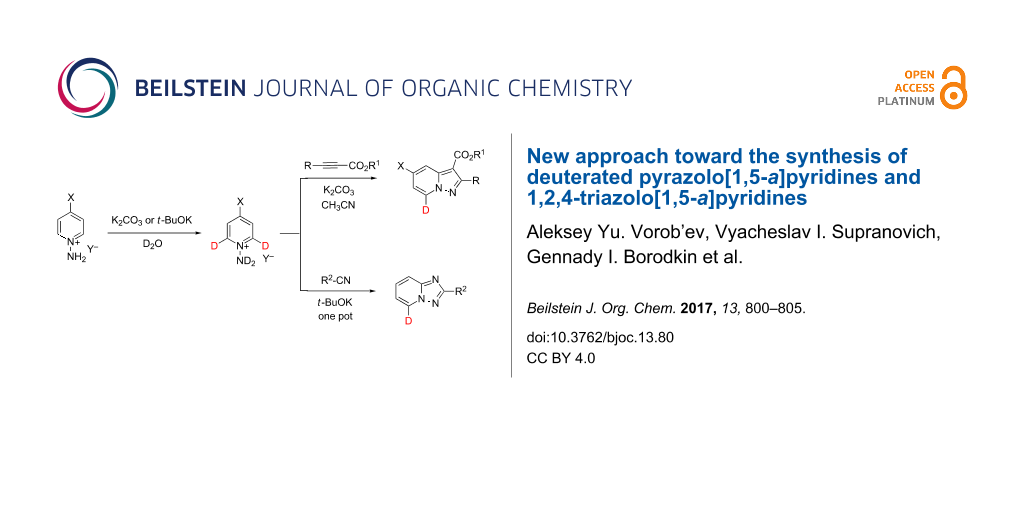
Abstract
An efficient and operationally simple synthesis of 7-deuteropyrazolo[1,5-a]pyridine and 7-deutero-1,2,4-triazolo[1,5-a]pyridine derivatives using α-H/D exchange of 1-aminopyridinium cations in basic D2O followed by a 1,3-cycloaddition of acetylenes and nitriles is presented. A high regioselectivity and a high degree of deuterium incorporation were achieved. The procedure was applied for several 4-R-1-aminopyridinium cations (R = H, Me, OMe).
Graphical Abstract
Introduction
Isotopically labeled compounds find broad applications in studies of chemical and biochemical reaction mechanisms and metabolism pathways. Deuterium is the most common used isotopic label in mechanistic studies. Deuterated organic compounds are widely used in biological [1] and pharmacological [2-5] investigations. In the last years deuteration became also an efficient tool in drug design [6].
Pyrazolo[1,5-a]pyridine and 1,2,4-triazolo[1,5-a]pyridine scaffolds attracted significant attention to the medicinal chemistry community during the past decade. For example, pyrazolo[1,5-a]pyridine derivatives were used in the design of antiviral [7,8], antimalarial [9] and antitubercular [10] agents. Also they were applied in the development of FIXa [11], PI3K [12], EGFR [13] and PDE [14] inhibitors and dopamine receptor ligands [15]. The nonselective PDE3,4 inhibitor ibudilast (MN-166) has been marketed in Japan for over 25 years for treating asthma and post-stroke patients [16]. 1,2,4-Triazolo[1,5-a]pyridines show antifungal [17], antitumor [18], and cytotoxic [19] activities. Both types of heterocyclic cores are readily available from N-aminopyridium salts and related pyridinium-N-imines via 1,3-cycloaddition reaction [20] or intramolecular ring closure [21-24]. The importance of these cores for medical chemistry studies suggests that isotopically labeled pyrazolo[1,5-a]pyridines and triazolo[1,5-a]pyridines could be of interest. Recently, deuterium-labeled pyridinium-N-imines were applied to mechanistic studies of the conversion to pyrazolo[1,5-a]pyridines [25,26]. Such labeled N-imines were obtained starting from commercially available pyridine-d5. Since substituted deuterated pyridines are less accessible new mild and simple methods of deuterium introduction into the pyridine ring are of great interest. The N-aminopyridinium cation has been shown to undergo a fast H/D exchange at the α-position of the pyridine ring [27]. Pyridine-N-imines could also be deuterated under significantly harder conditions [28,29]. In the present study we report mild and effective syntheses of 7-deuteropyrazolo[1,5-a]pyridine and 7-deutero-1,2,4-triazolo[1,5-a]pyridine derivatives by H/D exchange of 1-aminopyridinium cations followed by the reaction with acetylenes and nitriles.
