective of the presented work involves the design and further synthesis of 4,6-diaryl-substituted 2-(pyrrol-1-yl)pyrimidines, embedding 9-[ω-(4-methoxyphenoxy)alkyl]carbazole moieties as substituents, one of which is directly linked with a central pyrimidine core and another one is linked by a rigid thiophene cycle as a π-conjugated bridge. We have applied a usual synthetic protocol which includes eight successive steps: O-alkylation of 4-methoxyphenol (1), N-alkylation of carbazole (2), formylation or acetylation of a resulted N-alkyl carbazole (3), two-staged incorporation of thiophene moiety into obtained methyl ketone (4,5), condensation of the prepared aldehyde and methylketone (6), cyclization of thus prepared chalcone into 4,6-disubstituted 2-aminopyrimidine (7) and preparation of a target product – 4.6-disubstituted 2-(pyrrol-1-yl)pyrimidine via Clausson–Kaas condensation (8).
Alkylation of 4-methoxyphenol was realized with the help of the traditional base-catalyzed O-alkylation process in acetone media [11]. N-Alkylated carbazole derivatives can be obtained by different methods such as Mitsunobu reaction [12], Ullmann coupling [13,14], Buchwald–Hartwig amination [15] or N-alkylation of carbazole in the presence of alkali metal carbonates under MW irradiation [16]. In our case, the N-arylation was realized under phase transfer conditions using triethylbenzylammonium chloride (TEBA) as a catalyst [17,18] (Scheme 1).
Scheme 1: Synthesis of 9-[ω-(methoxyphenoxy)alkyl]-9H-carbazoles 1a,b.
Scheme 1: Synthesis of 9-[ω-(methoxyphenoxy)alkyl]-9H-carbazoles 1a,b.
9-[ω-(4-Methoxyphenoxy)alkyl]-9H-carbazole-3-carbaldehydes 2a and 2b, the carbonyl components for the future crotonic condensation, were prepared by Vilsmeier–Haack formylation of 9-[ω-(4-methoxyphenoxy)alkyl]-9H-carbazoles 1a,b [19] (Scheme 2). 3-Acetyl-9-[ω-(4-methoxyphenoxy)alkyl]-9H-carbazoles 3a,b were obtained via acetylation of the same carbazoles 1a,b [20]. The presence of an acetyl group in the resulted compounds 3a and 3b gives the opportunity to extend their conjugation chain via incorporation of an thiophene moiety with the help of a Vilsmeier–Haack–Arnold reaction. The reaction of POCl3, DMF and methylketones 3a and 3b resulted in formation of the corresponding 3-aryl-3-chloroprop-2-enals 4a,b, with subsequent sequential treating with Na2S and chloroacetone has given rise to 5-aryl-2-acetylthiophenes 5a,b [21] (Scheme 2).
Scheme 2: Synthesis of 9-[ω-(4-methoxyphenoxy)alkyl]-9H-carbazole-3-carbaldehydes 2a,b and 1-(5-arylthiophen-2-yl)ethanones 5a,b.
Scheme 2: Synthesis of 9-[ω-(4-methoxyphenoxy)alkyl]-9H-carbazole-3-carbaldehydes 2a,b and 1-(5-arylthiophen-...
Compounds 2 and 5 (Scheme 2) were further used to synthesize a series of D–π–A–D systems (6–8, Scheme 3), the so-called quadrupolar chromophores, which are known as prospective building blocks for nonlinear optical materials [22]. Crotonic condensation of N-substituted carbazole-3-carbaldehydes 2a,b and 1-(5-arylthiophene-2-yl)ethanones 5a,b in the alkaline ethanolic media resulted in the formation of 1,3-diarylsubstituted prop-2-en-1-ones 6a,b [23]. Cyclization of chalcones 6a,b with guanidine sulfate followed by oxidation with hydrogen peroxide gave rise to 2-amino-4,6-disubstituted pyrimidines 7a,b [24]. 2-(1H-Pyrrol-1-yl)pyrimidines 8a,b were synthesized via a Clausson–Kaas protocol using 2,5-dimethoxytetrahydrofuran (DMTHF) as a source of succinic aldehyde (Scheme 3) [25]. The purity of the prepared compounds was confirmed by elemental analysis and NMR and IR spectroscopic data.
Scheme 3: Synthesis of quadrupolar chromophores 6a,b−8a,b.
Scheme 3: Synthesis of quadrupolar chromophores 6a,b−8a,b.
Spectroscopic and luminescent properties of the synthesized compounds
The optical properties of all synthesized compounds were studied with the help of UV–vis absorption and fluorescence spectroscopy of their solutions in various solvents. It has been found out that all the prepared compounds bearing 9-[ω-(4-methoxyphenoxy)alkyl]carbazole moieties are readily soluble in dichloromethane (DCM), acetone (DMK), benzene, tetrahydrofuran (THF), dimethylformamide (DMF), dimethyl sulfoxide (DMSO), but are poorly soluble in hot ethanol and not soluble in hexane. On the enlargement of the conjugated system the colour of the compounds solutions in the sunlight expectedly changes from colourless (2a,b) to orange (7a,b). Under UV irradiation (λeх = 315–390 nm) the luminescence of solutions varies from blue (2a,b) to orange (7a,b). It has been found out that UV-irradiated solutions of the obtained chromophores 6a,b–8a,b change their colours in different solvents of various polarity; for example, compounds 7a,b demonstrate a yellow fluorescence in dichloromethane, DMF, acetone, and an orange one in ethanol, that is most likely connected with the enhancement of the intramolecular charge transfer under UV irradiation.
