Abstract
A π-extended “earring” subporphyrin 3 was synthesized from β,β′-diiodosubporphyrin and diboryltripyrrane via a Suzuki–Miyaura coupling and following oxidation. Its Pd complex 3Pd was also synthesized and both of the compounds were fully characterized by 1H NMR, MS and X-ray single crystal diffraction. The 1H NMR spectra and single crystal structures revealed that aromatic ring current did not extend to the “ear” in both of the two compounds. Their UV–vis/NIR spectra were recorded and the absorption of both compounds is extended to the NIR region and that the absorption of 3Pd is further red-shifted and more intense.
Graphical Abstract
Findings
Since its first synthesis in 2006 [1,2], subporphyrin, the lowest homolog of porphyrins, has received considerable attention [3-8] due to its 14π-electron configuration and bowl-shaped structure. In addition the intense absorption in the UV–vis region [9-24] makes it a promising building block in pigments. The functionalization of subporphyrin can proceed at various sites such as the central boron atom [25-30], meso- [31,32] and β-position [33-35]. By using the method developed by Osuka the β,β′-diborylsubporphyrins [36] can be obtained in high yields. A subsequent Suzuki–Miyaura coupling smoothly affords various β-aryl-substituted subporphyrins [37]. Alternatively, some β-aryl/vinyl-substituted subporphyrins can be synthesized from aryl/vinyl borate and the corresponding β,β′-dihalosubporphyrins, which can be obtained by the treatment of β,β′-diborylsubporphyrins with NBS/NIS in the presence of a Cu(I) salt [36].
Recently, our group successfully prepared multiple cavities π-extended “earring” porphyrins through the aforementioned Suzuki–Miyaura coupling reaction and subsequent oxidation [38]. In this case β,β′-dibromo/tetrabromoporphyrins and diboryltripyrrane were applied as reactants. We discovered that both the π-extended “earring” porphyrins and the corresponding Pd complexes exhibited remarkably near-infrared absorptions.
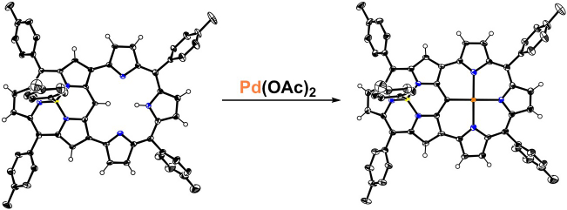
Based on our previous work, we herein designed a subporphyrin with one “earring”. The different geometry and properties could be envisioned due to the bowl-shaped structure and 14π-electron configuration of subporphyrin. To construct the skeleton of the “earring” subporphyrin, we performed a Suzuki–Miyaura coupling reaction between β,β′-diiodosubporphyrin 1 [37] and diboryltripyrrane 2 [38] (Scheme 1). Monitored by TLC, we merely observed a complicated mixture without any major band during the progress of the reaction. However, several clear bands emerged after stirring the mixture overnight at ambient conditions. This observation revealed that the coupling products could be oxidized by air and thereafter the target “earring” subporphyrin 3 was obtained in an isolated yield of 15% after column chromatography.
Scheme 1: Synthesis of “earring” subporphyrin and its Pd complex. Synthetic procedure: (i) Diboryltripyrrane (2.5 equiv), Pd2(dba)3 (10 mol %), SPhos (2-dicyclohexylphosphino-2',6'-dimethoxybiphenyl, 40 mol %), Cs2CO3 (2 equiv), CsF (2 equiv), Tol/DMF 2:1, reflux, 48 h; (ii) in air, rt, overnight; (iii) Pd(OAc)2 (3 equiv), CH2Cl2/MeOH 5:1, rt, overnight.
Scheme 1: Synthesis of “earring” subporphyrin and its Pd complex. Synthetic procedure: (i) Diboryltripyrrane ...
The mass spectrum of 3 exhibits a parent-ion peak at m/z = 911.4036 (calcd for C64H48BN6 [M]+ = 911.4028), which is in agreement with its structure. The 1H NMR spectrum of 3 (Figure 1) indicates it a symmetric structure. The peak appearing at 17.02 ppm is assigned to the NH since a D2O exchange experiment lead to its disappearance. While the peak at 15.82 ppm can be assigned to the meso-H of the subporphyrin moiety, which is shifted to a lower field region in comparison to 8.60 ppm of 4-tolyl-(5,10-di-(4-tolyl)-subporphyrinato)boron(III) (1a). These two signals at 17.02 ppm and 15.82 ppm can be attributed to the antiaromatic character of the ear-containing macrocycle, which is quite similar to the analogous “earring” porphyrin [38]. Furthermore, the antiaromaticity of the ear-containing macrocycle is proved by the large positive NICS value in the hole as well as the anticlockwise ring currency (see Supporting Information File 1 for details).