Results and Discussion
N-Aminopyridinium salts are easily available via direct N-amination of parent pyridines. Salt 1a was prepared by N-amination of pyridine with hydroxylamine-O-sulfonic acid followed by the reaction with HBF4 according to a previously described method [30]. Salts 1b,c were prepared by direct N-amination of the corresponding pyridines with O-mesitylsulfonylhydroxylamine. In view of difficulties in obtaining experimental pKa values of different positions of pyridinium cations we carried out DFT calculations [31] at the M06-2X 6-31+G(d,p) [32] level of theory with SMD [33] solvation (Figure 1, see also Supporting Information File 1).
Figure 1: pKa values for N-aminopyridinium cation hydrogen atoms according to DFT M06-2X 6-31+G(d,p) calculations.
Figure 1: pKa values for N-aminopyridinium cation hydrogen atoms according to DFT M06-2X 6-31+G(d,p) calculat...
As expected, in all cases the NH2 group is the most acidic. The NH2 group is usually ≈12–13 pKa units more acidic than α-C–H hydrogens. However, the difference in pKa of NH2 and CH3 groups of the 4-methyl-1-aminopyridinium cation is not so high and the NH2 group is only 2.7 units more acidic. 1-Aminopyridinium and 4-methyl-1-aminopyridinium cations have similar pKa values for NH2 and α-C–H groups. The 4-methoxy-N-aminopyridinium cation possesses significantly lower acidity for both N–H and C–H hydrogens possibly due to the electron-donating effect of the methoxy group. These quantum chemical data together with Zoltewicz’s work [27] suggest 7-deuterium-labeled pyrazolo[1,5-a]pyridines could be obtained from N-aminopyridium salts through an H/D exchange in basic D2O solution followed by the cycloaddition reaction with acetylenes (Scheme 1).
Scheme 1: H/D exchange of N-aminopyridinium salts 1a–c and their reaction with acetylenes.
Scheme 1: H/D exchange of N-aminopyridinium salts 1a–c and their reaction with acetylenes.
In the first step, the H/D exchange in 1a has been performed with a 0.67 M solution of K2CO3 in D2O at 80 °C for 5 min. After D2O evaporation and the reaction with dimethyl acetylenedicarboxylate (2a, DMAD) in MeCN D-labeled pyrazolo[1,5-a]pyridine 3 was obtained in 70% yield (Table 1). The deuteration at room temperature even for 24 h led to a significantly lower degree of deuteration (DD, 20%).
Table 1: Synthesis of deuteropyrazolo[1,5-a]pyridine derivativesa.
|
Product and
D content (%)b |
Yield (%) |
Product and
D content (%)b |
Yield (%) |
|---|---|---|---|
|
3 |
70 |
5 |
34c |
|
6 |
87 |
7 |
19d |
|
8, 9 |
63 | ||
aH/D-exchange: salt 1a–c (0.20 mmol), K2CO3 (1.0 mmol), D2O (1.5 mL), 80 °C, 5 min; 1,3-cycloaddition: acetylene compound (0.2 mmol), MeCN (5 mL), chloranil (0.15 mmol), rt, 1 h. bD content (%) was determined by 1H NMR. cDD and yield after two runs in D2O. dDD and yield after H/D exchange for 1 h in D2O at 80 °C.
