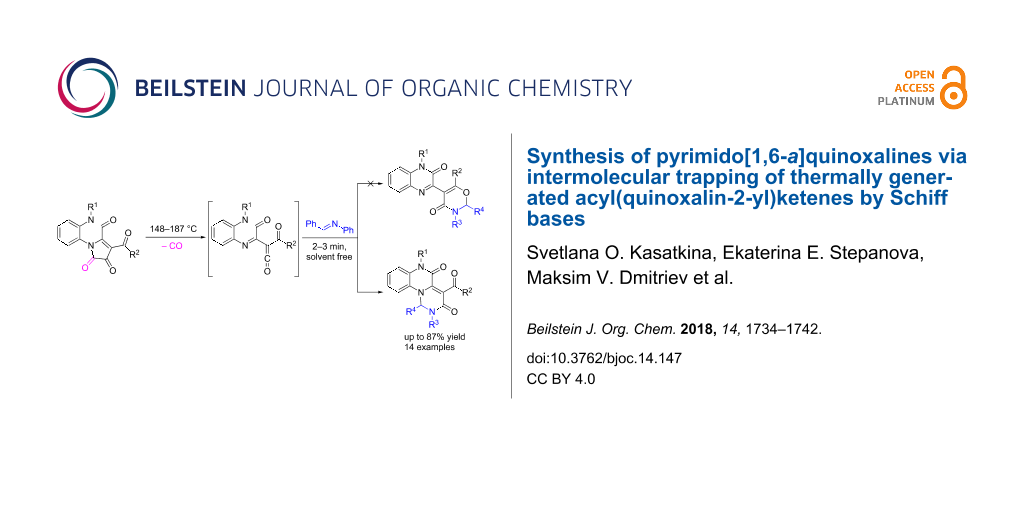
Abstract
Acyl(quinoxalin-2-yl)ketenes generated by thermal decarbonylation of 3-acylpyrrolo[1,2-a]quinoxaline-1,2,4(5H)-triones react regioselectively with Schiff bases under solvent-free conditions to form pyrimido[1,6-a]quinoxaline derivatives in good yields.
Graphical Abstract
Introduction
Quinoxaline is a 4-aza isostere of quinoline, which rarely occurs in structures of natural products. Its derivatives are gaining popularity in medicinal chemistry and pharmacology because many of them exhibit various biological activities [1,2].
Quinoxaline-based 6/6/6-angularly fused scaffolds (quinoxaline fused by a six-membered heterocycle at the [a]-side) are promising biologically active compounds. Recent research studies revealed that they can act as inhibitors of poly(ADP-ribose) polymerase (PARP) [3], inhibitors of hepatitis C virus [4], 5-HT2C agonists [5-7], substances for controlling intraocular pressure (IOP) [8] etc. (Figure 1).
Figure 1: Quinoxaline-based 6/6/6-angularly fused scaffolds and respective examples of biologically active compounds.
Figure 1: Quinoxaline-based 6/6/6-angularly fused scaffolds and respective examples of biologically active co...
Pyrimido[1,6-a]quinoxalines are one of the most intriguing and unexplored structures representing isosteres of this scaffold. Only few synthetic procedures towards these compounds are described in the literature: heterocyclizations of α-chloroisocyanates with quinoxalin-2-ylideneacetates [9], multicomponent Mannich–Ritter transformations of quinoxalin-2(1H)-ones under the action of nitriles and 3,4-dihydro-2H-pyran [10] and a microwave-assisted cascade strategy via in situ-generated N-acyliminium ion precursors and amines [11] (Figure 2).
Figure 2: Synthetic routes towards the pyrimido[1,6-a]quinoxaline scaffold.
Figure 2: Synthetic routes towards the pyrimido[1,6-a]quinoxaline scaffold.
To develop a new synthetic approach towards pyrimido[1,6-a]quinoxalines we looked through the procedures to their closest analogues – pyrido[1,2-a]quinoxalines, the synthesis of which has been explored more frequently [3,4,12-46]. The analysis helped us to disclose a tempting but challenging methodology, which has the potential to be extended for the synthesis of the desired heterocyclic system, via intermolecular trapping of thermally generated acyl(quinoxalin-2-yl)ketenes [20,21,23,24,28,29,38] (Figure 2).
Syntheses utilizing acylketenes are of practical and theoretical interest due to the high reactivity of acylketenes and the structural diversity of the reaction products [47-54]. The introduction of the quinoxalin-2-yl substituent into acylketenes results in the formation of a peculiar system of conjugated double bonds, which can potentially act as either oxo-diene or aza-diene (Figure 3).
To the best of our knowledge, there is no example of the involvement of the aza-diene fragment of acyl(quinoxalin-2-yl)ketenes into intermolecular trapping by hetero-dienophiles published so far. In this article we report a synthetic protocol towards pyrimido[1,6-a]quinoxalines via the intermolecular trapping of acyl(quinoxalin-2-yl)ketenes by Schiff bases.
Results and Discussion
The most convenient method for the generation of acyl(quinoxalin-2-yl)ketenes is the thermal decarbonylation (thermolysis) of five-membered 2,3-dioxoheterocycles having a quinoxaline fragment. Currently, three types of such precursors are known: 5-aryl-4-quinoxalin-2-ylfuran-2,3-diones I [21], 3-aroyl-4-arylpyrrolo[1,2-a]quinoxaline-1,2-diones II [55], and 3-acylpyrrolo[1,2-a]quinoxaline-1,2,4(5H)-triones III [23,56] (Scheme 1).
Scheme 1: Thermolysis of five-membered 2,3-dioxoheterocycles resulting in acyl(quinoxalin-2-yl)ketenes.
Scheme 1: Thermolysis of five-membered 2,3-dioxoheterocycles resulting in acyl(quinoxalin-2-yl)ketenes.
