Abstract
The structure of the sesquiterpene onchidal (6), a component of the defensive secretion of the shell-less mollusc Onchidella binneyi, contains a masked α,β-unsaturated 1,4-dialdehyde moiety, the presence of which has been proposed to be the cause of the feeding deterrent activity exhibited by the mollusc. We have found onchidal acts as an electrophile, reacting rapidly with the model nucleophile n-pentylamine forming diastereomeric aminated pyrrole adducts. Somewhat surprisingly, no reaction was observed between onchidal and n-pentanethiol. Structurally simplified n-pentyl 11–13 and cyclohexylmethyl 15–17 analogues of onchidal were prepared and demonstrated similar amine-selective reactivity. Onchidal and analogues reacted with the model protein lysozyme, forming covalent adducts and leading to protein cross-linking. These results provide preliminary evidence supporting the molecular mechanism of biological activity exhibited by onchidal.
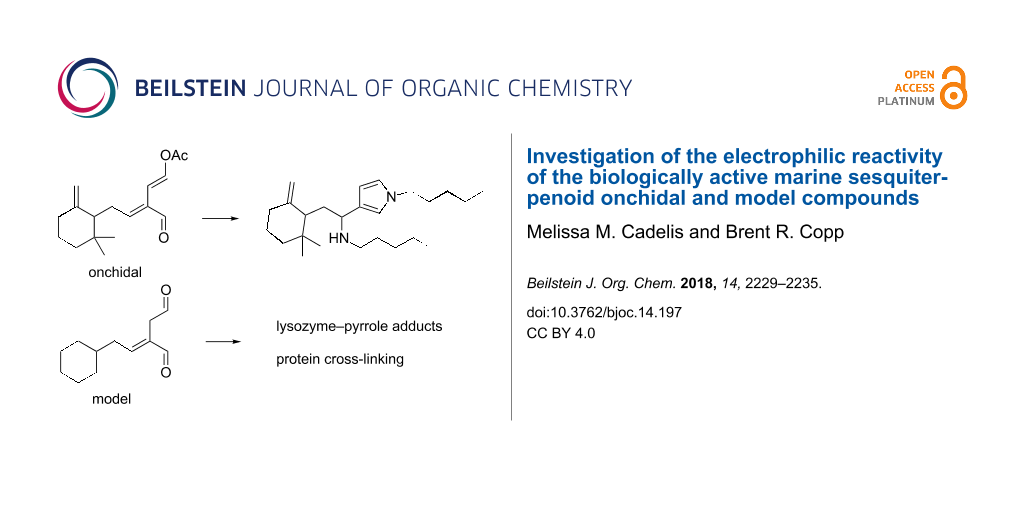
Graphical Abstract
Introduction
More than 80 terpenoid natural products containing the 1,4-dialdehyde moiety have been isolated from sources such as fungi, algae, sponges and molluscs [1]. Many of these natural products exhibit biological activity, ranging from anti-inflammatory to antimicrobial and antifeedant activities [1]. The prototypical examples polygodial (1) and scalaradial (2, Figure 1) both exhibit antifeedant activity against worms and fish [1,2], with recent studies also showing that 1 is a potential lead as a marine antifouling agent [3].
Figure 1: Masked and unmasked 1,4-dialdehyde natural products 1–6.
Figure 1: Masked and unmasked 1,4-dialdehyde natural products 1–6.
The ichthyotoxic masked dialdehyde caulerpenyne (3), a major component of extracts of the green alga Caulerpa taxifolia, exhibits antiproliferative activities as well as wound healing abilities with the latter resulting from rapid transformation to the highly reactive 1,4-dialdehyde, oxytoxin 2 (4) [4-6]. Oxytoxin 2 (4) is itself a natural product, produced by the mollusc Oxynoe olivacea from a diet-derived (Caulerpa algae) precursor and is predominantly present in the predator-deterring mucous secretion of the mollusc [7]. Two structurally-related masked dialdehydes, 5 (from Caulerpa ashmeadii) [8] and onchidal (6) [9,10] (from the defensive secretion of the mollusc Onchidella binneyi) also exhibit biological properties including feeding deterrence, antibacterial and anticholinesterase activities.