Salt 1b gave the corresponding 5-CD3-7-D-pyrazolopyridine 5 along with a 93% DD for the methyl group after two runs in D2O. The 4-methoxy derivative 1c slowly underwent an H/D exchange at the 2-position of the pyridinium ring probably by reason of a lower acidity due to the electron-donating effect of the methoxy group. Thus, 5-methoxypyrazolopyridine 6 was obtained in 25% yield with a DD of only 58%. A higher DD of 6 could be achieved by increasing the reaction time, however, the yield of pyrazolopyridine decreased possibly due to the hydrolysis of the methoxy group. The 4-dimethylamino-substituted pyridinium-N-amine salt (anion MesSO3−) did not undergo an H/D exchange under the present conditions. Both 4-CO2Me-substituted pyridinium-N-amine and N-aminoisoquinolinium mesitylenesulfonates failed deuteration owing to the formation of insoluble compounds in basic D2O solution.
Ethyl phenylpropiolate (2b) reacts similar to DMAD with the formation of the corresponding 7-D-pyrazolopyridine 7. When ethyl propiolate (2c) was used 2,7-dideuteropyrazolo[1,5-a]pyridine 8 was formed along with monodeuterated product 9. The deuterium atom may appear in position 2 of compound 8 in two different ways (Scheme 2). The first one includes deuterium atom migration from position 3a to position 2 in cycloadduct 10 (path a) with the formation of intermediate 11 which, on further oxidation, gives product 8. Intermediate 11 is the most stable isomer among other dihydro intermediates according to quantum chemistry calculations by the M-06-2X 6-31G+(d,p) method (Figure 2) and 3a -> 2 hydrogen atom migration is a highly exothermic process. However, the formation of 11 is probably kinetically unfavorable due to the prohibited 1,3-hydrogen shift. Thus, no NMR signals corresponding to 11 were found in the reaction mixture before oxidation. Another possible way of deuterium-atom incorporation includes the H/D exchange between the ND-group and the Csp–H hydrogen of ethyl propiolate before the formation of cycloadduct 12 (path b). Rearrangement of 12 into 13 and further oxidation leads to pyrazolopyridine 8.
Scheme 2: Possible pathways for the formation of 8.
Scheme 2: Possible pathways for the formation of 8.
Figure 2: Relative stability of 3-CO2Et-substituted dihydropyrazolo[1,5-a]pyridines by the M06-2X 6-31+G(d,p) method in kJ/mol.
Figure 2: Relative stability of 3-CO2Et-substituted dihydropyrazolo[1,5-a]pyridines by the M06-2X 6-31+G(d,p)...
In order to explore this approach for the synthesis of deutero-1,2,4-triazolo[1,5-a]pyridines the reaction of 1a and MeCN in basic D2O solution was studied (Scheme 3, Table 2). The previously reported conditions for such a reaction were applied [18] except the fact that t-BuOK was used instead of KOH for KOD generation to achieve a higher degree of deuteration. The reaction yielded the corresponding 7-D-triazolo[1,5-a]pyridines with high D content at position 7. However, an H/D exchange at the methyl group was also observed with only moderate DD. The use of MeCN-d3 in place of MeCN resulted in the same yield of triazolopyridine-d4 15. Other nitriles such as PhCN and 4-cyanopyridine also gave the desired 7-D-triazolopyridines 16 and 17, respectively.
Scheme 3: Synthesis of deutero 1,2,4-triazolo[1,5-a]pyridines.
Scheme 3: Synthesis of deutero 1,2,4-triazolo[1,5-a]pyridines.
Conclusion
We have developed an efficient protocol for the synthesis of deuterium labeled pyrazolo[1,5-a]pyridines and triazolo[1,5-a]pyridines. Readily available and cheap D2O was employed as the deuterium source. The established system displays notable efficacy under mild reaction conditions in a short reaction time. A comparative assessment of the pKa values of different positions of N-aminopyridinium cations by DFT calculations allows predicting the direction of deuterium exchange. We assume that this method could also be extended to tritium labelling of pharmaceutically interesting compounds for medicinal applications.