According to the literature data, precursors I and II are unsuitable for achieving the proposed goal as the generated ketene IV reacts only at its oxo-diene fragment in intermolecular trapping reactions with various dienophiles [57-62]. Under these circumstances precursors III generating ketenes V seemed to be the only suitable candidates for the development of a strategy towards pyrimido[1,6-a]quinoxalines.
First, we studied the decarbonylation of precursors III – 3-acylpyrrolo[1,2-a]quinoxaline-1,2,4(5H)-triones (PQTs, 1a–h) by simultaneous thermal analysis (STA, Table 1). According to the data obtained, PQTs 1a–h underwent thermal decomposition with a mass loss accompanied by an endothermic effect and CO evolution (Figure 4). The values of the mass loss corresponded to the elimination of a CO molecule from a PQT.
![[1860-5397-14-147-4]](/bjoc/content/figures/1860-5397-14-147-4.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 4: STA plot of thermolysis of PQT 1a. Blue solid curve: DSC; green solid curve: TG; greed dashed curve: DTG; violet solid curve: MID (m/z = 28); brown solid curve: MID (m/z = 44); heating rate: 5 °C/min.
Figure 4: STA plot of thermolysis of PQT 1a. Blue solid curve: DSC; green solid curve: TG; greed dashed curve...
Having taken into account the results of the thermal analysis, we examined the feasibility and conditions of the intermolecular reaction of the ketene generated from PQT 1a with benzalaniline (2a). The reaction mixtures obtained were investigated by UPLC–MS and the results are summarized in Table 2.
Table 2: Intermolecular trapping of ketene generated from PQT 1a by benzalaniline (2a)a.
|
|
|||
| entry | yield of 3a (%)b | time (min) | temp. (°C) |
| 1 | 65 | 5 | 190 |
| 2 | 50 | 15 | 190 |
| 3 | 57 | 5 | 200 |
| 4 | 30 | 60 | 200 |
| 5 | 80 | 3 | 175 |
| 6 | 85c | 2 | 187 |
aConditions: suspension of 1a (1 mmol) and 2a (1.1 mmol) in Dowtherm A (5 mL). bYields were determined by UPLC. cSolvent-free reaction.
The reaction mixtures contained only three types of products, and we succeeded to identify each of them. The structures of the reaction products were elucidated as the desired pyrimido[1,6-a]quinoxaline 3a, quinoxalinone 4a [29] and pyrido[1,2-a]quinoxaline 5a [29] (Scheme 2). Product IV of an alternative intermolecular trapping reaction (Table 1) was not detected.
Scheme 2: Side-reactions concurring with intermolecular trapping of ketene generated from PQT 1a by benzalaniline (2a).
Scheme 2: Side-reactions concurring with intermolecular trapping of ketene generated from PQT 1a by benzalani...
The most likely way of the formation of quinoxalinone 4a is hydration of the ketene with subsequent decarboxylation (Scheme 2); more careful drying the reaction vials and solvents easily reduced the amount of compound 4a.
The formation of pyrido[1,2-a]quinoxaline 5a can be explained by a concurrent process of ketene dimerization (Scheme 2) [29] in comparison to the intermolecular trapping of it by benzalaniline (2a). Since the yields of the target product 3a decreased and the yields of compound 5a increased at prolonged time of reaction, the formation of the target compounds deemed to be reversible.
Performing the reaction under solvent-free conditions at the onset decarbonylation temperature (Table 1) exceeded our expectations and gave the best yields of the target compound 3a (Table 2, entry 6).
Being inspired by the optimization results obtained, we examined the scope of the reaction applying the developed methodology with PQTs 1a–h and Schiff bases 2a–d. The results are shown in Figure 5.
Figure 5: Scope of the intermolecular trapping of ketenes generated from PQTs 1a–h by Schiff bases 2a–d under solvent-free conditions.
Figure 5: Scope of the intermolecular trapping of ketenes generated from PQTs 1a–h by Schiff bases 2a–d under...
Unfortunately, our attempts to involve Schiff bases synthesized from aliphatic aldehydes and ketones did not give any satisfactory results because of various nucleophilic side-reactions.
We found that the intermolecular trapping worked perfectly in case of N5-substituted PQTs 1a–f and did not work at all with N5-unsubstituted PQT 1g and 1h. The failure to obtain products 3o and 3p from PQTs 1g and 1h can be explained by the occurrence of intramolecular cyclization in these ketenes resulting in the formation of furoquinoxalines 6a,b [56,63,64] which were confirmed by UPLC–MS data as the sole products of the reaction (Scheme 3).
Scheme 3: Formation of furoquinoxalines 6a,b via intramolecular cyclization in ketenes generated from PQTs 1g,h.
Scheme 3: Formation of furoquinoxalines 6a,b via intramolecular cyclization in ketenes generated from PQTs 1g,...
The formation of pyrimido[1,6-a]quinoxalines 3a–n was unambiguously confirmed by the crystal structure of compounds 3g and 3j (CCDC 1834011, Figure 6; CCDC 1834012, Figure 7).
![[1860-5397-14-147-6]](/bjoc/content/figures/1860-5397-14-147-6.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 6: ORTEP drawing of compound 3g (CCDC 1834011) showing thermal ellipsoids at the 30% probability level.
Figure 6: ORTEP drawing of compound 3g (CCDC 1834011) showing thermal ellipsoids at the 30% probability level....
![[1860-5397-14-147-7]](/bjoc/content/figures/1860-5397-14-147-7.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 7: ORTEP drawing of compound 3j (CCDC 1834012) showing thermal ellipsoids at the 30% probability level.
Figure 7: ORTEP drawing of compound 3j (CCDC 1834012) showing thermal ellipsoids at the 30% probability level....
