Abstract
A one-pot synthesis of epoxides from commercially available benzyl alcohols and aldehydes is described. The reaction proceeds through in situ generation of sulfonium salts from benzyl alcohols and their subsequent deprotonation for use in Corey–Chaykovsky epoxidation of aldehydes. The generality of the method is exemplified by the synthesis of 34 epoxides that were made from an array of electronically and sterically varied alcohols and aldehydes.
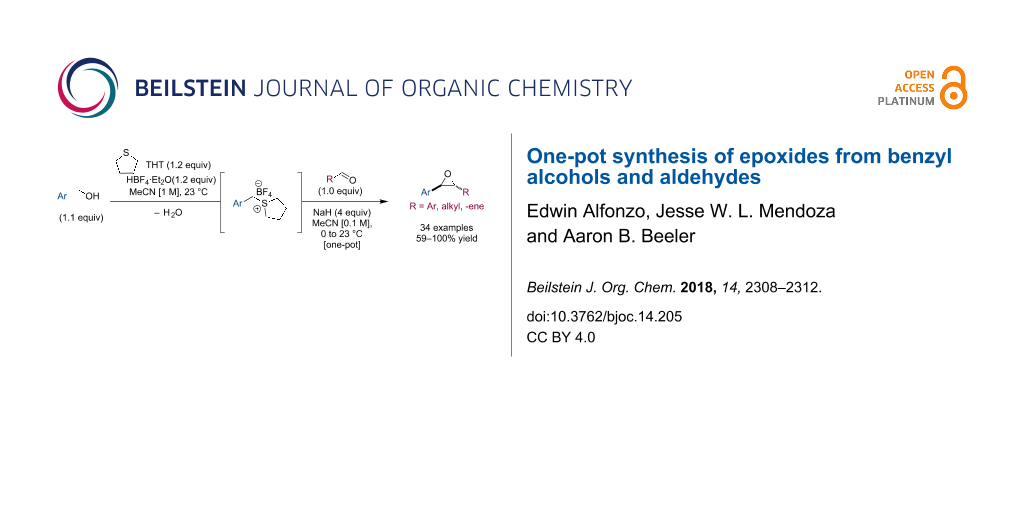
Graphical Abstract
Introduction
Epoxides have historically served as strategic functional groups in target-oriented synthesis [1-4]. Common examples of their utility include stereospecific ring opening [5-7], rearrangements into carbonyls [8-17], and application to cascade or domino reactions [18,19]. More recently, our group has used benzyl epoxides for the photoredox generation of carbonyl ylides which are leveraged in the synthesis of cyclic ethers [20]. This work has led us to search for a general and operationally simple method to generate benzyl epoxides. One of the most powerful methods to access epoxides is through the Corey–Chaykovsky reaction [21] which uses sulfonium ylides and their subsequent reaction with carbonyl groups. This reaction has seen major advancement since its original disclosure, particular in the area of asymmetric synthesis [22-24]. Other notable advancements include the expansion of its scope by using organic bases and a one-pot oxidation/epoxidation sequence of benzyl alcohols with manganese dioxide and an exogenous sulfonium salt [25,26]. Despite these efforts, the synthesis of epoxides using this powerful transformation still often requires multiple steps due to the need to independently synthesize the sulfonium salt. Typically, the salt is synthesized from nucleophilic displacement of benzyl halides but the work by Aggarwal and co-workers [27] has demonstrated that these can be generated from inexpensive benzyl alcohols in the presence of tetrafluoroboric acid and a thio-trapping agent. Unfortunately, isolation of the salt was still required for use in epoxidation of carbonyl groups.
Inspired by these aforementioned precedents, we hypothesized that a process, using commercially available starting materials, wherein the sulfonium salt could be generated in situ from benzyl alcohols and deprotonated would provide an efficient protocol for the synthesis of epoxides in a single reaction. Herein, we describe the realization of this methodology and its use in the synthesis of epoxides that are often unattainable by standard epoxidation methods.