The prepared compounds differ from each other only by the nature of the substituent at the C3 atom of the carbazole cycle: 2a,b – formyl group, 3a,b – acetyl group, 4a,b – 1-chloro-3-oxoprop-1-en-1-yl moiety and 5a,b – 5-acetylthiophen-2-yl unit. That’s why it is interesting to compare their optical properties. So, we have fulfilled the overlapping of the absorption and emission spectra curves shown in Figure 1a,b. The physicochemical characteristics and measurement data of compounds 2a,b–8a,b are presented in Table 1.
![[1860-5397-13-158-1]](/bjoc/content/figures/1860-5397-13-158-1.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 1: Comparison of UV–vis absorption and fluorescence spectra of compounds 2a–5a (a) and 2b–5b (b) in CHCl3 (c = 10−4 mol L−1).
Figure 1: Comparison of UV–vis absorption and fluorescence spectra of compounds 2a–5a (a) and 2b–5b (b) in CH...
It has been revealed that 9-[ω-(4-methoxyphenoxy)alkyl]-9H-carbazole-3-carbaldehydes 2a,b and 3-acetyl-9-[ω-(4-methoxyphenoxy)alkyl]-9H-carbazoles 3a,b are characterized by absorption in the 260–300 nm region, which corresponds to the π–π* electron transition, and also by the presence of less intensive absorption maxima in the 320–335 nm region corresponding to the n–π* electron transition (Table 1). The comparison of the absorption spectra of 3a,b and 5a,b has shown that incorporation of a thiophene unit into the structure of compounds 5a,b enforces the intramolecular charge transfer, which, in its turn, causes the decrease in the difference between the energy of the ground and excited states and the appearance of additional absorption bands in the 350–400 nm region corresponding to the π−π* electron transition. The absorption spectra of 3-aryl-3-chloroprop-2-enals 4a,b are of particular interest since there are practically no data concerning optical properties of such structures.
Table 1: Physicochemical characteristics of compounds 2a,b−8a,b.
| Compound | Absorption maxima (λmaxabs), nma | Emission maxima (λmaxem), nma | Stokes shift (Δλ), nm |
Band gaps width
(Egopt), eVb |
ФF,
%с |
|---|---|---|---|---|---|
| 2a | 267, 295, 322 | 418 | 96 | 3.25 | 6.3* |
| 2b | 269, 298, 325 | 419 | 94 | 3.21 | 1.7* |
| 3a | 247, 275, 290, 321 | 420 | 99 | 3.11 | – |
| 3b | 271, 298, 321, 334 | 378, 412, 434 | 100 | 3.25 | – |
| 4a | 267, 294, 340, 373, 470 | 473 | 100 | 2.19 | – |
| 4b | 247, 290, 346, 371, 482 | 492 | 121 | 2.03 | – |
| 5a | 257, 271, 291, 375 | 465 | 90 | 2.78 | 63.0** |
| 5b | 257, 273, 293, 351, 373 | 465 | 88 | 2.69 | 79.3** |
| 6a | 260, 298, 330, 419 | 505 | 86 | 2.55 | 19.5** |
| 6b | 258, 298, 336, 406 | 484 | 78 | 2.57 | 27.1** |
| 7a | 306, 346, 402, 477, 610 | 553 | 97 | 1.95 | − |
| 7b | 304, 340, 409, 463, 612 | 555 | 111 | 1.94 | − |
| 8a | 306, 351, 405, 521 | 483 | 78 | 1.97 | − |
| 8b | 316, 358, 407, 538 | 480 | 73 | 1.94 | − |
aAbsorption and emission spectra were measured for CHCl3 solutions (10−4 M); bEgopt was calculated on the basis of the absorption edge value (λonset), Egopt = 1240/ λonset; cfluorescence quantum yield was determined relative to quinine bisulfate* in 0.1 N H2SO4 or 3-aminophthalimide** in EtOH as a standard.
The absorption maxima in the spectra of chloropronenals 4a,b located within the 335–370 nm region correspond to the π–π* electron transition arising from the conjugation of the carbonyl group with a C2–C3 double bond and C3 chlorine atom. The broadened absorption maximum in the 460–510 nm region (at the interface between blue and green spectrum regions) characterizes the n–π* electron transition, that occurs most likely due to the presence of a chlorine atom with lone electron pairs.