Although some characteristic peaks in the 1H NMR spectrum of the “earring” subporphyrin are quite similar to those of “earring” porphyrin, there are some differences in their structures. This is mainly due to the fact that the bowl-shaped subporphyrin core differs significantly from the saddle-shaped or nearly planar porphyrin core. To elucidate the differences in their structures, we endeavored to cultivate single crystals of 3 and collected the data. The diffraction data unambiguously confirmed the designated structure (Figure 2) and revealed that all peripheral C–C bonds in the subporphyrin moiety are of similar lengths (1.383(6)–1.447(5) Å). In contrast, the C–C bond lengths in the tripyrrin moiety alternate (1.341(5)–1.472(5) Å). These data clearly indicate that the subporphyrin moiety remains its aromaticity while the tripyrrin moiety participates in an antiaromatic system as shown in Scheme 1. The cavity surrounded by the tripyrrin moiety owns a long axis of 4.229(4) Å and a short axis of 4.201(5) Å, which are almost the same. Despite of this the cavity is not circular because the three N atoms in the tripyrrin moiety and the nearest meso-C of the subporphyrin moiety are not ideally coplanar. This feature can be proved by the dihedral angles of two adjacent pyrrole units in the tripyrrin moiety, which are as large as 15.8(1)° and 16.1(1)°, respectively. We assume that the twisted structure results from the strain transmitted from the subporphyrin moiety. Furthermore, we speculate that this strain should be the origin of the much lower yield of 3 comparing to the corresponding “earring” porphyrins.
![[1860-5397-14-170-2]](/bjoc/content/figures/1860-5397-14-170-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: X-ray crystal structures of 3: a) top view; b) side view. Thermal ellipsoids are drawn at the 50% probability level. All hydrogens on tolyl groups are omitted for clarity.
Figure 2: X-ray crystal structures of 3: a) top view; b) side view. Thermal ellipsoids are drawn at the 50% p...
Concerning the diameter of the cavity and the strain of the skeleton, we assumed that some metal ions may insert into the hole surrounded by the tripyrrin moiety. Experimentally, 3Pd can be formed quantitatively by simply mixing Pd(OAc)2 with a CH2Cl2/MeOH solution of 3 at room temperature. This transformation is confirmed by MS with a parent-ion peak at m/z = 1015.24 (calcd for C64H46BN6Pd, [M]+ = 1015.29).
In the 1H NMR spectrum of 3Pd (Figure 3), no signals are detected in the very low field region. This indicates that the Pd is inserted in the cavity of 3 with the deprotonation of both N-H and meso-H. Meanwhile all signals belong to aromatic hydrogens shifted to a slightly higher field region after the complexation with Pd, which reveals that the insertion of the metal does not change the antiaromatic pathways in 3.
![[1860-5397-14-170-3]](/bjoc/content/figures/1860-5397-14-170-3.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 3: Partial 1H NMR spectrum of 3Pd.
Figure 3: Partial 1H NMR spectrum of 3Pd.
Fortunately we obtained single crystals of 3Pd from its CH2Cl2/CDCl3/MeOH solution via vapor diffusion. All of the bond lengths were carefully measured based on the diffraction results (Figure 4). We found that despite all peripheral C–C bond lengths in the tripyrrin moiety were still alternating (1.348(5)–1.441(4) Å), the differences among them were somewhat smaller compared to those of 3. While the cavity surrounded by the tripyrrin moiety in 3Pd has a long axis of 4.123(3) Å (N–N distance) and a short axis of 4.076(4) Å (N–C distance), both of which are shorter than that of 3. This contraction is probably due to a slight mismatch of the radii between the Pd center and the cavity. In addition, this mismatch also leads to a further twist of the pyrrole units in the tripyrrin moiety. The dihedral angles of two adjacent pyrrole units in the tripyrrin moiety are 13.7(1)° and 18.4(1)°, respectively. The difference between the two dihedral angles is much larger than that in 3.
