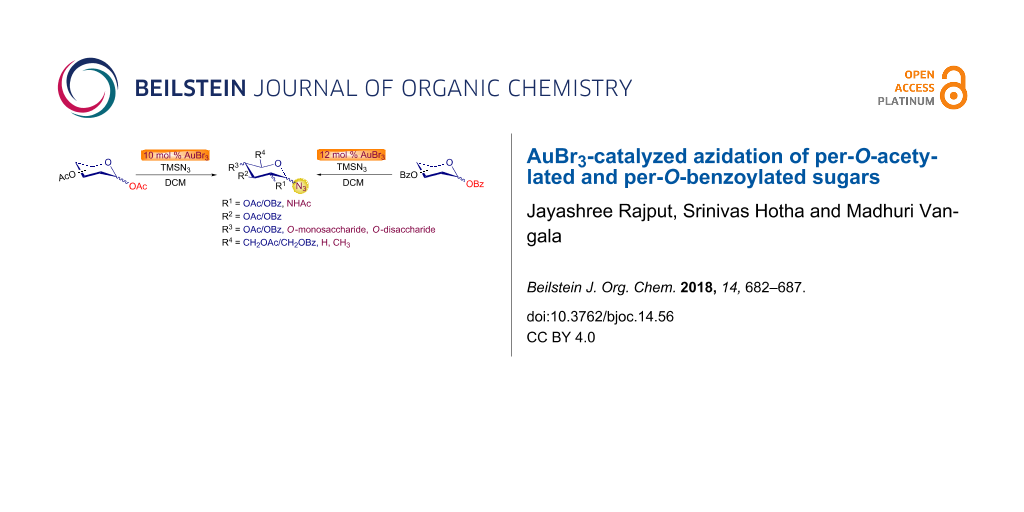
Abstract
Herein we report, for the first time, the successful anomeric azidation of per-O-acetylated and per-O-benzoylated sugars by catalytic amounts of oxophilic AuBr3 in good to excellent yields. The method is applicable to a wide range of easily accessible per-O-acetylated and per-O-benzoylated sugars. While reaction with per-O-acetylated and per-O-benzoylated monosaccharides was complete within 1–3 h at room temperature, the per-O-benzoylated disaccharides needed 2–3 h of heating at 55 °C.
Graphical Abstract
Introduction
The past few decades had seen the enrichment of transition metal complexes in various glycosylation strategies [1]. In particular, gold complexes with their operationally simple, safe and neutral reaction conditions, had widely contributed to the development of new glycosylation methods. Gold(I) and gold(III) complexes are usually alkynophilic [2], carbophilic and oxophilic because of their affinity towards the alkynes’ and C–O π systems [3-6]. Thus, various research groups employed either a remote alkyne group possessing versatile glycosyl donors [7-16] or used glycals [17] for effective O-, C-, and S-glycosylation reactions using gold(I) and gold(III) catalysts. Among the gold-catalyzed activation of non-alkynic glycosyl donors, glycosyl halides [18], armed O-methyl glycosides [19], armed and disarmed thioglycosides [20] as well as trichloroacetimidate [21,22] donors were successfully applied to O- and C-glycosylations.
Of the gold-catalyzed N-glycosylation reactions, Yu et al. demonstrated the effective purine and pyrimidine nucleoside synthesis using per-O-acyl/per-O-benzoyl furanosyl and pyranosyl o-hexynylbenzoates [23]. Subsequently, Hotha and co-workers utilized propargyl 1,2-orthoesters and alkynyl glycosyl carbonate donors for the synthesis of pyrimidine nucleosides [24,25]. In addition, N-glycosides are also accessible by AuCl3/phenylacetylene-promoted Ferrier rearrangement of glycals [17], thus, demonstrating the efficient catalysis by alkynophilic and carbophilic Au complexes. Although the alkynophilicity and carbophilicity of Au complexes are well explored, very little is known about the role played by the oxophilicity of gold [26] towards the glycosylation reactions.
Generally, easily accessible per-O-acetylated and per-O-benzoylated sugars are not regarded as effective glycosyl donors in glycosylation reactions since they require harsh reaction conditions due to the deactivating effect of the ester groups. In a recently reported gold(III)-mediated reaction Vankar and co-workers disclosed that a AuCl3–phenylacetylene complex promotes the O-glycosylation of armed 1-O-acetyl pyranosides and furanosides [17,27]. The authors also observed that 5 mol % AuCl3 alone promoted the O-glycosylation albeit in low yields, thus indicating the possible utility of the oxophilic character of Au(III) towards the acetylated sugars.