The above mentioned intramolecular charge transfer inherent to quadrupolar chromophores (D–A–D or A–D–A), also leads to the generation of the positive solvatochromism effects in these systems [26], which exhibit the redshift of a longwave absorption/emission maxima along with the increase of a solvent polarity. Maybe this is a result of a molecule symmetry breaking in its excited state [27]. Taking into account all the factors mentioned above, we have started the investigation of solvatochromic properties of chromophores 6a,b and obtained their absorption and emission spectra in a set of various solvents (diethyl ether, toluene, THF, CHCl3, DMF, DMSO). The choice of solvents was made on the basis of a solvatochromic parameter value (π*), which characterizes the ability of solvents to stabilize a neighboring charge or dipole by virtue of nonspecific dielectric interactions [28]. This parameter was determined by Kamlet, Abboud and Taft [29]. The measurement results are displayed in Figure 2.
![[1860-5397-13-158-2]](/bjoc/content/figures/1860-5397-13-158-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: Comparison of UV–vis absorption and fluorescence spectra of compounds 6a (a, b), 6b (c, d) in various solvents (c = 10−4 mol L−1).
Figure 2: Comparison of UV–vis absorption and fluorescence spectra of compounds 6a (a, b), 6b (c, d) in vario...
The obtained data demonstrate that the increase of solvent polarity affords the redshift of the longwave UV absorption maxima and of fluorescence maxima as well, and in the last case this shift is more pronounced. This indicates that the excited state of the molecule is more polar than the ground state, and, therefore, when a solvent is replaced by a more polar one, a large stabilization of the excited state in comparison with the ground state takes place, as evidenced by the quantum-chemical calculations carried out with the help of the Fire Fly package [30,31] on a PGU Tesla supercomputer.
All calculations of the dipole moments, HOMO and LUMO energies were performed on the B3LYP/6-31G* level for the gas phase and for solutions in toluene, chloroform and dimethyl sulfoxide. The solvent parameters were taken into account in the DPCM solvation model [30,31]. All geometries were initially fully optimized for energy in the ground state using the same method. Calculations of the absorption maxima values and the dipole moments in the first excited singlet state were performed on the TD-B3LYP/6-31G* level.
The results, which are presented in Table 2, show that the increase of the solvents polarity has brought about the increase of a molecule dipole moment both in the ground and excited states, and the redshift of an absorption band. The main contribution to the excitation energy of the S1 state in all cases corresponds to the electron transition from the highest occupied molecular orbital (HOMO) onto the lowest unoccupied molecular orbital (LUMO).
Table 2: The values of the dipole moments in the ground (μ0) and excited (μ*) states, the frontier orbitals energies and the absorption maxima in the gas phase and in various solvents calculated by B3LYP/6-31G* and TD-B3LYP/6-31G* methods for compound 6a.
| μ0, D | E(HOMO), eV | E(LUMO), eV | μ*, D | λmax, nm | |
|---|---|---|---|---|---|
| gaseous phase | 5.98 | −5.25 | −1.85 | 19.24 | 414 |
| toluene | 7.47 | −5.35 | −2.11 | 21.68 | 435 |
| CHCl3 | 8.43 | −5.39 | −2.26 | 22.90 | 448 |
| DMSO | 9.51 | −5.43 | −2.42 | 24.31 | 466 |
We have also investigated the solvatochromic behavior of chromophores 7a,b. An additional absorption maximum was found in the longwave region of the spectra recorded for chloroform and dimethyl sulfoxide solutions of these compounds (Figure 3). The replacement of diethyl ether by chloroform causes a bathochromic shift of the longwave absorption band. Further replacement of the solvents by more polar ones results in hypsochromic shifts.
![[1860-5397-13-158-3]](/bjoc/content/figures/1860-5397-13-158-3.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 3: Comparison of UV–vis absorption and fluorescence spectra of compounds 7a (a, b) and 7b (c, d) in various solvents (c = 10–4 mol L–1).
Figure 3: Comparison of UV–vis absorption and fluorescence spectra of compounds 7a (a, b) and 7b (c, d) in va...
The correlation between Kamlet–Taft π* parameters [29] and the absorption or emission maxima wavelength is clearly demonstrated by graphs presented in Figure 4. More detailed spectral data of chalcones 6a,b and 2-aminopyrimidines 7a,b are collected in a Table placed in Supporting Information File 1.
![[1860-5397-13-158-4]](/bjoc/content/figures/1860-5397-13-158-4.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 4: Correlation between Kamlet–Taft π* parameters [29] and the absorption and emission maxima wavelength of chalcones 6a,b (a) and 2-aminopyrimidines 7a,b (b) in the series of solvents.
Figure 4: Correlation between Kamlet–Taft π* parameters [29] and the absorption and emission maxima wavelength of...
Further it has been established that solvatochromism is inherent not only to these newly synthesized chromophores 7a,b, but also to the whole set of 2-amino-4,6-diarylsubstituted pyrimidines even of the less complicated structure. Here we present the results of solvatochromic studies of two previously synthesized 2-aminopyrimidines – symmetrical 2-amino-4,6-di(4-bromophenyl)pyrimidine and asymmetrical 2-amino-4-[4-(9H-carbazol-9-yl)phenyl]-6-[4-(dimethylamino)phenyl]pyrimidine. The measurements were done for the same set of solvents; the results are shown in Figure 5.