Results and Discussion
After evaluating numerous approaches toward the proposed reaction and subsequent optimization, we found that the sulfonium salt 2 (Scheme 1) could be generated in situ from benzyl alcohol (1) in the presence of slight excess of tetrafluoroboric acid in diethyl ether (HBF4·Et2O) and tetrahydrothiophene (THT). Notably, the use of acetonitrile (MeCN) as a solvent was critical for maintaining a homogeneous reaction and a successful outcome. We also observed that sodium hydride (NaH) was the only base that successfully afforded the desired epoxide 3, typically in excellent and reproducible yields (other bases screened included KOt-Bu, LiHMDS, TBD [25], and KOH). Furthermore, diluting the reaction after formation of the sulfonium salt, and cooling it in an ice bath, proved essential to control the exotherm caused by the deprotonation.
Scheme 1: One-pot synthesis of epoxides from benzyl alcohols and aldehydes.
Scheme 1: One-pot synthesis of epoxides from benzyl alcohols and aldehydes.
Having established a robust method, we then turned our attention to evaluating the scope of the reaction using three electronically varied benzyl alcohols 1, 4, and 5 (Figure 1) on a preparative scale (3 mmol, 5 mmol, 10 mmol). A range of aryl aldehydes worked well including para-nitro (7), ortho-methyl (8), and para-methoxy groups (9). Other notable examples include heterocycles, such as basic pyridines, thiophenes, and furans 16–18. Additionally, aliphatic 13 and 20 and alkenyl aldehydes 21 performed well providing synthetically useful quantities of the desired epoxides. Finally, epoxides containing electron-deficient aryl groups are also available with this method, as exemplified by the synthesis of compounds 22 and 23, which were obtained in good yields on a 10 mmol scale. With respect to the reactivity of the benzyl alcohols, electron-rich alcohol 4 showed faster reaction rates and yields, presumably due to faster and more efficient formation of the sulfonium salt through a para-quinonemethide (p-QM) intermediate. Furthermore, the lower diastereomeric ratios (dr) observed for benzyl alcohol 4 may be due to competing p-QM formation at the betaine intermediate prior to epoxide formation through displacement of THT. This is supported by example 9 which contained a para-methoxy group at the aldehyde component but was isolated as a single diastereomer [28]. Functional groups that are not compatible with this method include phenols, esters, and ketones (Figure 1). The latter is most likely due to competing enolization of the ketone leading to undesired reactivity [29].
Figure 1: Scope of the one-pot synthesis of epoxides from benzyl alcohols and aldehydes.
Figure 1: Scope of the one-pot synthesis of epoxides from benzyl alcohols and aldehydes.
Many of the epoxides that are of interest to us are highly oxygenated on the aryl rings and can be used for the synthesis of numerous bioactive molecules [27,30-34]. Attempts at synthesizing one of these epoxides with the standard mCPBA epoxidation (Scheme 2) led exclusively to rearranged aldehyde 24, presumably promoted by the carboxylic acid byproduct of mCPBA. Unfortunately, attempts to remedy this by using buffered conditions only led to an mCPBA-epoxide adduct 25 [35].
Scheme 2: mCPBA epoxidation of electron-rich stilbene derivatives.
Scheme 2: mCPBA epoxidation of electron-rich stilbene derivatives.
However, the one-pot reaction was highly successful with three electron-rich benzyl alcohols 26, 27, and 28 all bearing multiple oxygenation and with a large panel of electron-rich aldehydes (Figure 2). The reaction was highly successful even when both partners were poly-oxygenated (29–32). Other notable functionalities include heteroaromatics 34, alkenes 36, halides 33 and 37, and a benzyl protected alcohol 38. Furthermore, electron-donating groups were tolerated in all positions on the aryl groups (41–44). Although the Corey–Chaykovsky reaction has been well studied, nearly all the examples shown in Figure 2 represent new compounds and an extension to this methodology.
Figure 2: Scope of the reaction with electron-rich alcohols and aldehydes.