Chemical reactivity studies using polygodial (1), scalaradial (2) and caulerpenyne (3) have demonstrated evidence of pyrrole formation upon reaction with primary amines, with conclusions drawn attributing bioactivities such as antifeedant activity to this chemical reactivity [1,11-13]. In an effort to ascertain whether the mollusc metabolite onchidal is susceptible to nucleophilic attack in a similar manner, herein we report on the reactivity of onchidal and a library of simplified n-pentyl and cyclohexylmethyl model compounds towards thiol and amine nucleophiles as well as their reactivity towards a model protein target, lysozyme.
Results and Discussion
Preliminary studies of the reactivity of onchidal (6) towards 1-pentanethiol or 1-pentylamine were undertaken in CDCl3 solvent in an NMR tube. Somewhat to our surprise, no reaction was observed with 1-pentanethiol, even with incubation in the presence of excess thiol for one week [14]. In contrast, incubation with excess 1-pentylamine rapidly afforded a mixture of products, as identified by changes in the 1H NMR spectrum. Signals attributable to N-alkyl-3-substituted pyrroles 7–9 and N-pentylacetamide 10 [δH 7.62 t, J = 4.7 Hz; 2.28 m] were observed. Purification by silica gel column chromatography, eluting with CH2Cl2, afforded pyrrole adduct 7 as the free base. Elution with CH2Cl2/MeOH afforded two fractions with the first comprised of a single diastereomer as a salt 8, while a second fraction was obtained as a diastereomeric mixture (8:9, 3:1), again as salts (Scheme 1). Mass spectrometric data observed for 7 supported the formation of a diaminated pyrrole product, with a protonated molecular ion of m/z 373.3556 [M + H]+ corresponding to a formula of C25H45N2 (requires 373.3577). NMR data further supported such a structure, with pyrrole signals observed at [δH 6.57–6.55, m, H-1" and H-4"; 6.06, br s, H-3"; δC 120.6 (C-2" and C-4"); 118.5 (C-1"); 106.8 (C-3")] and pentylamine substitution at C-1 [δH 3.50–3.46, m; δC 54.1]. In the case of the more polar products 8 and 9, (+)-ESIMS derived the same formula as for 7, while differences observed in 1H NMR shifts for H-1/H-2/H-1' between 7 and 8 [δ8–7, Δδ +1.29–0.42] suggested 8/9 were purified as salts.
Scheme 1: Products of the reaction of onchidal (6) with 1-pentylamine. Reagents and conditions: 1-pentylamine (excess), CDCl3, overnight.
Scheme 1: Products of the reaction of onchidal (6) with 1-pentylamine. Reagents and conditions: 1-pentylamine...
A mechanism that leads to the formation of diaminated pyrrole adduct 7 starts with amine-induced formation of a 1,4-dialdehyde, which then undergoes Paal–Knorr pyrrole formation to give an azafulvinium intermediate (Scheme 2). This intermediate could then undergo trapping with an additional mole of amine nucleophile to give 7 as a mixture of diastereomers.
Scheme 2: Proposed mechanism for formation of onchidal diaminated adducts.
Scheme 2: Proposed mechanism for formation of onchidal diaminated adducts.
In an effort to reduce the complexity of the NMR spectra observed for the diastereomeric onchidal–pyrrole adducts, a range of simpler achiral n-pentyl 11–14 and cyclohexylmethyl 15–18 side-chained model compounds, as either the dialdehyde or masked dialdehyde variants, were prepared (Figure 2).
Figure 2: Target onchidal model compounds 11–18.
Figure 2: Target onchidal model compounds 11–18.