![[1860-5397-13-158-5]](/bjoc/content/figures/1860-5397-13-158-5.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 5: Comparison of UV–vis absorption spectra of 2-amino-4,6-di(4-bromophenyl)pyrimidine and 2-amino-4-[4-(9H-carbazol-9-yl)phenyl]-6-[4-(dimethylamino)phenyl]pyrimidine in various solvents (C = 10−4 mol L−1).
Figure 5: Comparison of UV–vis absorption spectra of 2-amino-4,6-di(4-bromophenyl)pyrimidine and 2-amino-4-[4...
The replacement of such a nonpolar solvent as toluene by some more polar solvent, chloroform, has resulted in the insignificant bathochromic shift of a longwave absorption maximum in the case of 2-amino-4,6-di(4-bromophenyl)pyrimidine (λmaxabs = 340 nm for the toluene solution and 362 nm for the chloroform one). In the case of asymmetrical 4-(4-N,N-dimethylamino)phenyl-6-[4-(9H-carbazolyl)phenyl]-2-aminopyrimidine this redshift was more significant ≈70 nm (λmaxabs = 371 nm for the toluene solution and 444 nm for the chloroform one). This fact can be explained by the presence of electron donating groups in the structure of substituents at C4 and C6 atoms of the pyrimidine core. At the same time, it is possible the protons of the NH2 group of the pyrimidine core interact with the N,N-dimethylamino group of another molecule, thus inducing the rearrangement of electronic density of both molecules [32]. The further increase in the solvent polarity leads in both cases to a hypsochromic shift of a long-wave absorption maximum.
Absorption and emission spectra of 2-(1H-pyrrol-1-yl)pyrimidines 8a,b are presented in Figure 6. Absorption bands in the 280–450 nm region characterizing π–π* electron transitions and a low-intensity absorption maxima (at 535 nm for 8a and at 540 nm for 8b) which corresponds to the effective intramolecular charge transfer, are typical for this series of compounds.
![[1860-5397-13-158-6]](/bjoc/content/figures/1860-5397-13-158-6.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 6: UV–vis absorption and fluorescence spectra of compounds 8a (a), 8b ( b) in CHCl3 (c = 10−4 mol L−1).
Figure 6: UV–vis absorption and fluorescence spectra of compounds 8a (a), 8b ( b) in CHCl3 (c = 10−4 mol L−1)....
Electrochemical properties of the synthesized compounds
The electrochemical behavior of the synthesized chromophores, each containing an electroactive carbazole unit in their structure, is of special interest. The presence of free C3 and C6 positions in this heterocyclic moiety makes it possible to form bicarbazyl structures as a result of electrochemical oxidation, which mechanism has been described in detail by K. Karon et al. [33]. Electrochemical properties of the obtained compounds were investigated by cyclic voltammetry (CV). The measurements were carried out for MeCN/CH2Cl2 (9:1) solutions containing Et4NClO4 (c = 10−3 mol L−1) as a supporting electrolyte in a three-electrode cell (RE – Ag|AgCl; SE – Pt wire; WE – carbon-pyroceramic disk or an ITO-covered glass plate). The redox potentials are referenced to the ferrocene/ferrocenium couple (Fc/Fc+). The data are presented in Figure 7 and Figure 8 and summarized in Table 3. The first cycle of the 9-[ω-(4-methoxyphenoxy)alkyl]-9H-carbazole-3-carbaldehydes 2a,b CV curves has shown two oxidation waves with potential peak values at 1.39 V (Eox1) and 1.81 V (Eox2) (Figure 7a). These peaks correspond to the formation of cation radical (Eox1) and dication (Eox2); the last one loses two protons and forms a dimer structure, which is depicted in the layout of a cathodic speck of a CV curve (Ered1 = 1.08 V, Ered2 = 1.35 V). The second and subsequent cycles are characterized by the presence of two reversible peaks (Eox1/Ered1 = 1.07/0.64 V, Eox2/Ered2 = 1.35/1.26 V), which correspond to the formation of cation radical (Eox1) and dication (Eox2) of a generated dimer. A similar picture was also observed for oxidation processes taking place in the solutions of 1-({5-{9-[6-(4-methoxyphenoxy)hexyl]-9H-carbazolyl}thiophen-2-yl})ethanone (5b) and 2b (Figure 7).
![[1860-5397-13-158-7]](/bjoc/content/figures/1860-5397-13-158-7.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 7: Cyclic voltammograms of compounds 2b (a), 5b (b); WE – carbon-pyroceramic electrode, 10 cycles, Et4NClO4, Vscan 50 mV∙s−1, CH3CN/CH2Cl2 (9:1, v/v).
Figure 7: Cyclic voltammograms of compounds 2b (a), 5b (b); WE – carbon-pyroceramic electrode, 10 cycles, Et4...