Figure 2: Scope of the reaction with electron-rich alcohols and aldehydes.
Conclusion
In conclusion, we have developed a general and simple method to access benzylic epoxides through the Corey–Chaykovsky reaction between benzyl alcohols and aldehydes. This method provides expedient access to epoxides from commercially available materials in a step and time economical fashion. In particular, we have demonstrated its applicability to the synthesis of epoxides that were generally unattainable using the standard mCPBA epoxidation.
Supporting Information
| Supporting Information File 1: Experimental procedures and characterization for all new compounds described herein. | ||
| Format: PDF | Size: 4.9 MB | Download |
Acknowledgements
Financial support from Boston University and National Science Foundation (ABB CHE-1555300) is gratefully acknowledged. We thank Dr. Norman Lee (Boston University) for high-resolution mass spectrometry data. NMR (CHE-0619339) and MS (CHE0443618) facilities at Boston University are supported by the NSF.
References
-
Parker, R. E.; Isaacs, N. S. Chem. Rev. 1959, 59, 737–799. doi:10.1021/cr50028a006
Return to citation in text: [1] -
Vilotijevic, I.; Jamison, T. F. Angew. Chem., Int. Ed. 2009, 48, 5250–5281. doi:10.1002/anie.200900600
Return to citation in text: [1] -
He, J.; Ling, J.; Chiu, P. Chem. Rev. 2014, 114, 8037–8128. doi:10.1021/cr400709j
Return to citation in text: [1] -
Heravi, M. M.; Leshaki, T. B.; Poorahmad, N. Tetrahedron: Asymmetry 2015, 26, 405–495. doi:10.1016/j.tetasy.2015.03.006
Return to citation in text: [1] -
Payne, G. B. J. Org. Chem. 1962, 27, 3819–3822. doi:10.1021/jo01058a015
Return to citation in text: [1] -
Rao, A. S.; Paknikar, S. K.; Kirtane, J. G. Tetrahedron 1983, 39, 2323–2367. doi:10.1016/S0040-4020(01)91961-1
Return to citation in text: [1] -
Paterson, I.; Berrisford, D. J. Angew. Chem., Int. Ed. Engl. 1992, 31, 1179–1180. doi:10.1002/anie.199211791
Return to citation in text: [1] -
House, H. O. J. Am. Chem. Soc. 1955, 77, 3070–3075. doi:10.1021/ja01616a041
Return to citation in text: [1] -
Meinwald, J.; Labana, S. S.; Chadha, M. S. J. Am. Chem. Soc. 1963, 85, 582–585. doi:10.1021/ja00888a022
Return to citation in text: [1] -
Rickborn, B.; Gerkin, R. M. J. Am. Chem. Soc. 1971, 93, 1693–1700. doi:10.1021/ja00736a021
Return to citation in text: [1] -
Suda, K.; Kikkawa, T.; Nakajima, S.-i.; Takanami, T. J. Am. Chem. Soc. 2004, 126, 9554–9555. doi:10.1021/ja047104k
Return to citation in text: [1] -
Gudla, V.; Balamurugan, R. Tetrahedron Lett. 2012, 53, 5243–5247. doi:10.1016/j.tetlet.2012.07.056
Return to citation in text: [1] -
Bah, J.; Naidu, V. R.; Teske, J.; Franzén, J. Adv. Synth. Catal. 2015, 357, 148–158. doi:10.1002/adsc.201400609