![[1860-5397-14-170-4]](/bjoc/content/figures/1860-5397-14-170-4.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 4: X-ray crystal structures of 3Pd: a) top view; b) side view. Thermal ellipsoids are at the 30% probability level. All hydrogens on tolyl are omitted for clarity.
Figure 4: X-ray crystal structures of 3Pd: a) top view; b) side view. Thermal ellipsoids are at the 30% proba...
The UV–vis/NIR absorption spectra of 3 and 3Pd are shown in Figure 5. However, no fluorescence emission can be observed for 3 and 3Pd. Both 3 and 3Pd display broad Soret bands and Q-like bands and all bands are red-shifted compared to the corresponding bands of 1a. In addition the absorptions of the Soret bands in 3 and 3Pd are much weaker than in case of 1a. For 3, its tail of Q-like band extends to over 1000 nm. While for 3Pd, its tail of Q-like band extends to over 1400 nm with several observable peaks. This remarkable absorption in the NIR region is comparable with that of the analogue “earring” porphyrins and reveals the π-conjugation between the subporphyrin and tripyrrin moiety.
![[1860-5397-14-170-5]](/bjoc/content/figures/1860-5397-14-170-5.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 5: UV–vis/NIR spectra of 3 and 3Pd.
Figure 5: UV–vis/NIR spectra of 3 and 3Pd.
Conclusion
In summary, we synthesized a π-extended “earring” subporphyrin from β,β′-diiodosubporphyrin and diboryltripyrrane via a Suzuki–Miyaura coupling and following oxidation. This “earring” subporphyrin’s cavity allows for complexation of Pd atom to form the corresponding Pd complex. The 1H NMR spectra of the compounds reveal that the aromatic ring current does not extend to the “ear”. Both the structure of “earring” subporphyrin and that of its Pd complex were elucidated by X-ray single crystal diffraction analysis. In addition, their UV–vis/NIR spectra revealed that the absorption region is extended to the NIR region and that the absorption of the Pd complex is further red-shifted and more intense. This work extends the research of “earring” porphyrins to “earring” subporphyrins. Investigations on their photophysical properties and further functionalization are underway.
Supporting Information
| Supporting Information File 1: Experimental part. | ||
| Format: PDF | Size: 1.5 MB | Download |
| Supporting Information File 2: Crystallographic information file of compound 3. | ||
| Format: CIF | Size: 34.9 KB | Download |
| Supporting Information File 3: Crystallographic information file of compound 3Pd. | ||
| Format: CIF | Size: 36.2 KB | Download |
Acknowledgements
The work was supported by the National Nature Science Foundation of China (Grant Nos. 21702057, 21602058, 21772036), Scientific Research Fund of Hunan Provincial Science and Technology Department (Grant No. 2017JJ3199), Scientific Research Fund of Hunan Provincial Education Department (Grant No. 17B156). We thank Prof. Shubin Liu and Mr. Donghai Yu for their help in DFT calculations.
References
-
Inokuma, Y.; Kwon, J. H.; Ahn, T. K.; Yoo, M.-C.; Kim, D.; Osuka, A. Angew. Chem., Int. Ed. 2006, 45, 961. doi:10.1002/anie.200503426
Return to citation in text: [1] -
Torres, T. Angew. Chem., Int. Ed. 2006, 45, 2834. doi:10.1002/anie.200504265
Return to citation in text: [1] -
Inokuma, Y.; Osuka, A. Dalton Trans. 2008, 2517. doi:10.1039/b719808f
Return to citation in text: [1] -
Xiao, J.; Jiang, H. Chin. J. Org. Chem. 2009, 29, 1750.