Supporting Information
| Supporting Information File 1: Experimental part, NMR spectra, and quantum calculation details. | ||
| Format: PDF | Size: 1.5 MB | Download |
References
-
Elmore, C. S. Annu. Rep. Med. Chem. 2009, 44, 515–534. doi:10.1016/S0065-7743(09)04425-X
Return to citation in text: [1] -
Schellekens, R. C. A.; Stellaard, F.; Woerdenbag, H. J.; Frijlink, H. W.; Kosterink, J. G. W. Br. J. Clin. Pharmacol. 2011, 72, 879–897. doi:10.1111/j.1365-2125.2011.04071.x
Return to citation in text: [1] -
Kushner, D. J.; Baker, A.; Dunstall, T. G. Can. J. Physiol. Pharmacol. 1999, 77, 79–88. doi:10.1139/y99-005
Return to citation in text: [1] -
Harbeson, S. L.; Tung, R. D. Annu. Rep. Med. Chem. 2011, 46, 403–417. doi:10.1016/B978-0-12-386009-5.00003-5
Return to citation in text: [1] -
Meanwell, N. A. J. Med. Chem. 2011, 54, 2529–2591. doi:10.1021/jm1013693
Return to citation in text: [1] -
Gant, T. G. J. Med. Chem. 2014, 57, 3595–3611. doi:10.1021/jm4007998
Return to citation in text: [1] -
Miller, J. F.; Chong, P. Y.; Shotwell, J. B.; Catalano, J. G.; Tai, V. W.-F.; Fang, J.; Banka, A. L.; Roberts, C. D.; Youngman, M.; Zhang, H.; Xiong, Z.; Mathis, A.; Pouliot, J. J.; Hamatake, R. K.; Price, D. J.; Seal, J. W., III; Stroup, L. L.; Creech, K. L.; Carballo, L. H.; Todd, D.; Spaltenstein, A.; Furst, S.; Hong, Z.; Peat, A. J. J. Med. Chem. 2014, 57, 2107–2120. doi:10.1021/jm400125h
Return to citation in text: [1] -
Tai, V. W.-F.; Garrido, D.; Price, D. J.; Maynard, A.; Pouliot, J. J.; Xiong, Z.; Seal, J. W., III; Creech, K. L.; Kryn, L. H.; Baughman, T. M.; Peat, A. J. Bioorg. Med. Chem. Lett. 2014, 24, 2288–2294. doi:10.1016/j.bmcl.2014.03.080
Return to citation in text: [1] -
Le Manach, C.; Paquet, T.; Brunschwig, C.; Njoroge, M.; Han, Z.; Gonzàlez Cabrera, D.; Bashyam, S.; Dhinakaran, R.; Taylor, D.; Reader, J.; Botha, M.; Churchyard, A.; Lauterbach, S.; Coetzer, T. L.; Birkholtz, L.-M.; Meister, S.; Winzeler, E. A.; Waterson, D.; Witty, M. J.; Wittlin, S.; Jiménez-Díaz, M.-B.; Santos Martinez, M.; Ferrer, S.; Angulo-Barturen, I.; Street, L. J.; Chibale, K. J. Med. Chem. 2015, 58, 8713–8722. doi:10.1021/acs.jmedchem.5b01605
Return to citation in text: [1] -
Tang, J.; Wang, B.; Wu, T.; Wan, J.; Tu, Z.; Njire, M.; Wan, B.; Franzblauc, S. G.; Zhang, T.; Lu, X.; Ding, K. ACS Med. Chem. Lett. 2015, 6, 814–818. doi:10.1021/acsmedchemlett.5b00176
Return to citation in text: [1] -
Meng, D.; Andre, P.; Bateman, T. J.; Berger, R.; Chen, Y.-H.; Desai, K.; Dewnani, S.; Ellsworth, K.; Feng, D.; Geissler, W. M.; Guo, L.; Hruza, A.; Jian, T.; Li, H.; Metzger, J.; Parker, D. L.; Reichert, P.; Sherer, E. C.; Smith, C. J.; Sonatore, L. M.; Tschirret-Guth, R.; Wu, J.; Xu, J.; Zhang, T.; Campeau, L.-C.; Orr, R.; Poirier, M.; McCabe-Dunn, J.; Araki, K.; Nishimura, T.; Sakurada, I.; Hirabayashi, T.; Wood, H. B. Bioorg. Med. Chem. Lett. 2015, 25, 5437–5443. doi:10.1016/j.bmcl.2015.07.078