Conclusion
We have developed a facile synthesis of pyrimido[1,6-a]quinoxaline derivatives via the intermolecular trapping of thermally generated acyl(quinoxalin-2-yl)ketenes by Schiff bases. The reaction proceeds under solvent-free conditions without any additives and catalysts. The elaborated method might be applicable to the syntheses of pharmaceutically important substances.
Supporting Information
| Supporting Information File 1: Experimental details, copies of 1H and 13C NMR spectra of pyrimido[1,6-a]quinoxalines 3a–n, STA plots of PQT 1a–h and X-ray crystal structure details of compounds 3g,j. | ||
| Format: PDF | Size: 2.5 MB | Download |
References
-
Pereira, J. A.; Pessoa, A. M.; Cordeiro, M. N. D. S.; Fernandes, R.; Prudêncio, C.; Noronha, J. P.; Vieira, M. Eur. J. Med. Chem. 2015, 97, 664–672. doi:10.1016/j.ejmech.2014.06.058
Return to citation in text: [1] -
Tariq, S.; Somakala, K.; Amir, M. Eur. J. Med. Chem. 2018, 143, 542–557. doi:10.1016/j.ejmech.2017.11.064
Return to citation in text: [1] -
Miyashiro, J.; Woods, K. W.; Park, C. H.; Liu, X.; Shi, Y.; Johnson, E. F.; Bouska, J. J.; Olson, A. M.; Luo, Y.; Fry, E. H.; Giranda, V. L.; Penning, T. D. Bioorg. Med. Chem. Lett. 2009, 19, 4050–4054. doi:10.1016/j.bmcl.2009.06.016
Return to citation in text: [1] [2] -
Liu, R.; Huang, Z.; Murray, M. G.; Guo, X.; Liu, G. J. Med. Chem. 2011, 54, 5747–5768. doi:10.1021/jm200394x
Return to citation in text: [1] [2] -
Sabb, A. L.; Welmaker, G. S.; Nelson, J. A. 2,3,4,4a-Tetrahydro-1H-pyrazino(1,2-a)quinoxalin-5(6H)one derivates being 5HT2C agonists. WO Patent WO0035922 A1, June 6, 2000.
Return to citation in text: [1] -
Rosenzweig-Lipson, S.; Zhang, J.; Mazandarani, H.; Harrison, B. L.; Sabb, A.; Sabalski, J.; Stack, G.; Welmaker, G.; Barrett, J. E.; Dunlop, J. Brain Res. 2006, 1073–1074, 240–251. doi:10.1016/j.brainres.2005.12.052
Return to citation in text: [1] -
Hayes, D. J.; Mosher, T. M.; Greenshaw, A. J. Behav. Brain Res. 2009, 197, 323–330. doi:10.1016/j.bbr.2008.08.034
Return to citation in text: [1] -
May, J. A.; Zinke, P. W. (R)-8,9-Dichloro-2,3,4,4a-tetrahydro-1H,6H-pyrazino[1,2-a]quinoxalin-5-one for controlling IOP and treating glaucoma. U.S. Patent US2006211700 A1, Sept 21, 2006.
Return to citation in text: [1] -
Kushnir, O. V.; Vovk, M. V. Russ. J. Org. Chem. 2010, 46, 890–893. doi:10.1134/s1070428010060187
Return to citation in text: [1] -
Preciado, S.; Vicente-García, E.; Llabrés, S.; Luque, F. J.; Lavilla, R. Angew. Chem. 2012, 124, 6980–6983. doi:10.1002/ange.201202927
Return to citation in text: [1] -
Sawant, R. T.; Stevens, M. Y.; Sköld, C.; Odell, L. R. Org. Lett. 2016, 18, 5392–5395. doi:10.1021/acs.orglett.6b02774
Return to citation in text: [1] -
Kappe, T.; Linnau, Y.; Stadlbauer, W. Monatsh. Chem. 1977, 108, 103–111. doi:10.1007/BF00900912
Return to citation in text: [1] -
Ames, D. E.; Brohi, M. I. J. Chem. Soc., Perkin Trans. 1 1980, 1384–1389. doi:10.1039/p19800001384
Return to citation in text: [1] -
Adegoke, E. A.; Alo, B. J. Heterocycl. Chem. 1983, 20, 1509–1512. doi:10.1002/jhet.5570200614
Return to citation in text: [1] -
Chernavskaya, L. N.; Kholodova, N. V.; Blagorodov, S. G.; Dmitrieva, N. A. Pharm. Chem. J. 1984, 18, 413–416. doi:10.1007/BF00776797
Return to citation in text: [1] -
Kurasawa, Y.; Nemoto, Y.; Sakakura, A.; Ogura, M.; Takada, A. Chem. Pharm. Bull. 1984, 32, 3366–3372. doi:10.1248/cpb.32.3366
Return to citation in text: [1] -
Kawahara, N.; Shimamori, T.; Itoh, T.; Takayanagi, H.; Ogura, H. Chem. Pharm. Bull. 1987, 35, 457–467. doi:10.1248/cpb.35.457
Return to citation in text: [1] -
Kawahara, N.; Shimamori, T.; Itoh, T.; Takayanagi, H.; Ogura, H. J. Heterocycl. Chem. 1989, 26, 847–852. doi:10.1002/jhet.5570260362