Return to citation in text: [1] -
Osuka, A.; Tsurumaki, E.; Tanaka, T. Bull. Chem. Soc. Jpn. 2011, 84, 679. doi:10.1246/bcsj.20110118
Return to citation in text: [1] -
Claessens, C. G.; González-Rodríguez, D.; Rodríguez-Morgade, S.; Medina, A.; Torres, T. Chem. Rev. 2014, 114, 2192. doi:10.1021/cr400088w
Return to citation in text: [1] -
Shimizu, S. Chem. Rev. 2017, 117, 2730. doi:10.1021/acs.chemrev.6b00403
Return to citation in text: [1] -
Mack, J. Chem. Rev. 2017, 117, 3444. doi:10.1021/acs.chemrev.6b00568
Return to citation in text: [1] -
Takeuchi, Y.; Matsuda, A.; Kobayashi, N. J. Am. Chem. Soc. 2007, 129, 8271. doi:10.1021/ja0712120
Return to citation in text: [1] -
Kobayashi, N.; Takeuchi, Y.; Matsuda, A. Angew. Chem., Int. Ed. 2007, 46, 758. doi:10.1002/anie.200603520
Return to citation in text: [1] -
Inokuma, Y.; Yoon, Z. S.; Kim, D.; Osuka, A. J. Am. Chem. Soc. 2007, 129, 4747. doi:10.1021/ja069324z
Return to citation in text: [1] -
Inokuma, Y.; Easwaramoorthi, S.; Yoon, Z. S.; Kim, D.; Osuka, A. J. Am. Chem. Soc. 2008, 130, 12234. doi:10.1021/ja804846v
Return to citation in text: [1] -
Inokuma, Y.; Easwaramoorthi, S.; Jang, S. Y.; Kim, K. S.; Kim, D.; Osuka, A. Angew. Chem., Int. Ed. 2008, 47, 4840. doi:10.1002/anie.200801192
Return to citation in text: [1] -
Makarova, E. A.; Shimizu, S.; Matsuda, A.; Luk’yanets, E. A.; Kobayashi, N. Chem. Commun. 2008, 2109. doi:10.1039/b801712c
Return to citation in text: [1] -
Xu, T.; Lu, R.; Liu, X.; Chen, P.; Qiu, X.; Zhao, Y. Eur. J. Org. Chem. 2008, 1065. doi:10.1002/ejoc.200700981
Return to citation in text: [1] -
Liu, X.; Lu, R.; Xu, T.; Xu, D.; Zhan, Y.; Chen, P.; Qiu, X.; Zhao, Y. Eur. J. Org. Chem. 2009, 53. doi:10.1002/ejoc.200800646
Return to citation in text: [1] -
Easwaramoorthi, S.; Shin, J.-Y.; Cho, S.; Kim, P.; Inokuma, Y.; Trurumaki, E.; Osuka, A.; Kim, D. Chem. – Eur. J. 2009, 15, 12005. doi:10.1002/chem.200901671
Return to citation in text: [1] -
Hayashi, S.-y.; Inokuma, Y.; Osuka, A. Org. Lett. 2010, 12, 4148. doi:10.1021/ol101746d
Return to citation in text: [1] -
Hayashi, S.-y.; Inokuma, Y.; Easwaramoorthi, S.; Kim, K. S.; Kim, D.; Osuka, A. Angew. Chem., Int. Ed. 2010, 49, 321. doi:10.1002/anie.200906005
Return to citation in text: [1] -
Remiro-Buenamañana, S.; Díaz-Moscoso, A.; Hughes, D. L.; Bochmann, M.; Tizzard, G. J.; Coles, S. J.; Cammidge, A. N. Angew. Chem., Int. Ed. 2015, 54, 7510. doi:10.1002/anie.201502662
Return to citation in text: [1] -
Copley, G.; Hwang, D.; Kim, D.; Osuka, A. Angew. Chem., Int. Ed. 2016, 55, 10287. doi:10.1002/anie.201604432
Return to citation in text: [1] -
Chandra, B.; Mondal, N.; Kumar, B. S.; Panda, P. K. J. Porphyrins Phthalocyanines 2016, 20, 429. doi:10.1142/S1088424616500255
Return to citation in text: [1] -
Tsukamoto, T.; Shimada, T.; Takagi, S. ACS Appl. Mater. Interfaces 2016, 8, 7522. doi:10.1021/acsami.5b11988