Return to citation in text: [1] -
Kendall, J. D.; Marshall, A. J.; Giddens, A. C.; Tsang, K. Y.; Boyd, M.; Frédérick, R.; Lill, C. L.; Lee, W.-J.; Kolekar, S.; Chao, M.; Malik, A.; Yu, S.; Chaussade, C.; Buchanan, C. M.; Rewcastle, G. W.; Baguley, B. C.; Flanagan, J. U.; Denny, W. A.; Shepherd, P. R. Med. Chem. Commun. 2014, 5, 41–46. doi:10.1039/C3MD00221G
Return to citation in text: [1] -
Finlay, M. R. V.; Anderton, M.; Ashton, S.; Ballard, P.; Bethel, P. A.; Box, M. R.; Bradbury, R. H.; Brown, S. J.; Butterworth, S.; Campbell, A.; Chorley, C.; Colclough, N.; Cross, D. A. E.; Currie, G. S.; Grist, M.; Hassall, L.; Hill, G. B.; James, D.; James, M.; Kemmitt, P.; Klinowska, T.; Lamont, G.; Lamont, S. G.; Martin, N.; McFarland, H. L.; Mellor, M. J.; Orme, J. P.; Perkins, D.; Perkins, P.; Richmond, G.; Smith, P.; Ward, R. A.; Waring, M. J.; Whittaker, D.; Wells, S.; Wrigley, G. L. J. Med. Chem. 2014, 57, 8249–8267. doi:10.1021/jm500973a
Return to citation in text: [1] -
Dore, A.; Asproni, B.; Scampuddu, A.; Pinna, G. A.; Christoffersen, C. T.; Langgård, M.; Kehler, J. Eur. J. Med. Chem. 2014, 84, 181–193. doi:10.1016/j.ejmech.2014.07.020
Return to citation in text: [1] -
Möller, D.; Kling, R. C.; Skultety, M.; Leuner, K.; Hübner, H.; Gmeiner, P. J. Med. Chem. 2014, 57, 4861–4875. doi:10.1021/jm5004039
Return to citation in text: [1] -
Rolan, P.; Hutchinson, M. R.; Johnson, K. W. Expert Opin. Pharmacother. 2009, 10, 2897–2904. doi:10.1517/14656560903426189
Return to citation in text: [1] -
Kuroyanagi, J.-i.; Kanai, K.; Sugimoto, Y.; Fujisawa, T.; Morita, C.; Suzuki, T.; Kawakami, K.; Takemura, M. Bioorg. Med. Chem. 2010, 18, 5845–5854. doi:10.1016/j.bmc.2010.06.096
Return to citation in text: [1] -
Tao, X.; Hu, Y. Med. Chem. 2010, 6, 65–69. doi:10.2174/157340610791321505
Return to citation in text: [1] [2] -
Zhang, G.; Chen, J. Lett. Org. Chem. 2011, 8, 180–183. doi:10.2174/157017811795038377
Return to citation in text: [1] -
Kendal, J. D. Curr. Org. Chem. 2011, 15, 2481–2518. doi:10.2174/138527211796150552
Return to citation in text: [1] -
Mousseau, J. J.; Fortier, A.; Charette, A. B. Org. Lett. 2010, 12, 516–519. doi:10.1021/ol902710f
Return to citation in text: [1] -
Mousseau, J. J.; Bull, J. A.; Ladd, C. L.; Fortier, A.; Roman, D. S.; Charette, A. B. J. Org. Chem. 2011, 76, 8243–8261. doi:10.1021/jo201303x
Return to citation in text: [1] -
Tamura, Y.; Ikeda, M. Adv. Heterocycl. Chem. 1981, 29, 71–139. doi:10.1016/S0065-2725(08)60786-2