Return to citation in text: [1] -
Robinson, M. W. C.; Davies, A. M.; Buckle, R.; Mabbett, I.; Taylor, S. H.; Graham, A. E. Org. Biomol. Chem. 2009, 7, 2559–2564. doi:10.1039/b900719a
Return to citation in text: [1] -
Robison, M. W. C.; Pillinger, K. S.; Graham, A. E. Tetrahedron Lett. 2006, 47, 5919–5921. doi:10.1016/j.tetlet.2006.06.055
Return to citation in text: [1] -
Karamé, I.; Tommasino, M. L.; Lemaire, M. Tetrahedron Lett. 2003, 44, 7687–7689. doi:10.1016/S0040-4039(03)01593-4
Return to citation in text: [1] -
Umeda, R.; Muraki, M.; Nakamura, Y.; Tanaka, T.; Kamiguchi, K.; Nishiyama, Y. Tetrahedron Lett. 2017, 58, 2393–2395. doi:10.1016/j.tetlet.2017.05.018
Return to citation in text: [1] -
Das, S. K. Asian J. Org. Chem. 2017, 6, 243–256. doi:10.1002/ajoc.201600440
Return to citation in text: [1] -
Zhu, W.; Ren, J.; Wang, Z. Eur. J. Org. Chem. 2014, 3561–3564. doi:10.1002/ejoc.201402160
Return to citation in text: [1] -
Alfonzo, E.; Alfonso, F. S.; Beeler, A. B. Org. Lett. 2017, 19, 2989–2992. doi:10.1021/acs.orglett.7b01222
Return to citation in text: [1] -
Corey, E. J.; Chaykovsky, M. J. Am. Chem. Soc. 1964, 87, 1353–1364. doi:10.1021/ja01084a034
Return to citation in text: [1] -
Aggarwal, V. K.; Winn, C. L. Acc. Chem. Res. 2004, 37, 611–620. doi:10.1021/ar030045f
Return to citation in text: [1] -
Davoust, M.; Brière, J.-F.; Jaffrès, P.-A.; Metzner, P. J. Org. Chem. 2005, 70, 4166–4169. doi:10.1021/jo0479260
Return to citation in text: [1] -
Illa, O.; Namutebi, M.; Saha, C.; Ostovar, M.; Chen, C. C.; Haddow, M. F.; Nocquet-Thibault, S.; Lusi, M.; McGarrigle, E. M.; Aggarwal, V. K. J. Am. Chem. Soc. 2013, 135, 11951–11966. doi:10.1021/ja405073w
Return to citation in text: [1] -
Phillips, D. J.; Graham, A. E. Synlett 2010, 769–773. doi:10.1055/s-0029-1219359
Return to citation in text: [1] [2] -
Phillips, D. J.; Kean, J. L.; Graham, A. E. Tetrahedron 2013, 69, 6196–6202. doi:10.1016/j.tet.2013.05.036
Return to citation in text: [1] -
Aggarwal, V. K.; Bae, I.; Lee, H.-Y.; Richardson, J.; Williams, D. T. Angew. Chem., Int. Ed. 2003, 42, 3274–3278. doi:10.1002/anie.200350968
Return to citation in text: [1] [2] -
Low diastereoselectivity of electron-rich sulfonium ylides have been previously observed by Aggarwal et al.; see references [22] and [27].
Return to citation in text: [1] -
Several byproducts were observed in the crude material. Attempts of using other bases did not produce the desired product.
Return to citation in text: [1] -
Hirsekorn, R. Tetrahedron Lett. 1990, 31, 7591–7594. doi:10.1016/S0040-4039(00)97306-4
Return to citation in text: [1] -
Hirserkorn, R.; Orlitsch, S. Process for the preparation of 1-alkylisoquinoline derivatives. Eur. Pat. Appl. EP0471303A1, Feb 19, 1992.