Return to citation in text: [1] -
Tsurumaki, E.; Hayashi, S.-y.; Tham, F. S.; Reed, C. A.; Osuka, A. J. Am. Chem. Soc. 2011, 133, 11956. doi:10.1021/ja2056566
Return to citation in text: [1] -
Saga, S.; Hayashi, S.-y.; Yoshida, K.; Tsurumaki, E.; Kim, P.; Sung, Y. M.; Sung, J.; Tanaka, T.; Kim, D.; Osuka, A. Chem. – Eur. J. 2013, 19, 11158. doi:10.1002/chem.201302454
Return to citation in text: [1] -
Zhao, S.; Liu, C.; Guo, Y.; Xiao, J.-C.; Chen, Q.-Y. Synthesis 2014, 46, 1674. doi:10.1055/s-0033-1341055
Return to citation in text: [1] -
Tsurumaki, E.; Sung, J.; Kim, D.; Osuka, A. J. Am. Chem. Soc. 2015, 137, 1056. doi:10.1021/ja5126269
Return to citation in text: [1] -
Tsurumaki, E.; Sung, J.; Kim, D.; Osuka, A. Angew. Chem., Int. Ed. 2016, 55, 2596. doi:10.1002/anie.201511590
Return to citation in text: [1] -
Kotani, R.; Yoshida, K.; Tsurumaki, E.; Osuka, A. Chem. – Eur. J. 2016, 22, 3320. doi:10.1002/chem.201504719
Return to citation in text: [1] -
Azarias, C.; Pawelek, M.; Jacquemin, D. J. Phys. Chem. A 2017, 121, 4306. doi:10.1021/acs.jpca.7b03644
Return to citation in text: [1] -
Kitano, M.; Hayashi, S.-y.; Tanaka, T.; Yorimitsu, H.; Aratani, N.; Osuka, A. Angew. Chem., Int. Ed. 2012, 51, 5593. doi:10.1002/anie.201201853
Return to citation in text: [1] -
Shimizu, D.; Mori, H.; Kitano, M.; Cha, W.-Y.; Oh, J.; Tanaka, T.; Kim, D.; Osuka, A. Chem. – Eur. J. 2014, 20, 16194. doi:10.1002/chem.201405110
Return to citation in text: [1] -
Tsurumaki, E.; Inokuma, Y.; Easwaramoorthi, S.; Lim, J. M.; Kim, D.; Osuka, A. Chem. – Eur. J. 2009, 15, 237. doi:10.1002/chem.200801802
Return to citation in text: [1] -
Tsurumaki, E.; Osuka, A. Chem. – Asian J. 2013, 8, 3042. doi:10.1002/asia.201300869
Return to citation in text: [1] -
Yoshida, K.; Osuka, A. Chem. – Asian J. 2015, 10, 1526. doi:10.1002/asia.201500225
Return to citation in text: [1] -
Kitano, M.; Okuda, Y.; Tsurumaki, E.; Tanaka, T.; Yorimitsu, H.; Osuka, A. Angew. Chem., Int. Ed. 2015, 54, 9275. doi:10.1002/anie.201503530
Return to citation in text: [1] [2] -
Kitano, M.; Tanaka, T.; Osuka, A. Organometallics 2017, 36, 2559. doi:10.1021/acs.organomet.7b00130
Return to citation in text: [1] [2] -
Rao, Y.; Kim, T.; Park, K. H.; Peng, F.; Liu, L.; Liu, Y.; Wen, B.; Liu, S.; Kirk, S. R.; Wu, L.; Chen, B.; Ma, M.; Zhou, M.; Yin, B.; Zhang, Y.; Kim, D.; Song, J. Angew. Chem., Int. Ed. 2016, 55, 6438. doi:10.1002/anie.201600955
Return to citation in text: [1] [2] [3]
| 1. | Inokuma, Y.; Kwon, J. H.; Ahn, T. K.; Yoo, M.-C.; Kim, D.; Osuka, A. Angew. Chem., Int. Ed. 2006, 45, 961. doi:10.1002/anie.200503426 |
| 2. | Torres, T. Angew. Chem., Int. Ed. 2006, 45, 2834. doi:10.1002/anie.200504265 |
| 31. | Kitano, M.; Hayashi, S.-y.; Tanaka, T.; Yorimitsu, H.; Aratani, N.; Osuka, A. Angew. Chem., Int. Ed. 2012, 51, 5593. doi:10.1002/anie.201201853 |