Return to citation in text: [1] -
Öcal, N.; Turgut, Z.; Kaban, Ş. J. Heterocycl. Chem. 1998, 35, 1349–1351. doi:10.1002/jhet.5570350620
Return to citation in text: [1] -
Maslivets, A. N.; Golovnina, O. V.; Krasnykh, O. P.; Aliev, Z. G. Chem. Heterocycl. Compd. 2000, 36, 615–616. doi:10.1007/bf02290858
Return to citation in text: [1] [2] -
Lisovenko, N. Y.; Krasnykh, O. P.; Aliev, Z. G.; Vostrov, E. S.; Tarasova, O. P.; Maslivets, A. N. Chem. Heterocycl. Compd. 2001, 37, 1314–1316. doi:10.1023/A:1013886602711
Return to citation in text: [1] [2] [3] -
Duffy, K. J.; Haltiwanger, R. C.; Freyer, A. J.; Li, F.; Luengo, J. I.; Cheng, H.-Y. J. Chem. Soc., Perkin Trans. 2 2002, 181–185. doi:10.1039/b102755g
Return to citation in text: [1] -
Maslivets, A. N.; Bozdyreva, K. S.; Smirnova, I. V.; Tolmacheva, I. A.; Mashevskaya, I. V. Chem. Heterocycl. Compd. 2002, 38, 498–499. doi:10.1023/A:1016056011167
Return to citation in text: [1] [2] [3] -
Maslivets, A. N.; Lisovenko, N. Y.; Krasnykh, O. P.; Tarasova, O. P.; Aliev, Z. G.; Atovmyan, L. O. Russ. Chem. Bull. 2002, 51, 850–853. doi:10.1023/A:1016097120253
Return to citation in text: [1] [2] -
García, M. B.; Orelli, L. R.; Magri, M. L.; Perillo, I. A. Synthesis 2002, 2687–2690. doi:10.1055/s-2002-35980
Return to citation in text: [1] -
Bunce, R. A.; Herron, D. M.; Hale, L. Y. J. Heterocycl. Chem. 2003, 40, 1031–1039. doi:10.1002/jhet.5570400611
Return to citation in text: [1] -
Chicharro, R.; de Castro, S.; Reino, J. L.; Arán, V. J. Eur. J. Org. Chem. 2003, 2314–2326. doi:10.1002/ejoc.200300028
Return to citation in text: [1] -
Maslivets, A. N.; Aliev, Z. G.; Krasnykh, O. P.; Golovnina, O. V.; Atovmyan, L. O. Chem. Heterocycl. Compd. 2004, 40, 1295–1299. doi:10.1007/s10593-005-0060-4
Return to citation in text: [1] [2] -
Bozdyreva, K. S.; Smirnova, I. V.; Maslivets, A. N. Russ. J. Org. Chem. 2005, 41, 1081–1088. doi:10.1007/s11178-005-0296-6
Return to citation in text: [1] [2] [3] [4] [5] -
Ma, Y.; Luo, W.; Camplo, M.; Liu, Z.; Hider, R. C. Bioorg. Med. Chem. Lett. 2005, 15, 3450–3452. doi:10.1016/j.bmcl.2005.05.010
Return to citation in text: [1] -
Tanimori, S.; Nishimura, T.; Kirihata, M. Bioorg. Med. Chem. Lett. 2009, 19, 4119–4121. doi:10.1016/j.bmcl.2009.06.007
Return to citation in text: [1] -
Yavari, I.; Souri, S.; Sirouspour, M.; Bayat, M. J. Synlett 2009, 1921–1922. doi:10.1055/s-0029-1217542
Return to citation in text: [1] -
Luo, X.; Chenard, E.; Martens, P.; Cheng, Y.-X.; Tomaszewski, M. J. Org. Lett. 2010, 12, 3574–3577. doi:10.1021/ol101454x
Return to citation in text: [1] -
Xu, L.; Jiang, Y.; Ma, D. Synlett 2010, 2285–2288. doi:10.1055/s-0030-1258030
Return to citation in text: [1] -
Tanimori, S.; Kashiwagi, H.; Nishimura, T.; Kirihata, M. Adv. Synth. Catal. 2010, 352, 2531–2537. doi:10.1002/adsc.201000323
Return to citation in text: [1] -
Gulevskaya, A. V.; Nguyen, H. T. L.; Tyaglivy, A. S.; Pozharskii, A. F. Tetrahedron 2012, 68, 488–498. doi:10.1016/j.tet.2011.11.018
Return to citation in text: [1] -
Xu, Z.; De Moliner, F.; Cappelli, A. P.; Hulme, C. Org. Lett. 2013, 15, 2738–2741. doi:10.1021/ol401068u
Return to citation in text: [1] -
Lisovenko, N. Y.; Yukova, Y. V.; Makhmudov, R. R. Pharm. Chem. J. 2014, 47, 593–595. doi:10.1007/s11094-014-1014-x
Return to citation in text: [1] [2] -
Nguyen, H. T. L.; Gulevskaya, A. V.; Pozharskii, A. F.; Nelina-Nemtseva, J. I. Tetrahedron 2014, 70, 4617–4625. doi:10.1016/j.tet.2014.05.023
Return to citation in text: [1] -
Obydennov, D. L.; Sosnovskikh, V. Y. Chem. Heterocycl. Compd. 2014, 50, 579–582. doi:10.1007/s10593-014-1510-7
Return to citation in text: [1] -
Tanimori, S.; Inaba, U.; Kato, Y.; Ura, H.; Kashiwagi, H.; Nishimura, T.; Kirihata, M. Res. Chem. Intermed. 2014, 40, 2157–2164. doi:10.1007/s11164-014-1593-x