Return to citation in text: [1] -
Lupattelli, P.; D’Auria, M.; Di Blasio, N.; Lenti, F. Eur. J. Org. Chem. 2009, 141–145. doi:10.1002/ejoc.200800957
Return to citation in text: [1] -
Aslam, S. N.; Stevenson, P. C.; Phythian, S. J.; Veitch, N. C.; Hall, D. R. Tetrahedron 2006, 62, 4214–4226. doi:10.1016/j.tet.2006.02.015
Return to citation in text: [1] -
Hadfield, J. A.; Gaukroger, K.; Hirst, N.; Weston, A. P.; Lawrence, N. J.; McGown, A. T. Eur. J. Med. Chem. 2005, 40, 529–541. doi:10.1016/j.ejmech.2004.12.008
Return to citation in text: [1] -
Anderson, W. K.; Veysoglu, T. J. Org. Chem. 1973, 38, 2267–2268. doi:10.1021/jo00952a046
Return to citation in text: [1]
| 1. | Parker, R. E.; Isaacs, N. S. Chem. Rev. 1959, 59, 737–799. doi:10.1021/cr50028a006 |
| 2. | Vilotijevic, I.; Jamison, T. F. Angew. Chem., Int. Ed. 2009, 48, 5250–5281. doi:10.1002/anie.200900600 |
| 3. | He, J.; Ling, J.; Chiu, P. Chem. Rev. 2014, 114, 8037–8128. doi:10.1021/cr400709j |
| 4. | Heravi, M. M.; Leshaki, T. B.; Poorahmad, N. Tetrahedron: Asymmetry 2015, 26, 405–495. doi:10.1016/j.tetasy.2015.03.006 |
| 20. | Alfonzo, E.; Alfonso, F. S.; Beeler, A. B. Org. Lett. 2017, 19, 2989–2992. doi:10.1021/acs.orglett.7b01222 |
| 22. | Aggarwal, V. K.; Winn, C. L. Acc. Chem. Res. 2004, 37, 611–620. doi:10.1021/ar030045f |
| 18. | Das, S. K. Asian J. Org. Chem. 2017, 6, 243–256. doi:10.1002/ajoc.201600440 |
| 19. | Zhu, W.; Ren, J.; Wang, Z. Eur. J. Org. Chem. 2014, 3561–3564. doi:10.1002/ejoc.201402160 |
| 27. | Aggarwal, V. K.; Bae, I.; Lee, H.-Y.; Richardson, J.; Williams, D. T. Angew. Chem., Int. Ed. 2003, 42, 3274–3278. doi:10.1002/anie.200350968 |
| 8. | House, H. O. J. Am. Chem. Soc. 1955, 77, 3070–3075. doi:10.1021/ja01616a041 |
| 9. | Meinwald, J.; Labana, S. S.; Chadha, M. S. J. Am. Chem. Soc. 1963, 85, 582–585. doi:10.1021/ja00888a022 |
| 10. | Rickborn, B.; Gerkin, R. M. J. Am. Chem. Soc. 1971, 93, 1693–1700. doi:10.1021/ja00736a021 |
| 11. | Suda, K.; Kikkawa, T.; Nakajima, S.-i.; Takanami, T. J. Am. Chem. Soc. 2004, 126, 9554–9555. doi:10.1021/ja047104k |
| 12. | Gudla, V.; Balamurugan, R. Tetrahedron Lett. 2012, 53, 5243–5247. doi:10.1016/j.tetlet.2012.07.056 |
| 13. | Bah, J.; Naidu, V. R.; Teske, J.; Franzén, J. Adv. Synth. Catal. 2015, 357, 148–158. doi:10.1002/adsc.201400609 |
| 14. | Robinson, M. W. C.; Davies, A. M.; Buckle, R.; Mabbett, I.; Taylor, S. H.; Graham, A. E. Org. Biomol. Chem. 2009, 7, 2559–2564. doi:10.1039/b900719a |
| 15. | Robison, M. W. C.; Pillinger, K. S.; Graham, A. E. Tetrahedron Lett. 2006, 47, 5919–5921. doi:10.1016/j.tetlet.2006.06.055 |
| 16. | Karamé, I.; Tommasino, M. L.; Lemaire, M. Tetrahedron Lett. 2003, 44, 7687–7689. doi:10.1016/S0040-4039(03)01593-4 |
| 17. | Umeda, R.; Muraki, M.; Nakamura, Y.; Tanaka, T.; Kamiguchi, K.; Nishiyama, Y. Tetrahedron Lett. 2017, 58, 2393–2395. doi:10.1016/j.tetlet.2017.05.018 |