Horner–Wadsworth–Emmons (H.W.E.) reaction of n-hexanal with phosphonoester 19 [15] afforded an E/Z mixture of olefinic diesters, purification of which by silica gel column chromatography afforded a fraction of the desired E diester 20 (60%), a second fraction comprised of a 5:1 E/Z mixture and a third fraction of Z diester 21 (10%, Scheme 3). The reduction of diesters 20 (E) and 21 (Z) with LiAlH4 afforded diols 22 and 23 in 63% and 67% yield, respectively. Subsequent oxidation of 22 with DMP afforded dialdehyde 11 in 31% yield. Correspondingly, the reaction of diol 23 with DMP afforded a mixture of dialdehyde 11 with dialdehyde 12 (1:1). Attempts at chromatographic separation of these two isomers resulted in degradation of 12. Final conversion of 11 to enol acetate 13 was achieved by overnight reaction with pyridine and acetic anhydride. Purification by silica gel column chromatography afforded the desired E,E enol acetate 13 in 17% yield. A lack of purified dialdehyde 12 prevented any attempt at the preparation of enolacetate 14.
Scheme 3: Synthesis of n-pentyl dialdehydes 11 and 12 and enol acetate 13. Reagents and conditions: a) n-hexanal (0.8 equiv), LiOH·H2O (1.2 equiv), THF, 4 h, 60% (20), 10% (21); b) LiAlH4 (2.5 equiv), Et2O, 0 °C, 1 h, 63% (22), 67% (23); c) DMP (2.5 equiv), CH2Cl2, 4 h, 31% (11); d) Ac2O (2 equiv), pyridine (4 equiv), overnight, 17% (13).
Scheme 3: Synthesis of n-pentyl dialdehydes 11 and 12 and enol acetate 13. Reagents and conditions: a) n-hexa...
Having developed a successful synthetic route to n-pentyl side-chain dialdehyde 11 and enol acetate 13, the synthesis of analogues 15–18 with a side-chain more comparable to onchidal (6) were attempted. H.W.E reaction of 2-cyclohexylacetaldehyde (24) [16] with phosphonoester 19 afforded a fraction of the desired E diester 25 in 15% yield, a fraction of Z diester 26 in 1.5% yield and another fraction of a mixture of the two (5:1) (Scheme 4). The reaction of diesters 25 and 26 with LiAlH4 afforded the corresponding diols 27 and 28 in 61% and 71% yield, respectively, which upon oxidation (DMP) afforded dialdehydes 15 and 16 in 49% and 73% yield, respectively. The reaction of dialdehyde 15 with Ac2O and pyridine afforded enol acetate 17 in 43% yield after purification. Interestingly, the reaction of dialdehyde 16 with Ac2O/pyridine only afforded decomposition products, failing to give 18.
Scheme 4: Synthesis of cyclohexylmethyl dialdehydes 15 and 16 and enol acetate 17. Reagents and conditions: a) phosphonate 19 (1.3 equiv), LiOH·H2O (1.5 equiv), THF, 4 h, 15% (25), 1.5% (26); b) LiAlH4 (2.5 equiv), Et2O, 0 °C, 1 h, 61% (27), 71% (28); c) DMP (2.5 equiv), CH2Cl2, 4 h, 49% (15), 73% (16); d) Ac2O (2 equiv), pyridine (4 equiv), overnight, 43% (17).
Scheme 4: Synthesis of cyclohexylmethyl dialdehydes 15 and 16 and enol acetate 17. Reagents and conditions: a...
The electrophilic reactivity of model dialdehydes 11 and 15 and enol acetates 13 and 17 towards 1-pentanethiol and 1-pentylamine were then studied. As found for onchidal, no reaction (NMR tube) between 11/13/15/17 and 1-pentanethiol was detected, even after one week of incubation. In direct contrast, all four model compounds reacted rapidly with 1-pentylamine, forming pyrrole adducts. The reaction of dialdehyde 11 with 1-pentylamine afforded pyrrole adduct 29 almost instantaneously as determined by 1H NMR. Purification of the crude reaction product gave 29 as the free base (15% yield) and as the salt, 30 (also 15% yield, Figure 3). Spectroscopic and spectrometric analysis of 29 confirmed the formation of a diamine adduct, with detection of a protonated molecular ion in the (+)-ESI mass spectrum at m/z 307.3097 (C20H39N2 requires 307.3108) and NMR signals appropriate for a 3-substituted N-alkylpyrrole [δH 6.57 dd, J = 2.3, 2.3 Hz, H-4"; 6.54 br s, H-1"; 6.04 dd, J = 2.3, 2.3 Hz, H-3"; δC 120.8 (C-2"), 120.3 (C-4"), 118.4 (C-1"), 106.2 (C-3")].