| 32. | Shimizu, D.; Mori, H.; Kitano, M.; Cha, W.-Y.; Oh, J.; Tanaka, T.; Kim, D.; Osuka, A. Chem. – Eur. J. 2014, 20, 16194. doi:10.1002/chem.201405110 |
| 25. | Saga, S.; Hayashi, S.-y.; Yoshida, K.; Tsurumaki, E.; Kim, P.; Sung, Y. M.; Sung, J.; Tanaka, T.; Kim, D.; Osuka, A. Chem. – Eur. J. 2013, 19, 11158. doi:10.1002/chem.201302454 |
| 26. | Zhao, S.; Liu, C.; Guo, Y.; Xiao, J.-C.; Chen, Q.-Y. Synthesis 2014, 46, 1674. doi:10.1055/s-0033-1341055 |
| 27. | Tsurumaki, E.; Sung, J.; Kim, D.; Osuka, A. J. Am. Chem. Soc. 2015, 137, 1056. doi:10.1021/ja5126269 |
| 28. | Tsurumaki, E.; Sung, J.; Kim, D.; Osuka, A. Angew. Chem., Int. Ed. 2016, 55, 2596. doi:10.1002/anie.201511590 |
| 29. | Kotani, R.; Yoshida, K.; Tsurumaki, E.; Osuka, A. Chem. – Eur. J. 2016, 22, 3320. doi:10.1002/chem.201504719 |
| 30. | Azarias, C.; Pawelek, M.; Jacquemin, D. J. Phys. Chem. A 2017, 121, 4306. doi:10.1021/acs.jpca.7b03644 |
| 9. | Takeuchi, Y.; Matsuda, A.; Kobayashi, N. J. Am. Chem. Soc. 2007, 129, 8271. doi:10.1021/ja0712120 |
| 10. | Kobayashi, N.; Takeuchi, Y.; Matsuda, A. Angew. Chem., Int. Ed. 2007, 46, 758. doi:10.1002/anie.200603520 |
| 11. | Inokuma, Y.; Yoon, Z. S.; Kim, D.; Osuka, A. J. Am. Chem. Soc. 2007, 129, 4747. doi:10.1021/ja069324z |
| 12. | Inokuma, Y.; Easwaramoorthi, S.; Yoon, Z. S.; Kim, D.; Osuka, A. J. Am. Chem. Soc. 2008, 130, 12234. doi:10.1021/ja804846v |
| 13. | Inokuma, Y.; Easwaramoorthi, S.; Jang, S. Y.; Kim, K. S.; Kim, D.; Osuka, A. Angew. Chem., Int. Ed. 2008, 47, 4840. doi:10.1002/anie.200801192 |
| 14. | Makarova, E. A.; Shimizu, S.; Matsuda, A.; Luk’yanets, E. A.; Kobayashi, N. Chem. Commun. 2008, 2109. doi:10.1039/b801712c |
| 15. | Xu, T.; Lu, R.; Liu, X.; Chen, P.; Qiu, X.; Zhao, Y. Eur. J. Org. Chem. 2008, 1065. doi:10.1002/ejoc.200700981 |
| 16. | Liu, X.; Lu, R.; Xu, T.; Xu, D.; Zhan, Y.; Chen, P.; Qiu, X.; Zhao, Y. Eur. J. Org. Chem. 2009, 53. doi:10.1002/ejoc.200800646 |
| 17. | Easwaramoorthi, S.; Shin, J.-Y.; Cho, S.; Kim, P.; Inokuma, Y.; Trurumaki, E.; Osuka, A.; Kim, D. Chem. – Eur. J. 2009, 15, 12005. doi:10.1002/chem.200901671 |
| 18. | Hayashi, S.-y.; Inokuma, Y.; Osuka, A. Org. Lett. 2010, 12, 4148. doi:10.1021/ol101746d |
| 19. | Hayashi, S.-y.; Inokuma, Y.; Easwaramoorthi, S.; Kim, K. S.; Kim, D.; Osuka, A. Angew. Chem., Int. Ed. 2010, 49, 321. doi:10.1002/anie.200906005 |
| 20. | Remiro-Buenamañana, S.; Díaz-Moscoso, A.; Hughes, D. L.; Bochmann, M.; Tizzard, G. J.; Coles, S. J.; Cammidge, A. N. Angew. Chem., Int. Ed. 2015, 54, 7510. doi:10.1002/anie.201502662 |
| 21. | Copley, G.; Hwang, D.; Kim, D.; Osuka, A. Angew. Chem., Int. Ed. 2016, 55, 10287. doi:10.1002/anie.201604432 |
| 22. | Chandra, B.; Mondal, N.; Kumar, B. S.; Panda, P. K. J. Porphyrins Phthalocyanines 2016, 20, 429. doi:10.1142/S1088424616500255 |