The CV curves of the first potential scan obtained in the process of electrochemical oxidation of chromophores 6–8 are characterized by lower values of the oxidation potentials if compared with those of compounds 2a,b and 5a,b: Eox1 = 1.12 V, Eox2 = 1.68 V (6b); Eox1 = 1.06 V, Eox2 = 1.65 V (7b), Eox1 = 1.05 V, Eox2 = 1.34 V (8b). This fact can be explained by elongation of a conjugation chain in heterocycles 6 and 8. Further potential sweep demonstrates a broadened oxidation peak (6b: Eox1 = 0.91 V, 7b: Eox1 = 0.95 V, 8b: Eox1 = 1.04 V), which represents the formation of a stable dication of a 6,6’-bicarbazyl structure (Figure 8 and Table 3).
![[1860-5397-13-158-8]](/bjoc/content/figures/1860-5397-13-158-8.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 8: Cyclic voltammograms of compounds 6b (a), 7b (b), 8b (с); WE – carbon-pyroceramic electrode, 10 cycles, Et4NClO4, Vscan 50 mV∙s−1, CH3CN/CH2Cl2 (9:1, v/v).
Figure 8: Cyclic voltammograms of compounds 6b (a), 7b (b), 8b (с); WE – carbon-pyroceramic electrode, 10 cyc...
Table 3: Electrochemical characteristics of compounds 6–8 versus Fc/Fc+.
| Compounds | 6a | 6b | 7b | 8a | 8b |
|---|---|---|---|---|---|
| oxidation potential (Eox), Va | 1.11, 1.61 | 1.12, 1.68 | 1.06, 1.65 | 1.06, 1.32, 1.67 | 1.05, 1.34 |
| reduction potential (Ered), Va | 0.60 | 0.59 | 0.56 | 0.58 | 0.62 |
| oxidation onset potential (Eoxonset), Va | 1.10 | 1.06 | 1.10 | 1.12 | 1.10 |
| reduction onset potential (Eredonset), Va | −0.98 | −1.04 | 0.85 | −0.84 | −0.83 |
| EHOMO, eVb | −5.57 | −5.53 | −5.57 | −5.59 | −5.57 |
| ELUMO, eVb | −3.49 | −3.43 | −3.62 | −3.63 | −3.64 |
| Egel, eVc | 2.08 | 2.1 | 1.95 | 1.96 | 1.93 |
aThe electrochemical measurements were carried for monomer solution (c = 10−3 M) in a CH3CN/CH2Cl2 (9:1) mixture at room temperature with Et4N+ClO4− as a background electrolyte (c = 0.1 mol/L) («Potentiostat/Galvanostat/ZRA Interface 1000» and three electrode electrochemical cell with Si(C)-disk, carbon-pyroceramic electrode as working electrodes, Pt wire as auxiliary electrode, silver chloride electrode as reference electrode); bEHOMO = −(Eoxonset versus Ag/AgCl − EFc versus AgCl + 4.8) eV; ELUMO = −(Eredonset versus Ag/AgCl − EFc versus AgCl + 4.8) eV; cEgel = (EHOMO − ELUMO) eV.
In addition to these studies, we have executed the electrochemical oxidation of compounds 6–8b using ITO covered glass plate as a working electrode. These processes resulted in the formation of thin films on the surface of the working electrode. Cyclic voltammograms, film images and the study of the morphology of their surface are presented in detail in Supporting Information File 1.
Conclusion
To summarize, we have developed an efficient synthetic approach to a family of highly luminescent D–π–A–D conjugated chromophores each containing the flexible N-[ω-(4-methoxyphenoxy)alkyl]carbazole fragment. It has been revealed that positive solvatochromism is inherent to the chromophores of the chalcone group, whereas chromophores of 2-aminopyrimidine group exhibit both positive and negative solvatochromism. For some of the compounds, the fluorescence quantum yields were experimentally determined, which values for 1-(5-arylthiophen-2-yl)ethanones series lie in the range of 60–80%. Electrochemical oxidation of these chromophores has given rise to coloured thin oligomeric films, which are formed by the packed in stacks fibers variously oriented toward each other.
Supporting Information
| Supporting Information File 1: Synthetic procedures and characterization data for all compounds 1–8 (a,b). | ||
| Format: PDF | Size: 1.7 MB | Download |
References
-
Organic Electronics for a Better Tomorrow: Innovation, Accessibility, Sustainability, A White Paper from the Chemical Sciences and Society Summit (CS3), San Francisco, California, United States, September 17-20, 2012.
Return to citation in text: [1] -
Wise, D. L.; Wnek, G. E.; Trantolo, D. J.; Cooper, T. M.; Gresser, J. D., Eds. Photonic Polymer Systems: Fundamentals: Methods, and Applications; Marcel Dekker, Inc.: New York, 1998.
Return to citation in text: [1] -
Morin, J.-F.; Leclerc, M.; Adès, D.; Siove, A. Macromol. Rapid Commun. 2005, 26, 761–778. doi:10.1002/marc.200500096
Return to citation in text: [1] [2] -
Skotheim, T. A.; Elsenbaumer, R. L.; Reynolds, J. R., Eds. Handbook of Conducting Polymers, 2nd ed.; Marcel Dekker: New York, 2007.