Return to citation in text: [1] -
Johnston, K. A.; Allcock, R. W.; Jiang, Z.; Collier, I. D.; Blakli, H.; Rosair, G. M.; Bailey, P. D.; Morgan, K. M.; Kohno, Y.; Adams, D. R. Org. Biomol. Chem. 2008, 6, 175–186. doi:10.1039/B713638B
Return to citation in text: [1] -
Ding, S.; Yan, Y.; Jiao, N. Chem. Commun. 2013, 49, 4250–4252. doi:10.1039/C2CC33706A
Return to citation in text: [1] -
Ling, L.; Chen, J.; Song, J.; Zhang, Y.; Li, X.; Song, L.; Shi, F.; Li, Y.; Wu, C. Org. Biomol. Chem. 2013, 11, 3894–3902. doi:10.1039/c3ob40448j
Return to citation in text: [1] -
Zoltewicz, J. A.; Helmick, L. S. J. Am. Chem. Soc. 1970, 92, 7547–7552. doi:10.1021/ja00729a006
Return to citation in text: [1] [2] -
Mousseau, J. J.; Bull, J. A.; Charette, A. B. Angew. Chem., Int. Ed. 2010, 49, 1115–1118. doi:10.1002/anie.200906020
Return to citation in text: [1] -
Carceller, R.; García-Navío, J. L.; Izquierdo, M. L.; Alvarez-Builla, J.; Fajardo, M.; Gómez-Sal, P.; Gago, F. Tetrahedron 1994, 50, 4995–5012. doi:10.1016/S0040-4020(01)90411-9
Return to citation in text: [1] -
Lämsä, M.; Huuskonen, J.; Rissanen, K.; Pursiainen, J. Chem. – Eur. J. 1998, 4, 84–92. doi:10.1002/(SICI)1521-3765(199801)4:1<84::AID-CHEM84>3.0.CO;2-T
Return to citation in text: [1] -
Schmidt, M. W.; Baldridge, K. K.; Boatz, J. A.; Elbert, S. T.; Gordon, M. S.; Jensen, J. H.; Koseki, S.; Matsunaga, N.; Nguyen, K. A.; Su, S.; Windus, T. L.; Dupuis, M.; Montgomery, J. A., Jr. J. Comput. Chem. 1993, 14, 1347–1363. doi:10.1002/jcc.540141112
Return to citation in text: [1] -
Zhao, Y.; Truhlar, D. G. Theor. Chem. Acc. 2008, 120, 215–241. doi:10.1007/s00214-007-0310-x
Return to citation in text: [1] -
Marenich, A. V.; Cramer, C. J.; Truhlar, D. G. J. Phys. Chem. B 2009, 113, 6378–6396. doi:10.1021/jp810292n
Return to citation in text: [1]
| 1. | Elmore, C. S. Annu. Rep. Med. Chem. 2009, 44, 515–534. doi:10.1016/S0065-7743(09)04425-X |
| 9. | Le Manach, C.; Paquet, T.; Brunschwig, C.; Njoroge, M.; Han, Z.; Gonzàlez Cabrera, D.; Bashyam, S.; Dhinakaran, R.; Taylor, D.; Reader, J.; Botha, M.; Churchyard, A.; Lauterbach, S.; Coetzer, T. L.; Birkholtz, L.-M.; Meister, S.; Winzeler, E. A.; Waterson, D.; Witty, M. J.; Wittlin, S.; Jiménez-Díaz, M.-B.; Santos Martinez, M.; Ferrer, S.; Angulo-Barturen, I.; Street, L. J.; Chibale, K. J. Med. Chem. 2015, 58, 8713–8722. doi:10.1021/acs.jmedchem.5b01605 |
| 19. | Zhang, G.; Chen, J. Lett. Org. Chem. 2011, 8, 180–183. doi:10.2174/157017811795038377 |