Return to citation in text: [1] -
Miyamaru, S.; Umezu, K.; Ito, A.; Shimizu, M. Eur. J. Org. Chem. 2015, 3327–3337. doi:10.1002/ejoc.201500225
Return to citation in text: [1] -
Rezvanian, A. Tetrahedron 2016, 72, 6428–6435. doi:10.1016/j.tet.2016.08.049
Return to citation in text: [1] -
Azev, Y. A.; Kodess, M. I.; Ezhikova, M. A.; Ermakova, O. S.; Berseneva, V. S.; Bakulev, V. A. Mendeleev Commun. 2017, 27, 97–98. doi:10.1016/j.mencom.2017.01.032
Return to citation in text: [1] -
Azev, Y. A.; Ermakova, O. S.; Berseneva, V. S.; Kodess, M. I.; Ezhikova, M. A.; Ganebnykh, I. N. Mendeleev Commun. 2017, 27, 637–639. doi:10.1016/j.mencom.2017.11.034
Return to citation in text: [1] -
Soozani, A.; Keivanloo, A.; Bakherad, M. Tetrahedron 2018, 74, 150–156. doi:10.1016/j.tet.2017.11.055
Return to citation in text: [1] -
Reber, K. P.; Tilley, S. D.; Sorensen, E. J. Chem. Soc. Rev. 2009, 38, 3022–3034. doi:10.1039/B912599J
Return to citation in text: [1] -
Presset, M.; Coquerel, Y.; Rodriguez, J. Org. Lett. 2009, 11, 5706–5709. doi:10.1021/ol9024056
Return to citation in text: [1] -
Presset, M.; Coquerel, Y.; Rodriguez, J. Org. Lett. 2010, 12, 4212–4215. doi:10.1021/ol101938r
Return to citation in text: [1] -
Leber, S.; Kollenz, G.; Wentrup, C. Beilstein J. Org. Chem. 2012, 8, 738–743. doi:10.3762/bjoc.8.83
Return to citation in text: [1] -
Galvez, J.; Castillo, J.-C.; Quiroga, J.; Rajzmann, M.; Rodriguez, J.; Coquerel, Y. Org. Lett. 2014, 16, 4126–4129. doi:10.1021/ol5018245
Return to citation in text: [1] -
Khlebnikov, A. F.; Novikov, M. S.; Pakalnis, V. V.; Iakovenko, R. O.; Yufit, D. S. Beilstein J. Org. Chem. 2014, 10, 784–793. doi:10.3762/bjoc.10.74
Return to citation in text: [1] -
Cookson, R.; Barrett, T. N.; Barrett, A. G. M. Acc. Chem. Res. 2015, 48, 628–642. doi:10.1021/ar5004169
Return to citation in text: [1] -
Kollenz, G.; Wentrup, C. Beilstein J. Org. Chem. 2018, 14, 1–10. doi:10.3762/bjoc.14.1
Return to citation in text: [1] -
Silaichev, P. S.; Maslivets, A. N. Russ. J. Org. Chem. 2012, 48, 1261–1262. doi:10.1134/S1070428012090229
Return to citation in text: [1] -
Mashevskaya, I. V.; Mokrushin, I. G.; Bozdyreva, K. S.; Maslivets, A. N. Russ. J. Org. Chem. 2011, 47, 253–257. doi:10.1134/S1070428011020151
Return to citation in text: [1] [2] -
Lisovenko, N. Y.; Maslivets, A. N.; Aliev, Z. G. Chem. Heterocycl. Compd. 2003, 39, 132–134. doi:10.1023/A:1023097414711
Return to citation in text: [1] -
Lisovenko, N. Y.; Maslivets, A. N. Chem. Heterocycl. Compd. 2004, 40, 247–248. doi:10.1023/B:COHC.0000027901.78882.86
Return to citation in text: [1] -
Lisovenko, N. Y.; Maslivets, A. N.; Aliev, Z. G. Russ. J. Org. Chem. 2004, 40, 1053–1057. doi:10.1023/B:RUJO.0000045203.69639.83
Return to citation in text: [1] -
Lisovenko, N. Y.; Maslivets, A. N.; Aliev, Z. G. Russ. J. Org. Chem. 2007, 43, 117–120. doi:10.1134/s1070428007010150
Return to citation in text: [1] -
Nekrasov, D. D.; Obukhova, A. S.; Lisovenko, N. Y.; Roubtsov, A. E. Chem. Heterocycl. Compd. 2010, 46, 413–418. doi:10.1007/s10593-010-0525-y
Return to citation in text: [1] -
Lisovenko, N. Y.; Nekrasov, D. D.; Karmanov, V. I. Chem. Heterocycl. Compd. 2012, 48, 1357–1360. doi:10.1007/s10593-012-1144-6
Return to citation in text: [1] -
Masilvets, A. N.; Golovnina, O. V.; Krasnykh, O. P.; Aliev, Z. G. Chem. Heterocycl. Compd. 2000, 36, 355–356. doi:10.1007/BF02256878
Return to citation in text: [1] -
Aliev, Z. G.; Maslivets, A. N.; Golovnina, O. V.; Krasnykh, O. P.; Atovmyan, L. O. Russ. Chem. Bull. 2001, 50, 1317–1319. doi:10.1023/A:1014091731016
Return to citation in text: [1]
| 56. | Mashevskaya, I. V.; Mokrushin, I. G.; Bozdyreva, K. S.; Maslivets, A. N. Russ. J. Org. Chem. 2011, 47, 253–257. doi:10.1134/S1070428011020151 |