Figure 3: Pyrrole product 29 and salt 30 obtained from the reaction of dialdehyde 11 with n-pentylamine.
Figure 3: Pyrrole product 29 and salt 30 obtained from the reaction of dialdehyde 11 with n-pentylamine.
As proposed for the onchidal–diamine adduct, the formation of 29 is presumably a consequence of dialdehyde 11 undergoing Paal–Knorr pyrrole formation to form an azafulvenium intermediate which is subsequently quenched with another mole of amine nucleophile to form the observed product (Scheme 5).
Scheme 5: Reaction of dialdehyde 11 with excess 1-pentylamine to form 29. Reagents and conditions: (a) 1-pentylamine (excess), CDCl3, overnight.
Scheme 5: Reaction of dialdehyde 11 with excess 1-pentylamine to form 29. Reagents and conditions: (a) 1-pent...
Similar reactivity profiles were observed for each of cyclohexylmethyl dialdehyde 15, and enol acetates 13 and 17, with no reactivity towards 1-pentanethiol being detected, but with rapid reaction with 1-pentyamine to form pyrrole adducts. In the case of dialdehyde 15, the reaction product was determined to be 31 (12% plus 18% as the salt, 32, Figure 4), while enol esters 13 and 17 gave 29 and 31 (7% and 5% yields), respectively, upon reaction with the amine nucleophile.
Figure 4: Pyrrole product 31 and salt 32 obtained from reaction of dialdehyde 15 with n-pentylamine.
Figure 4: Pyrrole product 31 and salt 32 obtained from reaction of dialdehyde 15 with n-pentylamine.
We next investigated the reactivity of onchidal (6) and analogues 11–13 and 15–17 towards the lysine-rich model protein lysozyme. Previous studies have reported hen egg white lysozyme (HEWL) as a suitable target of electrophiles due to its commercial availability, a well-characterized amino acid sequence and the ability for routine (+)-ESIMS analysis to identify covalent adduct formation [17].
Reactivity studies were conducted with commercially available HEWL, in a solution of MeOH/H2O (+ 0.5% formic acid), and the reaction products were investigated by (+)-ESIMS. Preliminary reaction of onchidal (6) with lysozyme was conducted in a solvent mixture of MeOH/H2O (1:15) at 20 °C and examined regularly by (+)-ESIMS. No adducts were detected at 20 hours, but by day 3 (72 h), three new peaks representing mass additions of +198 mu, +216 mu, and +230 mu were detected (Figure 5 and Table 1). These adducts are likely the result of the reaction of lysine residues present in the enzyme [17]. The latter two adducts are proposed to be pyrrole adducts with incorporation of solvolytic H2O and methanol, respectively. The +198 mu adduct could have arisen via elimination of H2O or methanol from the corresponding adducts, or alternatively, from deprotonation of the anticipated lysozyme-onchidal azafulvenium intermediate. The adduct product distributions were calculated from the deconvoluted (+)-ESI mass spectrum, identifying a total lysozyme modification yield of 15% (Table 1). The presence of a large amount of unmodified lysozyme (85%), even after 72 h, was attributed to the slow reactivity of the enol acetate functionality of onchidal, as observed in the original model studies.
Figure 5: Lysine adducts arising from the reaction of onchidal (6) with lysozyme.
Figure 5: Lysine adducts arising from the reaction of onchidal (6) with lysozyme.