Return to citation in text: [1] -
Michinobu, T.; Osako, H.; Shigehara, K. Polymers (Basel, Switz.) 2010, 2, 159–173. doi:10.3390/polym2030159
Return to citation in text: [1] [2] -
Kim, J.; Kwon, Y. S.; Shin, W. S.; Moon, S.-J.; Park, T. Macromolecules 2011, 44, 1909–1919. doi:10.1021/ma102467w
Return to citation in text: [1] -
Vacareanu, L.; Catargiu, A.-M.; Grigoras, M. High Perform. Polym. 2014, 27, 476–485. doi:10.1177/0954008314555529
Return to citation in text: [1] -
Mei, J.; Bao, Z. Chem. Mater. 2014, 26, 604–615. doi:10.1021/cm4020805
Return to citation in text: [1] [2] -
Koguchi, R.; Kobayashi, N.; Shinnai, T.; Oikawa, K.; Tsuchiya, K.; Kijima, M. Macromol. Chem. Phys. 2008, 209, 439–449. doi:10.1002/macp.200700448
Return to citation in text: [1] -
Jin, J.-I.; Park, C.-K.; Shim, H.-K. Polymer 1994, 35, 480–489. doi:10.1016/0032-3861(94)90500-2
Return to citation in text: [1] -
Abashev, G. G.; Galkin, A. V.; Syutkin, R. V.; Usath, V. A.; Shklyaeva, E. V. The synthesis of derivatives of p-hydroquinone and p-methoxyphenol and the thiophene - and carbazole-containing compounds based on them. In Proceedings of the international scientific conference on the innovative potential of the natural sciences, Perm; 2006.
vol. 1, pp 133–140.
Return to citation in text: [1] -
Bombrun, A.; Casi, G. Tetrahedron Lett. 2002, 43, 2187–2190. doi:10.1016/S0040-4039(02)00217-4
Return to citation in text: [1] -
Zhang, H.; Cai, Q.; Ma, D. J. Org. Chem. 2005, 70, 5164–5173. doi:10.1021/jo0504464
Return to citation in text: [1] -
Bissember, A. C.; Lundgren, R. J.; Creutz, S. E.; Peters, J. C.; Fu, G. C. Angew. Chem. 2013, 125, 5233–5237. doi:10.1002/ange.201301202
Return to citation in text: [1] -
Watanabe, T.; Ueda, S.; Inuki, S.; Oishi, S.; Fujii, N.; Ohno, H. Chem. Commun. 2007, 4516–4518. doi:10.1039/B707899d
Return to citation in text: [1] -
Milen, M.; Grün, A.; Bálint, E.; Dancsó, A.; Keglevich, G. Synth. Commun. 2010, 40, 2291–2301. doi:10.1080/00397910903226166
Return to citation in text: [1] -
Li, X.; Mintz, E. A.; Bu, X. R.; Zehnder, O.; Bosshard, C.; Günter, P. Tetrahedron 2000, 56, 5785–5791. doi:10.1016/S0040-4020(00)00536-6
Return to citation in text: [1] -
Syutkin, R. V.; Abashev, G. G.; Shklyaeva, E. V. Butlerov Soobshch. 2008, 13, 11–16.
Return to citation in text: [1] -
Deane, F. M.; Miller, C. M.; Maguire, A. R.; McCarthy, F. O. J. Heterocycl. Chem. 2011, 48, 814–823. doi:10.1002/jhet.598
Return to citation in text: [1] -
Reshetova, M. D.; Borisova, N. E. The Moscow University Herald. Series “Chemistry” 1999, 40, 43–45.
Return to citation in text: [1] -
Herbivo, C.; Comel, A.; Kirsch, G.; Raposo, M. M. M. Tetrahedron 2009, 65, 2079–2086. doi:10.1016/j.tet.2008.12.078
Return to citation in text: [1] -
Terenziani, F.; Painelli, A.; Katan, C.; Charlot, M.; Blanchard-Desce, M. J. Am. Chem. Soc. 2006, 128, 15742–15755. doi:10.1021/ja064521j
Return to citation in text: [1] -
Bushueva, A. Y.; Shklyaeva, E. V.; Abashev, G. G. Mendeleev Commun. 2009, 19, 329–331. doi:10.1016/j.mencom.2009.11.012
Return to citation in text: [1] -
Varga, L.; Nagy, T.; Kövesdi, I.; Benet-Buchholz, J.; Dormán, G.; Ürge, L.; Darvas, F. Tetrahedron 2003, 59, 655–662. doi:10.1016/S0040-4020(02)01560-0
Return to citation in text: [1] -
Nakazaki, J.; Chung, I.; Matsushita, M. M.; Sugawara, T.; Watanabe, R.; Izuoka, A.; Kawada, Y. J. Mater. Chem. 2003, 13, 1011–1022. doi:10.1039/b211986b
Return to citation in text: [1] -
Herbivo, C.; Comel, A.; Kirsch, G.; Fonseca, A. M. C.; Belsley, M.; Raposo, M. M. M. Dyes Pigm. 2010, 86, 217–226. doi:10.1016/j.dyepig.2010.01.006
Return to citation in text: [1] -
Painelli, A.; Terenziani, F.; Soos, Z. G. Theor. Chem. Acc. 2007, 117, 915–931. doi:10.1007/s00214-006-0210-5
Return to citation in text: [1] -
Reichardt, C. Chem. Rev. 1994, 94, 2319–2358. doi:10.1021/cr00032a005
Return to citation in text: [1] -
Kamlet, M. J.; Abboud, J. L. M.; Abraham, M. H.; Taft, R. W. J. Org. Chem. 1983, 48, 2877–2887. doi:10.1021/jo00165a018
Return to citation in text: [1] [2] [3] -
Granovsky, A. A. Firefly version 8. http://classic.chem.msu.su/gran/firefly/index.html.