| 63. | Masilvets, A. N.; Golovnina, O. V.; Krasnykh, O. P.; Aliev, Z. G. Chem. Heterocycl. Compd. 2000, 36, 355–356. doi:10.1007/BF02256878 |
| 64. | Aliev, Z. G.; Maslivets, A. N.; Golovnina, O. V.; Krasnykh, O. P.; Atovmyan, L. O. Russ. Chem. Bull. 2001, 50, 1317–1319. doi:10.1023/A:1014091731016 |
| 29. | Bozdyreva, K. S.; Smirnova, I. V.; Maslivets, A. N. Russ. J. Org. Chem. 2005, 41, 1081–1088. doi:10.1007/s11178-005-0296-6 |
| 29. | Bozdyreva, K. S.; Smirnova, I. V.; Maslivets, A. N. Russ. J. Org. Chem. 2005, 41, 1081–1088. doi:10.1007/s11178-005-0296-6 |
| 1. | Pereira, J. A.; Pessoa, A. M.; Cordeiro, M. N. D. S.; Fernandes, R.; Prudêncio, C.; Noronha, J. P.; Vieira, M. Eur. J. Med. Chem. 2015, 97, 664–672. doi:10.1016/j.ejmech.2014.06.058 |
| 2. | Tariq, S.; Somakala, K.; Amir, M. Eur. J. Med. Chem. 2018, 143, 542–557. doi:10.1016/j.ejmech.2017.11.064 |
| 8. | May, J. A.; Zinke, P. W. (R)-8,9-Dichloro-2,3,4,4a-tetrahydro-1H,6H-pyrazino[1,2-a]quinoxalin-5-one for controlling IOP and treating glaucoma. U.S. Patent US2006211700 A1, Sept 21, 2006. |
| 57. | Lisovenko, N. Y.; Maslivets, A. N.; Aliev, Z. G. Chem. Heterocycl. Compd. 2003, 39, 132–134. doi:10.1023/A:1023097414711 |
| 58. | Lisovenko, N. Y.; Maslivets, A. N. Chem. Heterocycl. Compd. 2004, 40, 247–248. doi:10.1023/B:COHC.0000027901.78882.86 |
| 59. | Lisovenko, N. Y.; Maslivets, A. N.; Aliev, Z. G. Russ. J. Org. Chem. 2004, 40, 1053–1057. doi:10.1023/B:RUJO.0000045203.69639.83 |
| 60. | Lisovenko, N. Y.; Maslivets, A. N.; Aliev, Z. G. Russ. J. Org. Chem. 2007, 43, 117–120. doi:10.1134/s1070428007010150 |
| 61. | Nekrasov, D. D.; Obukhova, A. S.; Lisovenko, N. Y.; Roubtsov, A. E. Chem. Heterocycl. Compd. 2010, 46, 413–418. doi:10.1007/s10593-010-0525-y |
| 62. | Lisovenko, N. Y.; Nekrasov, D. D.; Karmanov, V. I. Chem. Heterocycl. Compd. 2012, 48, 1357–1360. doi:10.1007/s10593-012-1144-6 |
| 5. | Sabb, A. L.; Welmaker, G. S.; Nelson, J. A. 2,3,4,4a-Tetrahydro-1H-pyrazino(1,2-a)quinoxalin-5(6H)one derivates being 5HT2C agonists. WO Patent WO0035922 A1, June 6, 2000. |
| 6. | Rosenzweig-Lipson, S.; Zhang, J.; Mazandarani, H.; Harrison, B. L.; Sabb, A.; Sabalski, J.; Stack, G.; Welmaker, G.; Barrett, J. E.; Dunlop, J. Brain Res. 2006, 1073–1074, 240–251. doi:10.1016/j.brainres.2005.12.052 |
| 7. | Hayes, D. J.; Mosher, T. M.; Greenshaw, A. J. Behav. Brain Res. 2009, 197, 323–330. doi:10.1016/j.bbr.2008.08.034 |
| 29. | Bozdyreva, K. S.; Smirnova, I. V.; Maslivets, A. N. Russ. J. Org. Chem. 2005, 41, 1081–1088. doi:10.1007/s11178-005-0296-6 |
| 4. | Liu, R.; Huang, Z.; Murray, M. G.; Guo, X.; Liu, G. J. Med. Chem. 2011, 54, 5747–5768. doi:10.1021/jm200394x |
| 55. | Silaichev, P. S.; Maslivets, A. N. Russ. J. Org. Chem. 2012, 48, 1261–1262. doi:10.1134/S1070428012090229 |
| 3. | Miyashiro, J.; Woods, K. W.; Park, C. H.; Liu, X.; Shi, Y.; Johnson, E. F.; Bouska, J. J.; Olson, A. M.; Luo, Y.; Fry, E. H.; Giranda, V. L.; Penning, T. D. Bioorg. Med. Chem. Lett. 2009, 19, 4050–4054. doi:10.1016/j.bmcl.2009.06.016 |
| 23. | Maslivets, A. N.; Bozdyreva, K. S.; Smirnova, I. V.; Tolmacheva, I. A.; Mashevskaya, I. V. Chem. Heterocycl. Compd. 2002, 38, 498–499. doi:10.1023/A:1016056011167 |
| 56. | Mashevskaya, I. V.; Mokrushin, I. G.; Bozdyreva, K. S.; Maslivets, A. N. Russ. J. Org. Chem. 2011, 47, 253–257. doi:10.1134/S1070428011020151 |
| 3. | Miyashiro, J.; Woods, K. W.; Park, C. H.; Liu, X.; Shi, Y.; Johnson, E. F.; Bouska, J. J.; Olson, A. M.; Luo, Y.; Fry, E. H.; Giranda, V. L.; Penning, T. D. Bioorg. Med. Chem. Lett. 2009, 19, 4050–4054. doi:10.1016/j.bmcl.2009.06.016 |
| 4. | Liu, R.; Huang, Z.; Murray, M. G.; Guo, X.; Liu, G. J. Med. Chem. 2011, 54, 5747–5768. doi:10.1021/jm200394x |