Table 1: Summary of lysozyme modifications by onchidal (6) and analogues 11–13 and 15–17.a
| No. | unmod (%)b | +1 (%)c | +2 (%)c | ||||
|---|---|---|---|---|---|---|---|
| alkened | OHd | OCH3d | alkened | OHd | OCH3d | ||
| 6a,e | 85 | 5 | 4 | 6 | 0 | 0 | 0 |
| 11f | 37 | 24 | 18 | 21 | 0 | 0 | 0 |
| 12 | 13 | 10 | 8 | 21 | 5 | 0 | 14 |
| 13 | 82 | 0 | 18 | 0 | 0 | 0 | 0 |
| 15 | 10 | 14 | 7 | 15 | 11 | 0 | 11 |
| 16 | 30 | 18 | 30 | 22 | 0 | 0 | 0 |
| 17e | 93 | 2 | 3 | 2 | 0 | 0 | 0 |
aStandard reaction conditions: 50 µM substrate, 10 µM lysozyme, in MeOH/H2O at 20 °C for 20 hours (unless otherwise noted). Product distribution determined from deconvoluted (+)-HRESIMS data. bPercentage of unmodified lysozyme. cPercentage of mono-adduct (+1) and di-adduct (+2) products detected by (+)-ESIMS. dAlkene-, hydroxy and methoxy group containing adducts detected. In the case of di-adducts, ions observed consistent for mixed nucleophilic quenching products, i.e., one hydroxy and one methoxy group are not reported in the Table. eIncubation time of 3 days. fIncubation time of 4 hours.
Next, the reactivity of dialdehydes 11, 12, 15 and 16 and enol acetates 13 and 17 with lysozyme were examined in a similar manner with mass spectrometry identifying varying degrees of modification. Of the dialdehydes, 11 was the most reactive leading to rapid formation of a white precipitate, speculated to be due to formation of insoluble higher order protein adducts. ESIMS analysis of the supernatant identified only a trace of unreacted lysozyme and detection of ions arising from extensive modification of the enzyme. To simplify the analysis of these adducts, the incubation time for 11 was shortened to 4 hours, with resultant ESIMS analysis identifying the presence of the three expected pyrrole adducts with mass additions of +132, +150 and +164 (Table 1). Interestingly, Z-dialdehyde 12, formed the same adducts as 11 but at a much slower rate, requiring overnight incubation. In addition to the expected mono-adducts [+132, +150, +164], lysozyme di-adducts were also detected at +264 (2 × alkene), +282 (alkene and OH), +296 (alkene and OMe), +314 (OH and OMe), and +328 (2 × OMe).
Similar reactivity was observed for cyclohexylmethyl E-dialdehyde 15, leading to the formation of a range of mono- (+158, +176, +190) and di-adducts (+316 [2 × alkene], +334 [alkene and OH], +348 [alkene and OMe], +366 [OH and OMe], +380 [2 × OMe]) (Table 1), while Z-dialdehyde 16 was comparatively less reactive, forming only mono-adducts. As expected, enol acetates 13 and 17 were only slowly reactive, giving 18% and 7% yield of adducts, respectively, with 17 requiring 72 hour incubation.
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) was used to look for the presence of protein crosslinking arising from the incubation of dialdehydes 11 and 15 and enol acetate 13 with lysozyme. Bands corresponding to dimers (28 kDa) were evident for both the n-pentyl and cyclohexylmethyl dialdehydes, with a faint band at 50 kDa also evident in the n-pentyl dialdehyde incubation reaction, indicating the presence of lysozyme trimers (Figure 6). No crosslinking was detected for enol acetate 13, likely due to its low reactivity as determined from the n-pentylamine incubation studies.
![[1860-5397-14-197-6]](/bjoc/content/figures/1860-5397-14-197-6.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 6: SDS-PAGE separation of lysozyme after modification with 11 (left), 13 (middle), 15 (right).
Figure 6: SDS-PAGE separation of lysozyme after modification with 11 (left), 13 (middle), 15 (right).
Conclusion
A chemical reactivity study of the opisthobranch mollusc metabolite onchidal (6) has identified that it can react with amines to form pyrrole products. The reaction was presumed to proceed via amine-mediated conversion of the enolester containing natural product to a 1,4-dialdehyde, which then undergoes Paal–Knorr pyrrole formation. Structurally simplified n-pentyl- and cyclohexylmethyl-dialdehydes were synthesized and found to undergo similar pyrrole forming reactions with pentylamine. These reactions were also apparent with the lysine-rich enzyme hen egg white lysozyme, with onchidal (6) and model compounds 11–13 and 15–17 affording pyrrole adducts of the enzyme that were detected by (+)-ESIMS. The more reactive dialdehydes were also found to lead to protein crosslinking with formation of lysozyme dimers and trimers. Taken together, these results support the hypothesis that onchidal (6) could be used in chemical defense in a similar manner to related sesquiterpenoid dialdehydes and enol esters.