Return to citation in text: [1] [2] -
Schmidt, M. W.; Baldridge, K. K.; Boatz, J. A.; Elbert, S. T.; Gordon, M. S.; Jensen, J. H.; Koseki, S.; Matsunaga, N.; Nguyen, K. A.; Su, S.; Windus, T. L.; Dupuis, M.; Montgomery, J. A., Jr. J. Comput. Chem. 1993, 14, 1347–1363. doi:10.1002/jcc.540141112
Return to citation in text: [1] [2] -
Joseph, J.; Jemmis, E. D. J. Am. Chem. Soc. 2007, 129, 4620–4632. doi:10.1021/ja067545z
Return to citation in text: [1] -
Karon, K.; Lapkowski, M. J. Solid State Electrochem. 2015, 19, 2601–2610. doi:10.1007/s10008-015-2973-x
Return to citation in text: [1]
| 29. | Kamlet, M. J.; Abboud, J. L. M.; Abraham, M. H.; Taft, R. W. J. Org. Chem. 1983, 48, 2877–2887. doi:10.1021/jo00165a018 |
| 32. | Joseph, J.; Jemmis, E. D. J. Am. Chem. Soc. 2007, 129, 4620–4632. doi:10.1021/ja067545z |
| 33. | Karon, K.; Lapkowski, M. J. Solid State Electrochem. 2015, 19, 2601–2610. doi:10.1007/s10008-015-2973-x |
| 1. | Organic Electronics for a Better Tomorrow: Innovation, Accessibility, Sustainability, A White Paper from the Chemical Sciences and Society Summit (CS3), San Francisco, California, United States, September 17-20, 2012. |
| 20. | Reshetova, M. D.; Borisova, N. E. The Moscow University Herald. Series “Chemistry” 1999, 40, 43–45. |
| 5. | Michinobu, T.; Osako, H.; Shigehara, K. Polymers (Basel, Switz.) 2010, 2, 159–173. doi:10.3390/polym2030159 |
| 21. | Herbivo, C.; Comel, A.; Kirsch, G.; Raposo, M. M. M. Tetrahedron 2009, 65, 2079–2086. doi:10.1016/j.tet.2008.12.078 |
| 3. | Morin, J.-F.; Leclerc, M.; Adès, D.; Siove, A. Macromol. Rapid Commun. 2005, 26, 761–778. doi:10.1002/marc.200500096 |
| 5. | Michinobu, T.; Osako, H.; Shigehara, K. Polymers (Basel, Switz.) 2010, 2, 159–173. doi:10.3390/polym2030159 |
| 6. | Kim, J.; Kwon, Y. S.; Shin, W. S.; Moon, S.-J.; Park, T. Macromolecules 2011, 44, 1909–1919. doi:10.1021/ma102467w |
| 7. | Vacareanu, L.; Catargiu, A.-M.; Grigoras, M. High Perform. Polym. 2014, 27, 476–485. doi:10.1177/0954008314555529 |
| 17. | Li, X.; Mintz, E. A.; Bu, X. R.; Zehnder, O.; Bosshard, C.; Günter, P. Tetrahedron 2000, 56, 5785–5791. doi:10.1016/S0040-4020(00)00536-6 |
| 18. | Syutkin, R. V.; Abashev, G. G.; Shklyaeva, E. V. Butlerov Soobshch. 2008, 13, 11–16. |
| 2. | Wise, D. L.; Wnek, G. E.; Trantolo, D. J.; Cooper, T. M.; Gresser, J. D., Eds. Photonic Polymer Systems: Fundamentals: Methods, and Applications; Marcel Dekker, Inc.: New York, 1998. |
| 3. | Morin, J.-F.; Leclerc, M.; Adès, D.; Siove, A. Macromol. Rapid Commun. 2005, 26, 761–778. doi:10.1002/marc.200500096 |
| 4. | Skotheim, T. A.; Elsenbaumer, R. L.; Reynolds, J. R., Eds. Handbook of Conducting Polymers, 2nd ed.; Marcel Dekker: New York, 2007. |
| 19. | Deane, F. M.; Miller, C. M.; Maguire, A. R.; McCarthy, F. O. J. Heterocycl. Chem. 2011, 48, 814–823. doi:10.1002/jhet.598 |
| 12. | Bombrun, A.; Casi, G. Tetrahedron Lett. 2002, 43, 2187–2190. doi:10.1016/S0040-4039(02)00217-4 |
| 15. | Watanabe, T.; Ueda, S.; Inuki, S.; Oishi, S.; Fujii, N.; Ohno, H. Chem. Commun. 2007, 4516–4518. doi:10.1039/B707899d |
| 11. |
Abashev, G. G.; Galkin, A. V.; Syutkin, R. V.; Usath, V. A.; Shklyaeva, E. V. The synthesis of derivatives of p-hydroquinone and p-methoxyphenol and the thiophene - and carbazole-containing compounds based on them. In Proceedings of the international scientific conference on the innovative potential of the natural sciences, Perm; 2006.