| 27. | Aggarwal, V. K.; Bae, I.; Lee, H.-Y.; Richardson, J.; Williams, D. T. Angew. Chem., Int. Ed. 2003, 42, 3274–3278. doi:10.1002/anie.200350968 |
| 30. | Hirsekorn, R. Tetrahedron Lett. 1990, 31, 7591–7594. doi:10.1016/S0040-4039(00)97306-4 |
| 31. | Hirserkorn, R.; Orlitsch, S. Process for the preparation of 1-alkylisoquinoline derivatives. Eur. Pat. Appl. EP0471303A1, Feb 19, 1992. |
| 32. | Lupattelli, P.; D’Auria, M.; Di Blasio, N.; Lenti, F. Eur. J. Org. Chem. 2009, 141–145. doi:10.1002/ejoc.200800957 |
| 33. | Aslam, S. N.; Stevenson, P. C.; Phythian, S. J.; Veitch, N. C.; Hall, D. R. Tetrahedron 2006, 62, 4214–4226. doi:10.1016/j.tet.2006.02.015 |
| 34. | Hadfield, J. A.; Gaukroger, K.; Hirst, N.; Weston, A. P.; Lawrence, N. J.; McGown, A. T. Eur. J. Med. Chem. 2005, 40, 529–541. doi:10.1016/j.ejmech.2004.12.008 |
| 5. | Payne, G. B. J. Org. Chem. 1962, 27, 3819–3822. doi:10.1021/jo01058a015 |
| 6. | Rao, A. S.; Paknikar, S. K.; Kirtane, J. G. Tetrahedron 1983, 39, 2323–2367. doi:10.1016/S0040-4020(01)91961-1 |
| 7. | Paterson, I.; Berrisford, D. J. Angew. Chem., Int. Ed. Engl. 1992, 31, 1179–1180. doi:10.1002/anie.199211791 |
| 35. | Anderson, W. K.; Veysoglu, T. J. Org. Chem. 1973, 38, 2267–2268. doi:10.1021/jo00952a046 |
| 27. | Aggarwal, V. K.; Bae, I.; Lee, H.-Y.; Richardson, J.; Williams, D. T. Angew. Chem., Int. Ed. 2003, 42, 3274–3278. doi:10.1002/anie.200350968 |
| 28. | Low diastereoselectivity of electron-rich sulfonium ylides have been previously observed by Aggarwal et al.; see references [22] and [27]. |
| 25. | Phillips, D. J.; Graham, A. E. Synlett 2010, 769–773. doi:10.1055/s-0029-1219359 |
| 26. | Phillips, D. J.; Kean, J. L.; Graham, A. E. Tetrahedron 2013, 69, 6196–6202. doi:10.1016/j.tet.2013.05.036 |
| 29. | Several byproducts were observed in the crude material. Attempts of using other bases did not produce the desired product. |
| 22. | Aggarwal, V. K.; Winn, C. L. Acc. Chem. Res. 2004, 37, 611–620. doi:10.1021/ar030045f |
| 23. | Davoust, M.; Brière, J.-F.; Jaffrès, P.-A.; Metzner, P. J. Org. Chem. 2005, 70, 4166–4169. doi:10.1021/jo0479260 |
| 24. | Illa, O.; Namutebi, M.; Saha, C.; Ostovar, M.; Chen, C. C.; Haddow, M. F.; Nocquet-Thibault, S.; Lusi, M.; McGarrigle, E. M.; Aggarwal, V. K. J. Am. Chem. Soc. 2013, 135, 11951–11966. doi:10.1021/ja405073w |
| 21. | Corey, E. J.; Chaykovsky, M. J. Am. Chem. Soc. 1964, 87, 1353–1364. doi:10.1021/ja01084a034 |
| 25. | Phillips, D. J.; Graham, A. E. Synlett 2010, 769–773. doi:10.1055/s-0029-1219359 |
© 2018 Alfonzo et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0). Please note that the reuse, redistribution and reproduction in particular requires that the authors and source are credited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (https://www.beilstein-journals.org/bjoc)