| 12. | Kappe, T.; Linnau, Y.; Stadlbauer, W. Monatsh. Chem. 1977, 108, 103–111. doi:10.1007/BF00900912 |
| 13. | Ames, D. E.; Brohi, M. I. J. Chem. Soc., Perkin Trans. 1 1980, 1384–1389. doi:10.1039/p19800001384 |
| 14. | Adegoke, E. A.; Alo, B. J. Heterocycl. Chem. 1983, 20, 1509–1512. doi:10.1002/jhet.5570200614 |
| 15. | Chernavskaya, L. N.; Kholodova, N. V.; Blagorodov, S. G.; Dmitrieva, N. A. Pharm. Chem. J. 1984, 18, 413–416. doi:10.1007/BF00776797 |
| 16. | Kurasawa, Y.; Nemoto, Y.; Sakakura, A.; Ogura, M.; Takada, A. Chem. Pharm. Bull. 1984, 32, 3366–3372. doi:10.1248/cpb.32.3366 |
| 17. | Kawahara, N.; Shimamori, T.; Itoh, T.; Takayanagi, H.; Ogura, H. Chem. Pharm. Bull. 1987, 35, 457–467. doi:10.1248/cpb.35.457 |
| 18. | Kawahara, N.; Shimamori, T.; Itoh, T.; Takayanagi, H.; Ogura, H. J. Heterocycl. Chem. 1989, 26, 847–852. doi:10.1002/jhet.5570260362 |
| 19. | Öcal, N.; Turgut, Z.; Kaban, Ş. J. Heterocycl. Chem. 1998, 35, 1349–1351. doi:10.1002/jhet.5570350620 |
| 20. | Maslivets, A. N.; Golovnina, O. V.; Krasnykh, O. P.; Aliev, Z. G. Chem. Heterocycl. Compd. 2000, 36, 615–616. doi:10.1007/bf02290858 |
| 21. | Lisovenko, N. Y.; Krasnykh, O. P.; Aliev, Z. G.; Vostrov, E. S.; Tarasova, O. P.; Maslivets, A. N. Chem. Heterocycl. Compd. 2001, 37, 1314–1316. doi:10.1023/A:1013886602711 |
| 22. | Duffy, K. J.; Haltiwanger, R. C.; Freyer, A. J.; Li, F.; Luengo, J. I.; Cheng, H.-Y. J. Chem. Soc., Perkin Trans. 2 2002, 181–185. doi:10.1039/b102755g |
| 23. | Maslivets, A. N.; Bozdyreva, K. S.; Smirnova, I. V.; Tolmacheva, I. A.; Mashevskaya, I. V. Chem. Heterocycl. Compd. 2002, 38, 498–499. doi:10.1023/A:1016056011167 |
| 24. | Maslivets, A. N.; Lisovenko, N. Y.; Krasnykh, O. P.; Tarasova, O. P.; Aliev, Z. G.; Atovmyan, L. O. Russ. Chem. Bull. 2002, 51, 850–853. doi:10.1023/A:1016097120253 |
| 25. | García, M. B.; Orelli, L. R.; Magri, M. L.; Perillo, I. A. Synthesis 2002, 2687–2690. doi:10.1055/s-2002-35980 |
| 26. | Bunce, R. A.; Herron, D. M.; Hale, L. Y. J. Heterocycl. Chem. 2003, 40, 1031–1039. doi:10.1002/jhet.5570400611 |
| 27. | Chicharro, R.; de Castro, S.; Reino, J. L.; Arán, V. J. Eur. J. Org. Chem. 2003, 2314–2326. doi:10.1002/ejoc.200300028 |
| 28. | Maslivets, A. N.; Aliev, Z. G.; Krasnykh, O. P.; Golovnina, O. V.; Atovmyan, L. O. Chem. Heterocycl. Compd. 2004, 40, 1295–1299. doi:10.1007/s10593-005-0060-4 |
| 29. | Bozdyreva, K. S.; Smirnova, I. V.; Maslivets, A. N. Russ. J. Org. Chem. 2005, 41, 1081–1088. doi:10.1007/s11178-005-0296-6 |
| 30. | Ma, Y.; Luo, W.; Camplo, M.; Liu, Z.; Hider, R. C. Bioorg. Med. Chem. Lett. 2005, 15, 3450–3452. doi:10.1016/j.bmcl.2005.05.010 |
| 31. | Tanimori, S.; Nishimura, T.; Kirihata, M. Bioorg. Med. Chem. Lett. 2009, 19, 4119–4121. doi:10.1016/j.bmcl.2009.06.007 |
| 32. | Yavari, I.; Souri, S.; Sirouspour, M.; Bayat, M. J. Synlett 2009, 1921–1922. doi:10.1055/s-0029-1217542 |
| 33. | Luo, X.; Chenard, E.; Martens, P.; Cheng, Y.-X.; Tomaszewski, M. J. Org. Lett. 2010, 12, 3574–3577. doi:10.1021/ol101454x |
| 34. | Xu, L.; Jiang, Y.; Ma, D. Synlett 2010, 2285–2288. doi:10.1055/s-0030-1258030 |
| 35. | Tanimori, S.; Kashiwagi, H.; Nishimura, T.; Kirihata, M. Adv. Synth. Catal. 2010, 352, 2531–2537. doi:10.1002/adsc.201000323 |
| 36. | Gulevskaya, A. V.; Nguyen, H. T. L.; Tyaglivy, A. S.; Pozharskii, A. F. Tetrahedron 2012, 68, 488–498. doi:10.1016/j.tet.2011.11.018 |
| 37. | Xu, Z.; De Moliner, F.; Cappelli, A. P.; Hulme, C. Org. Lett. 2013, 15, 2738–2741. doi:10.1021/ol401068u |
| 38. | Lisovenko, N. Y.; Yukova, Y. V.; Makhmudov, R. R. Pharm. Chem. J. 2014, 47, 593–595. doi:10.1007/s11094-014-1014-x |
| 39. | Nguyen, H. T. L.; Gulevskaya, A. V.; Pozharskii, A. F.; Nelina-Nemtseva, J. I. Tetrahedron 2014, 70, 4617–4625. doi:10.1016/j.tet.2014.05.023 |