vol. 1, pp 133–140. |
| 16. | Milen, M.; Grün, A.; Bálint, E.; Dancsó, A.; Keglevich, G. Synth. Commun. 2010, 40, 2291–2301. doi:10.1080/00397910903226166 |
| 10. | Jin, J.-I.; Park, C.-K.; Shim, H.-K. Polymer 1994, 35, 480–489. doi:10.1016/0032-3861(94)90500-2 |
| 8. | Mei, J.; Bao, Z. Chem. Mater. 2014, 26, 604–615. doi:10.1021/cm4020805 |
| 9. | Koguchi, R.; Kobayashi, N.; Shinnai, T.; Oikawa, K.; Tsuchiya, K.; Kijima, M. Macromol. Chem. Phys. 2008, 209, 439–449. doi:10.1002/macp.200700448 |
| 13. | Zhang, H.; Cai, Q.; Ma, D. J. Org. Chem. 2005, 70, 5164–5173. doi:10.1021/jo0504464 |
| 14. | Bissember, A. C.; Lundgren, R. J.; Creutz, S. E.; Peters, J. C.; Fu, G. C. Angew. Chem. 2013, 125, 5233–5237. doi:10.1002/ange.201301202 |
| 24. | Varga, L.; Nagy, T.; Kövesdi, I.; Benet-Buchholz, J.; Dormán, G.; Ürge, L.; Darvas, F. Tetrahedron 2003, 59, 655–662. doi:10.1016/S0040-4020(02)01560-0 |
| 22. | Terenziani, F.; Painelli, A.; Katan, C.; Charlot, M.; Blanchard-Desce, M. J. Am. Chem. Soc. 2006, 128, 15742–15755. doi:10.1021/ja064521j |
| 23. | Bushueva, A. Y.; Shklyaeva, E. V.; Abashev, G. G. Mendeleev Commun. 2009, 19, 329–331. doi:10.1016/j.mencom.2009.11.012 |
| 30. | Granovsky, A. A. Firefly version 8. http://classic.chem.msu.su/gran/firefly/index.html. |
| 31. | Schmidt, M. W.; Baldridge, K. K.; Boatz, J. A.; Elbert, S. T.; Gordon, M. S.; Jensen, J. H.; Koseki, S.; Matsunaga, N.; Nguyen, K. A.; Su, S.; Windus, T. L.; Dupuis, M.; Montgomery, J. A., Jr. J. Comput. Chem. 1993, 14, 1347–1363. doi:10.1002/jcc.540141112 |
| 29. | Kamlet, M. J.; Abboud, J. L. M.; Abraham, M. H.; Taft, R. W. J. Org. Chem. 1983, 48, 2877–2887. doi:10.1021/jo00165a018 |
| 29. | Kamlet, M. J.; Abboud, J. L. M.; Abraham, M. H.; Taft, R. W. J. Org. Chem. 1983, 48, 2877–2887. doi:10.1021/jo00165a018 |
| 30. | Granovsky, A. A. Firefly version 8. http://classic.chem.msu.su/gran/firefly/index.html. |
| 31. | Schmidt, M. W.; Baldridge, K. K.; Boatz, J. A.; Elbert, S. T.; Gordon, M. S.; Jensen, J. H.; Koseki, S.; Matsunaga, N.; Nguyen, K. A.; Su, S.; Windus, T. L.; Dupuis, M.; Montgomery, J. A., Jr. J. Comput. Chem. 1993, 14, 1347–1363. doi:10.1002/jcc.540141112 |
| 27. | Painelli, A.; Terenziani, F.; Soos, Z. G. Theor. Chem. Acc. 2007, 117, 915–931. doi:10.1007/s00214-006-0210-5 |
| 25. | Nakazaki, J.; Chung, I.; Matsushita, M. M.; Sugawara, T.; Watanabe, R.; Izuoka, A.; Kawada, Y. J. Mater. Chem. 2003, 13, 1011–1022. doi:10.1039/b211986b |
| 26. | Herbivo, C.; Comel, A.; Kirsch, G.; Fonseca, A. M. C.; Belsley, M.; Raposo, M. M. M. Dyes Pigm. 2010, 86, 217–226. doi:10.1016/j.dyepig.2010.01.006 |
© 2017 Selivanova et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)