| 23. | Tsukamoto, T.; Shimada, T.; Takagi, S. ACS Appl. Mater. Interfaces 2016, 8, 7522. doi:10.1021/acsami.5b11988 |
| 24. | Tsurumaki, E.; Hayashi, S.-y.; Tham, F. S.; Reed, C. A.; Osuka, A. J. Am. Chem. Soc. 2011, 133, 11956. doi:10.1021/ja2056566 |
| 38. | Rao, Y.; Kim, T.; Park, K. H.; Peng, F.; Liu, L.; Liu, Y.; Wen, B.; Liu, S.; Kirk, S. R.; Wu, L.; Chen, B.; Ma, M.; Zhou, M.; Yin, B.; Zhang, Y.; Kim, D.; Song, J. Angew. Chem., Int. Ed. 2016, 55, 6438. doi:10.1002/anie.201600955 |
| 3. | Inokuma, Y.; Osuka, A. Dalton Trans. 2008, 2517. doi:10.1039/b719808f |
| 4. | Xiao, J.; Jiang, H. Chin. J. Org. Chem. 2009, 29, 1750. |
| 5. | Osuka, A.; Tsurumaki, E.; Tanaka, T. Bull. Chem. Soc. Jpn. 2011, 84, 679. doi:10.1246/bcsj.20110118 |
| 6. | Claessens, C. G.; González-Rodríguez, D.; Rodríguez-Morgade, S.; Medina, A.; Torres, T. Chem. Rev. 2014, 114, 2192. doi:10.1021/cr400088w |
| 7. | Shimizu, S. Chem. Rev. 2017, 117, 2730. doi:10.1021/acs.chemrev.6b00403 |
| 8. | Mack, J. Chem. Rev. 2017, 117, 3444. doi:10.1021/acs.chemrev.6b00568 |
| 36. | Kitano, M.; Okuda, Y.; Tsurumaki, E.; Tanaka, T.; Yorimitsu, H.; Osuka, A. Angew. Chem., Int. Ed. 2015, 54, 9275. doi:10.1002/anie.201503530 |
| 37. | Kitano, M.; Tanaka, T.; Osuka, A. Organometallics 2017, 36, 2559. doi:10.1021/acs.organomet.7b00130 |
| 37. | Kitano, M.; Tanaka, T.; Osuka, A. Organometallics 2017, 36, 2559. doi:10.1021/acs.organomet.7b00130 |
| 38. | Rao, Y.; Kim, T.; Park, K. H.; Peng, F.; Liu, L.; Liu, Y.; Wen, B.; Liu, S.; Kirk, S. R.; Wu, L.; Chen, B.; Ma, M.; Zhou, M.; Yin, B.; Zhang, Y.; Kim, D.; Song, J. Angew. Chem., Int. Ed. 2016, 55, 6438. doi:10.1002/anie.201600955 |
| 36. | Kitano, M.; Okuda, Y.; Tsurumaki, E.; Tanaka, T.; Yorimitsu, H.; Osuka, A. Angew. Chem., Int. Ed. 2015, 54, 9275. doi:10.1002/anie.201503530 |
| 33. | Tsurumaki, E.; Inokuma, Y.; Easwaramoorthi, S.; Lim, J. M.; Kim, D.; Osuka, A. Chem. – Eur. J. 2009, 15, 237. doi:10.1002/chem.200801802 |
| 34. | Tsurumaki, E.; Osuka, A. Chem. – Asian J. 2013, 8, 3042. doi:10.1002/asia.201300869 |
| 35. | Yoshida, K.; Osuka, A. Chem. – Asian J. 2015, 10, 1526. doi:10.1002/asia.201500225 |
| 38. | Rao, Y.; Kim, T.; Park, K. H.; Peng, F.; Liu, L.; Liu, Y.; Wen, B.; Liu, S.; Kirk, S. R.; Wu, L.; Chen, B.; Ma, M.; Zhou, M.; Yin, B.; Zhang, Y.; Kim, D.; Song, J. Angew. Chem., Int. Ed. 2016, 55, 6438. doi:10.1002/anie.201600955 |
© 2018 Guan et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0). Please note that the reuse, redistribution and reproduction in particular requires that the authors and source are credited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (https://www.beilstein-journals.org/bjoc)





![[1860-5397-14-170-1]](/bjoc/content/figures/1860-5397-14-170-1.png?scale=2.5&max-width=1024&background=FFFFFF)