| 40. | Obydennov, D. L.; Sosnovskikh, V. Y. Chem. Heterocycl. Compd. 2014, 50, 579–582. doi:10.1007/s10593-014-1510-7 |
| 41. | Tanimori, S.; Inaba, U.; Kato, Y.; Ura, H.; Kashiwagi, H.; Nishimura, T.; Kirihata, M. Res. Chem. Intermed. 2014, 40, 2157–2164. doi:10.1007/s11164-014-1593-x |
| 42. | Miyamaru, S.; Umezu, K.; Ito, A.; Shimizu, M. Eur. J. Org. Chem. 2015, 3327–3337. doi:10.1002/ejoc.201500225 |
| 43. | Rezvanian, A. Tetrahedron 2016, 72, 6428–6435. doi:10.1016/j.tet.2016.08.049 |
| 44. | Azev, Y. A.; Kodess, M. I.; Ezhikova, M. A.; Ermakova, O. S.; Berseneva, V. S.; Bakulev, V. A. Mendeleev Commun. 2017, 27, 97–98. doi:10.1016/j.mencom.2017.01.032 |
| 45. | Azev, Y. A.; Ermakova, O. S.; Berseneva, V. S.; Kodess, M. I.; Ezhikova, M. A.; Ganebnykh, I. N. Mendeleev Commun. 2017, 27, 637–639. doi:10.1016/j.mencom.2017.11.034 |
| 46. | Soozani, A.; Keivanloo, A.; Bakherad, M. Tetrahedron 2018, 74, 150–156. doi:10.1016/j.tet.2017.11.055 |
| 47. | Reber, K. P.; Tilley, S. D.; Sorensen, E. J. Chem. Soc. Rev. 2009, 38, 3022–3034. doi:10.1039/B912599J |
| 48. | Presset, M.; Coquerel, Y.; Rodriguez, J. Org. Lett. 2009, 11, 5706–5709. doi:10.1021/ol9024056 |
| 49. | Presset, M.; Coquerel, Y.; Rodriguez, J. Org. Lett. 2010, 12, 4212–4215. doi:10.1021/ol101938r |
| 50. | Leber, S.; Kollenz, G.; Wentrup, C. Beilstein J. Org. Chem. 2012, 8, 738–743. doi:10.3762/bjoc.8.83 |
| 51. | Galvez, J.; Castillo, J.-C.; Quiroga, J.; Rajzmann, M.; Rodriguez, J.; Coquerel, Y. Org. Lett. 2014, 16, 4126–4129. doi:10.1021/ol5018245 |
| 52. | Khlebnikov, A. F.; Novikov, M. S.; Pakalnis, V. V.; Iakovenko, R. O.; Yufit, D. S. Beilstein J. Org. Chem. 2014, 10, 784–793. doi:10.3762/bjoc.10.74 |
| 53. | Cookson, R.; Barrett, T. N.; Barrett, A. G. M. Acc. Chem. Res. 2015, 48, 628–642. doi:10.1021/ar5004169 |
| 54. | Kollenz, G.; Wentrup, C. Beilstein J. Org. Chem. 2018, 14, 1–10. doi:10.3762/bjoc.14.1 |
| 11. | Sawant, R. T.; Stevens, M. Y.; Sköld, C.; Odell, L. R. Org. Lett. 2016, 18, 5392–5395. doi:10.1021/acs.orglett.6b02774 |
| 21. | Lisovenko, N. Y.; Krasnykh, O. P.; Aliev, Z. G.; Vostrov, E. S.; Tarasova, O. P.; Maslivets, A. N. Chem. Heterocycl. Compd. 2001, 37, 1314–1316. doi:10.1023/A:1013886602711 |
| 10. | Preciado, S.; Vicente-García, E.; Llabrés, S.; Luque, F. J.; Lavilla, R. Angew. Chem. 2012, 124, 6980–6983. doi:10.1002/ange.201202927 |
| 9. | Kushnir, O. V.; Vovk, M. V. Russ. J. Org. Chem. 2010, 46, 890–893. doi:10.1134/s1070428010060187 |
| 20. | Maslivets, A. N.; Golovnina, O. V.; Krasnykh, O. P.; Aliev, Z. G. Chem. Heterocycl. Compd. 2000, 36, 615–616. doi:10.1007/bf02290858 |
| 21. | Lisovenko, N. Y.; Krasnykh, O. P.; Aliev, Z. G.; Vostrov, E. S.; Tarasova, O. P.; Maslivets, A. N. Chem. Heterocycl. Compd. 2001, 37, 1314–1316. doi:10.1023/A:1013886602711 |
| 23. | Maslivets, A. N.; Bozdyreva, K. S.; Smirnova, I. V.; Tolmacheva, I. A.; Mashevskaya, I. V. Chem. Heterocycl. Compd. 2002, 38, 498–499. doi:10.1023/A:1016056011167 |
| 24. | Maslivets, A. N.; Lisovenko, N. Y.; Krasnykh, O. P.; Tarasova, O. P.; Aliev, Z. G.; Atovmyan, L. O. Russ. Chem. Bull. 2002, 51, 850–853. doi:10.1023/A:1016097120253 |
| 28. | Maslivets, A. N.; Aliev, Z. G.; Krasnykh, O. P.; Golovnina, O. V.; Atovmyan, L. O. Chem. Heterocycl. Compd. 2004, 40, 1295–1299. doi:10.1007/s10593-005-0060-4 |
| 29. | Bozdyreva, K. S.; Smirnova, I. V.; Maslivets, A. N. Russ. J. Org. Chem. 2005, 41, 1081–1088. doi:10.1007/s11178-005-0296-6 |
| 38. | Lisovenko, N. Y.; Yukova, Y. V.; Makhmudov, R. R. Pharm. Chem. J. 2014, 47, 593–595. doi:10.1007/s11094-014-1014-x |
© 2018 Kasatkina et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (https://www.beilstein-journals.org/bjoc)