| 7. | Miller, J. F.; Chong, P. Y.; Shotwell, J. B.; Catalano, J. G.; Tai, V. W.-F.; Fang, J.; Banka, A. L.; Roberts, C. D.; Youngman, M.; Zhang, H.; Xiong, Z.; Mathis, A.; Pouliot, J. J.; Hamatake, R. K.; Price, D. J.; Seal, J. W., III; Stroup, L. L.; Creech, K. L.; Carballo, L. H.; Todd, D.; Spaltenstein, A.; Furst, S.; Hong, Z.; Peat, A. J. J. Med. Chem. 2014, 57, 2107–2120. doi:10.1021/jm400125h |
| 8. | Tai, V. W.-F.; Garrido, D.; Price, D. J.; Maynard, A.; Pouliot, J. J.; Xiong, Z.; Seal, J. W., III; Creech, K. L.; Kryn, L. H.; Baughman, T. M.; Peat, A. J. Bioorg. Med. Chem. Lett. 2014, 24, 2288–2294. doi:10.1016/j.bmcl.2014.03.080 |
| 20. | Kendal, J. D. Curr. Org. Chem. 2011, 15, 2481–2518. doi:10.2174/138527211796150552 |
| 17. | Kuroyanagi, J.-i.; Kanai, K.; Sugimoto, Y.; Fujisawa, T.; Morita, C.; Suzuki, T.; Kawakami, K.; Takemura, M. Bioorg. Med. Chem. 2010, 18, 5845–5854. doi:10.1016/j.bmc.2010.06.096 |
| 2. | Schellekens, R. C. A.; Stellaard, F.; Woerdenbag, H. J.; Frijlink, H. W.; Kosterink, J. G. W. Br. J. Clin. Pharmacol. 2011, 72, 879–897. doi:10.1111/j.1365-2125.2011.04071.x |
| 3. | Kushner, D. J.; Baker, A.; Dunstall, T. G. Can. J. Physiol. Pharmacol. 1999, 77, 79–88. doi:10.1139/y99-005 |
| 4. | Harbeson, S. L.; Tung, R. D. Annu. Rep. Med. Chem. 2011, 46, 403–417. doi:10.1016/B978-0-12-386009-5.00003-5 |
| 5. | Meanwell, N. A. J. Med. Chem. 2011, 54, 2529–2591. doi:10.1021/jm1013693 |
| 13. | Finlay, M. R. V.; Anderton, M.; Ashton, S.; Ballard, P.; Bethel, P. A.; Box, M. R.; Bradbury, R. H.; Brown, S. J.; Butterworth, S.; Campbell, A.; Chorley, C.; Colclough, N.; Cross, D. A. E.; Currie, G. S.; Grist, M.; Hassall, L.; Hill, G. B.; James, D.; James, M.; Kemmitt, P.; Klinowska, T.; Lamont, G.; Lamont, S. G.; Martin, N.; McFarland, H. L.; Mellor, M. J.; Orme, J. P.; Perkins, D.; Perkins, P.; Richmond, G.; Smith, P.; Ward, R. A.; Waring, M. J.; Whittaker, D.; Wells, S.; Wrigley, G. L. J. Med. Chem. 2014, 57, 8249–8267. doi:10.1021/jm500973a |
| 15. | Möller, D.; Kling, R. C.; Skultety, M.; Leuner, K.; Hübner, H.; Gmeiner, P. J. Med. Chem. 2014, 57, 4861–4875. doi:10.1021/jm5004039 |
| 12. | Kendall, J. D.; Marshall, A. J.; Giddens, A. C.; Tsang, K. Y.; Boyd, M.; Frédérick, R.; Lill, C. L.; Lee, W.-J.; Kolekar, S.; Chao, M.; Malik, A.; Yu, S.; Chaussade, C.; Buchanan, C. M.; Rewcastle, G. W.; Baguley, B. C.; Flanagan, J. U.; Denny, W. A.; Shepherd, P. R. Med. Chem. Commun. 2014, 5, 41–46. doi:10.1039/C3MD00221G |
| 16. | Rolan, P.; Hutchinson, M. R.; Johnson, K. W. Expert Opin. Pharmacother. 2009, 10, 2897–2904. doi:10.1517/14656560903426189 |
| 11. | Meng, D.; Andre, P.; Bateman, T. J.; Berger, R.; Chen, Y.-H.; Desai, K.; Dewnani, S.; Ellsworth, K.; Feng, D.; Geissler, W. M.; Guo, L.; Hruza, A.; Jian, T.; Li, H.; Metzger, J.; Parker, D. L.; Reichert, P.; Sherer, E. C.; Smith, C. J.; Sonatore, L. M.; Tschirret-Guth, R.; Wu, J.; Xu, J.; Zhang, T.; Campeau, L.-C.; Orr, R.; Poirier, M.; McCabe-Dunn, J.; Araki, K.; Nishimura, T.; Sakurada, I.; Hirabayashi, T.; Wood, H. B. Bioorg. Med. Chem. Lett. 2015, 25, 5437–5443. doi:10.1016/j.bmcl.2015.07.078 |