References
-
Dasari, R.; De Carvalho, A.; Medellin, D. C.; Middleton, K. N.; Hague, F.; Volmar, M. N. M.; Frolova, L. V.; Rossato, M. F.; De La Chapa, J. J.; Dybdal-Hargreaves, N. F.; Pillai, A.; Mathieu, V.; Rogelj, S.; Gonzales, C. B.; Calixto, J. B.; Evidente, A.; Gautier, M.; Munirathinam, G.; Glass, R.; Burth, P.; Pelly, S. C.; van Otterlo, W. A. L.; Kiss, R.; Kornienko, A. ChemMedChem 2015, 10, 2014–2026. doi:10.1002/cmdc.201500360
Return to citation in text: [1] [2] [3] [4] -
Cimino, G.; Sodano, G.; Spinella, A. Tetrahedron 1987, 43, 5401–5410. doi:10.1016/S0040-4020(01)87721-8
Return to citation in text: [1] -
Moodie, L. W. K.; Trepos, R.; Cervin, G.; Larsen, L.; Larsen, D. S.; Pavia, H.; Hellio, C.; Cahill, P.; Svenson, J. J. Nat. Prod. 2017, 80, 515–525. doi:10.1021/acs.jnatprod.6b01056
Return to citation in text: [1] -
Barbier, P.; Guise, S.; Huitorel, P.; Amade, P.; Pesando, D.; Briand, C.; Peyrot, V. Life Sci. 2001, 70, 415–429. doi:10.1016/S0024-3205(01)01396-0
Return to citation in text: [1] -
Adolph, S.; Jung, V.; Rattke, J.; Pohnert, G. Angew. Chem., Int. Ed. 2005, 44, 2806–2808. doi:10.1002/anie.200462276
Return to citation in text: [1] -
Jung, V.; Pohnert, G. Tetrahedron 2001, 57, 7169–7172. doi:10.1016/S0040-4020(01)00692-5
Return to citation in text: [1] -
Cimino, G.; Crispino, A.; Di Marzo, V.; Gavagnin, M.; Ros, J. D. Experientia 1990, 46, 767–770. doi:10.1007/BF01939963
Return to citation in text: [1] -
Paul, V. J.; Littler, M. M.; Littler, D. S.; Fenical, W. J. Chem. Ecol. 1987, 13, 1171–1185. doi:10.1007/BF01020547
Return to citation in text: [1] -
Ireland, C.; Faulkner, D. J. Bioorg. Chem. 1978, 7, 125–131. doi:10.1016/0045-2068(78)90043-3
Return to citation in text: [1] -
Abramson, S. N.; Radic, Z.; Manker, D.; Faulkner, D. J.; Taylor, P. Mol. Pharmacol. 1989, 36, 349–354.
Return to citation in text: [1] -
Guerriero, A.; Depentori, D.; D’Ambrosio, M.; Pietra, F. Helv. Chim. Acta 1995, 78, 1755–1762. doi:10.1002/hlca.19950780709
Return to citation in text: [1] -
Potts, B. C. M.; Faulkner, D. J.; De Carvalho, M. S.; Jacobs, R. S. J. Am. Chem. Soc. 1992, 114, 5093–5100. doi:10.1021/ja00039a021
Return to citation in text: [1] -
Cimino, G.; Spinella, A.; Sodano, G. Tetrahedron Lett. 1984, 25, 4151–4152. doi:10.1016/S0040-4039(01)90207-2
Return to citation in text: [1] -
Avonto, C.; Taglialatela-Scafati, O.; Pollastro, F.; Minassi, A.; Di Marzo, V.; De Petrocellis, L.; Appendino, G. Angew. Chem., Int. Ed. 2011, 50, 467–471. doi:10.1002/anie.201005959
Return to citation in text: [1] -
Li, S.; Wang, X.; Liu, L.; Kang, J.; Wang, L.; Liu, H.; Ruan, C.; Nie, A.; Zheng, Z.; Xie, Y.; Zhao, G.; Xiao, J.; Hu, Y.; Zhong, W.; Cui, H.; Zhou, X. 5-Membered-S-Heterocyclic Compounds and Their Use in Ppreparing of Medicines for Treating or Preventing the Obesity-Relating Diseases. CN Pat. PCT/CN2004/001118, April 6, 2006.