| 10. | Tang, J.; Wang, B.; Wu, T.; Wan, J.; Tu, Z.; Njire, M.; Wan, B.; Franzblauc, S. G.; Zhang, T.; Lu, X.; Ding, K. ACS Med. Chem. Lett. 2015, 6, 814–818. doi:10.1021/acsmedchemlett.5b00176 |
| 14. | Dore, A.; Asproni, B.; Scampuddu, A.; Pinna, G. A.; Christoffersen, C. T.; Langgård, M.; Kehler, J. Eur. J. Med. Chem. 2014, 84, 181–193. doi:10.1016/j.ejmech.2014.07.020 |
| 27. | Zoltewicz, J. A.; Helmick, L. S. J. Am. Chem. Soc. 1970, 92, 7547–7552. doi:10.1021/ja00729a006 |
| 21. | Mousseau, J. J.; Fortier, A.; Charette, A. B. Org. Lett. 2010, 12, 516–519. doi:10.1021/ol902710f |
| 22. | Mousseau, J. J.; Bull, J. A.; Ladd, C. L.; Fortier, A.; Roman, D. S.; Charette, A. B. J. Org. Chem. 2011, 76, 8243–8261. doi:10.1021/jo201303x |
| 23. | Tamura, Y.; Ikeda, M. Adv. Heterocycl. Chem. 1981, 29, 71–139. doi:10.1016/S0065-2725(08)60786-2 |
| 24. | Johnston, K. A.; Allcock, R. W.; Jiang, Z.; Collier, I. D.; Blakli, H.; Rosair, G. M.; Bailey, P. D.; Morgan, K. M.; Kohno, Y.; Adams, D. R. Org. Biomol. Chem. 2008, 6, 175–186. doi:10.1039/B713638B |
| 25. | Ding, S.; Yan, Y.; Jiao, N. Chem. Commun. 2013, 49, 4250–4252. doi:10.1039/C2CC33706A |
| 26. | Ling, L.; Chen, J.; Song, J.; Zhang, Y.; Li, X.; Song, L.; Shi, F.; Li, Y.; Wu, C. Org. Biomol. Chem. 2013, 11, 3894–3902. doi:10.1039/c3ob40448j |
| 33. | Marenich, A. V.; Cramer, C. J.; Truhlar, D. G. J. Phys. Chem. B 2009, 113, 6378–6396. doi:10.1021/jp810292n |
| 27. | Zoltewicz, J. A.; Helmick, L. S. J. Am. Chem. Soc. 1970, 92, 7547–7552. doi:10.1021/ja00729a006 |
| 31. | Schmidt, M. W.; Baldridge, K. K.; Boatz, J. A.; Elbert, S. T.; Gordon, M. S.; Jensen, J. H.; Koseki, S.; Matsunaga, N.; Nguyen, K. A.; Su, S.; Windus, T. L.; Dupuis, M.; Montgomery, J. A., Jr. J. Comput. Chem. 1993, 14, 1347–1363. doi:10.1002/jcc.540141112 |
| 32. | Zhao, Y.; Truhlar, D. G. Theor. Chem. Acc. 2008, 120, 215–241. doi:10.1007/s00214-007-0310-x |
| 28. | Mousseau, J. J.; Bull, J. A.; Charette, A. B. Angew. Chem., Int. Ed. 2010, 49, 1115–1118. doi:10.1002/anie.200906020 |
| 29. | Carceller, R.; García-Navío, J. L.; Izquierdo, M. L.; Alvarez-Builla, J.; Fajardo, M.; Gómez-Sal, P.; Gago, F. Tetrahedron 1994, 50, 4995–5012. doi:10.1016/S0040-4020(01)90411-9 |
| 30. | Lämsä, M.; Huuskonen, J.; Rissanen, K.; Pursiainen, J. Chem. – Eur. J. 1998, 4, 84–92. doi:10.1002/(SICI)1521-3765(199801)4:1<84::AID-CHEM84>3.0.CO;2-T |
© 2017 Vorob’ev et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)