Return to citation in text: [1] -
Peterlin, Z.; Li, Y.; Sun, G.; Shah, R.; Firestein, S.; Ryan, K. Chem. Biol. 2008, 15, 1317–1327. doi:10.1016/j.chembiol.2008.10.014
Return to citation in text: [1] -
Schnermann, M. J.; Beaudry, C. M.; Genung, N. E.; Canham, S. M.; Untiedt, N. L.; Karanikolas, B. D. W.; Sütterlin, C.; Overman, L. E. J. Am. Chem. Soc. 2011, 133, 17494–17503. doi:10.1021/ja207727h
Return to citation in text: [1] [2]
| 1. | Dasari, R.; De Carvalho, A.; Medellin, D. C.; Middleton, K. N.; Hague, F.; Volmar, M. N. M.; Frolova, L. V.; Rossato, M. F.; De La Chapa, J. J.; Dybdal-Hargreaves, N. F.; Pillai, A.; Mathieu, V.; Rogelj, S.; Gonzales, C. B.; Calixto, J. B.; Evidente, A.; Gautier, M.; Munirathinam, G.; Glass, R.; Burth, P.; Pelly, S. C.; van Otterlo, W. A. L.; Kiss, R.; Kornienko, A. ChemMedChem 2015, 10, 2014–2026. doi:10.1002/cmdc.201500360 |
| 4. | Barbier, P.; Guise, S.; Huitorel, P.; Amade, P.; Pesando, D.; Briand, C.; Peyrot, V. Life Sci. 2001, 70, 415–429. doi:10.1016/S0024-3205(01)01396-0 |
| 5. | Adolph, S.; Jung, V.; Rattke, J.; Pohnert, G. Angew. Chem., Int. Ed. 2005, 44, 2806–2808. doi:10.1002/anie.200462276 |
| 6. | Jung, V.; Pohnert, G. Tetrahedron 2001, 57, 7169–7172. doi:10.1016/S0040-4020(01)00692-5 |
| 3. | Moodie, L. W. K.; Trepos, R.; Cervin, G.; Larsen, L.; Larsen, D. S.; Pavia, H.; Hellio, C.; Cahill, P.; Svenson, J. J. Nat. Prod. 2017, 80, 515–525. doi:10.1021/acs.jnatprod.6b01056 |
| 1. | Dasari, R.; De Carvalho, A.; Medellin, D. C.; Middleton, K. N.; Hague, F.; Volmar, M. N. M.; Frolova, L. V.; Rossato, M. F.; De La Chapa, J. J.; Dybdal-Hargreaves, N. F.; Pillai, A.; Mathieu, V.; Rogelj, S.; Gonzales, C. B.; Calixto, J. B.; Evidente, A.; Gautier, M.; Munirathinam, G.; Glass, R.; Burth, P.; Pelly, S. C.; van Otterlo, W. A. L.; Kiss, R.; Kornienko, A. ChemMedChem 2015, 10, 2014–2026. doi:10.1002/cmdc.201500360 |
| 2. | Cimino, G.; Sodano, G.; Spinella, A. Tetrahedron 1987, 43, 5401–5410. doi:10.1016/S0040-4020(01)87721-8 |
| 17. | Schnermann, M. J.; Beaudry, C. M.; Genung, N. E.; Canham, S. M.; Untiedt, N. L.; Karanikolas, B. D. W.; Sütterlin, C.; Overman, L. E. J. Am. Chem. Soc. 2011, 133, 17494–17503. doi:10.1021/ja207727h |
| 1. | Dasari, R.; De Carvalho, A.; Medellin, D. C.; Middleton, K. N.; Hague, F.; Volmar, M. N. M.; Frolova, L. V.; Rossato, M. F.; De La Chapa, J. J.; Dybdal-Hargreaves, N. F.; Pillai, A.; Mathieu, V.; Rogelj, S.; Gonzales, C. B.; Calixto, J. B.; Evidente, A.; Gautier, M.; Munirathinam, G.; Glass, R.; Burth, P.; Pelly, S. C.; van Otterlo, W. A. L.; Kiss, R.; Kornienko, A. ChemMedChem 2015, 10, 2014–2026. doi:10.1002/cmdc.201500360 |
| 17. | Schnermann, M. J.; Beaudry, C. M.; Genung, N. E.; Canham, S. M.; Untiedt, N. L.; Karanikolas, B. D. W.; Sütterlin, C.; Overman, L. E. J. Am. Chem. Soc. 2011, 133, 17494–17503. doi:10.1021/ja207727h |
| 1. | Dasari, R.; De Carvalho, A.; Medellin, D. C.; Middleton, K. N.; Hague, F.; Volmar, M. N. M.; Frolova, L. V.; Rossato, M. F.; De La Chapa, J. J.; Dybdal-Hargreaves, N. F.; Pillai, A.; Mathieu, V.; Rogelj, S.; Gonzales, C. B.; Calixto, J. B.; Evidente, A.; Gautier, M.; Munirathinam, G.; Glass, R.; Burth, P.; Pelly, S. C.; van Otterlo, W. A. L.; Kiss, R.; Kornienko, A. ChemMedChem 2015, 10, 2014–2026. doi:10.1002/cmdc.201500360 |
| 11. | Guerriero, A.; Depentori, D.; D’Ambrosio, M.; Pietra, F. Helv. Chim. Acta 1995, 78, 1755–1762. doi:10.1002/hlca.19950780709 |
| 12. | Potts, B. C. M.; Faulkner, D. J.; De Carvalho, M. S.; Jacobs, R. S. J. Am. Chem. Soc. 1992, 114, 5093–5100. doi:10.1021/ja00039a021 |
| 13. | Cimino, G.; Spinella, A.; Sodano, G. Tetrahedron Lett. 1984, 25, 4151–4152. doi:10.1016/S0040-4039(01)90207-2 |
| 15. | Li, S.; Wang, X.; Liu, L.; Kang, J.; Wang, L.; Liu, H.; Ruan, C.; Nie, A.; Zheng, Z.; Xie, Y.; Zhao, G.; Xiao, J.; Hu, Y.; Zhong, W.; Cui, H.; Zhou, X. 5-Membered-S-Heterocyclic Compounds and Their Use in Ppreparing of Medicines for Treating or Preventing the Obesity-Relating Diseases. CN Pat. PCT/CN2004/001118, April 6, 2006. |
| 9. | Ireland, C.; Faulkner, D. J. Bioorg. Chem. 1978, 7, 125–131. doi:10.1016/0045-2068(78)90043-3 |
| 10. | Abramson, S. N.; Radic, Z.; Manker, D.; Faulkner, D. J.; Taylor, P. Mol. Pharmacol. 1989, 36, 349–354. |
| 16. | Peterlin, Z.; Li, Y.; Sun, G.; Shah, R.; Firestein, S.; Ryan, K. Chem. Biol. 2008, 15, 1317–1327. doi:10.1016/j.chembiol.2008.10.014 |
| 8. | Paul, V. J.; Littler, M. M.; Littler, D. S.; Fenical, W. J. Chem. Ecol. 1987, 13, 1171–1185. doi:10.1007/BF01020547 |
| 7. | Cimino, G.; Crispino, A.; Di Marzo, V.; Gavagnin, M.; Ros, J. D. Experientia 1990, 46, 767–770. doi:10.1007/BF01939963 |
| 14. | Avonto, C.; Taglialatela-Scafati, O.; Pollastro, F.; Minassi, A.; Di Marzo, V.; De Petrocellis, L.; Appendino, G. Angew. Chem., Int. Ed. 2011, 50, 467–471. doi:10.1002/anie.201005959 |
© 2018 Cadelis and Copp; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0). Please note that the reuse, redistribution and reproduction in particular requires that the authors and source are credited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (https://www.beilstein-journals.org/bjoc)