Among the N-glycosides, anomeric azido glycosides are important intermediates due to various applications in the synthesis of various glycosyl amides [28,29], glycoconjugates [30-32], N-glycosyl heterocycles [33,34], N-glycosyl triazole [35,36], etc. Glycosyl azides can be accessed from the corresponding glycosyl halides [37-40] by nucleophilic displacement with NaN3 or using trimethylsilyl azide in the presence of a phase transfer catalyst [41-45]. More commonly, glycosyl azides are synthesized from per-O-acetylated sugars using trimethylsilyl azide in the presence of a variety of Lewis acids such as SnCl4 [46], TiCl4 [47,48], BF3·OEt2 [49], TMSOTf [50,51], etc. However, at higher concentration Lewis acids can potentially lead to slow anomerization [52]. In 2011, Chen’s group reported that 5 mol % FeCl3 can catalyze the reaction of trimethylsilyl azide with per-O-acetylated β-monosaccharides to afford glycosyl azides in 3–7 h, whereas per-O-acetylated β-di- and trisaccharides required 22–28 h for complete conversion [53]. Despite the use of various Lewis acid catalysts, gold(III)-catalyzed azidation reactions remain rather underexplored till date. In our efforts towards the syntheses of glycoderivatives [54-56], we found that AuBr3 activates per-O-acetylated and per-O-benzoylated sugars towards anomeric azidation in good to excellent yields.
Results and Discussion
We began our studies by treating per-O-acetylated glucose with 3 equiv trimethylsilyl azide in the presence of 10 mol % AuBr3 in dichloromethane at room temperature. The reaction proceeded smoothly giving 2,3,4,6-tetra-O-acetyl-β-D-glucopyranosyl azide (1) within 3 h in 91% yield (Scheme 1).
Scheme 1: Azidation of per O-acetylated glucose.
Scheme 1: Azidation of per O-acetylated glucose.
Next we tested the slightly more Lewis acidic AuCl3 in this reaction, and found that 10 mol % of AuCl3 were essential for complete consumption of the starting material. As AuBr3 is less hygroscopic than AuCl3 and thus easier to handle, all further experiments were conducted with AuBr3 only. Interestingly, we noticed that, when stirring the reaction mixture with 4 Å molecular sieves powder to remove moisture prior to the addition of the catalyst no product was formed. This observation suggested that in addition to the coordination of AuBr3 to the lone pairs of the anomeric acetate carbonyl oxygen, probably the Brønsted acid, HBr, generated from AuBr3 and water present in the reaction medium is also participating in the catalytic cycle.
Also no reaction was observed when peracetylated galactose and 3 equiv of trimethylsilyl azide were stirred at room temperature in the absence of AuBr3 as the catalyst. Additionally, the treatment of peracetylated galactose with NaN3 instead of trimethylsilyl azide at room temperature for 6 h also yielded no product. In view of the above observations, a plausible catalytic cycle is proposed in Supporting Information File 1.
In a similar fashion using 10 mol % AuBr3, 2,3,4,6-tetra-O-acetyl-β-D-galactopyranosyl azide (2) and 2,3,4,6-tetra-O-acetyl-β-D-mannopyranosyl azide (3) were obtained from their corresponding per-O-acetylated sugar precursors (Table 1, entries 1 and 2) in 3 h at 25 °C in 87% and 90% yield, respectively. Interestingly, the reaction of per-O-acetylated xylopyranose (Table 1, entry 3) also proceeded smoothly affording 2,3,4-tri-O-acetyl-β-D-xylopyranosyl azide in 85% yield after 1 h. Conversely, the azidation of peracetylated L-fucopyranose gave an α/β mixture (1:6), with 2,3,4-tri-O-acetyl-β-L-fucopyranosyl azide (5) obtained in 71% yield in 1 h [57]. Gratifyingly, the disaccharide, β-D-cellobiosyl azide (6), could be conveniently synthesized from the commercially available α-D-peracetylated cellobiose in 3 h at room temperature in an excellent yield. Additionally, peracetylated maltotriose took 5 h for completion to afford the corresponding azido compound 7 in 82% yield.
Table 1: Scope of AuBr3-catalyzed azido glycosylation of peracetates.
|
|
||||
| Entrya | Substrate | Product | Time (h)/ temp |
α:β ratio/
yieldb |
|---|---|---|---|---|
| 1 |
|
2 |
3/rt |
α:β
1:19 β-87% |
| 2 |
|
3 |
3/rt |
α:β
19:1 α-90% |
| 3 |
|
4 |
1/rt |
β-only
β-85% |
| 4 |
|
5 |
1/rt |
α:β
1:6 β-71% |
| 5 |
|
6 |
3/rt |
β-only
β-92% |
| 6c |
|
7 |
5/rt |
β-only
β-82% |
| 7 |
|
8 |
2/0 °C |
β-only
β-93% |
| 8d |
|
9 |
48/55 °C |
β-only
β-74% |
aAll reactions were carried out on a 300 mg scale, using 10 mol % AuBr3 and 3 equiv TMSN3 in 4 mL of CH2Cl2; bisolated purified yield; c30 mol % AuBr3 were used; d1 equiv AuBr3 was used; rt: room temperature.
As anticipated, the anomeric azidation of peracetylated ribofuranose (Table 1, entry 7) proceeded well even at 0 °C within 2 h to give the product in 93% yield. However, the azidation of peracetylated 2-deoxy-D-glucosamine was slow and required one equivalent of AuBr3 and heating at 55 °C for 48 h to reach completion. In this case the desired product β-azido 2,3,4,6-acetyl-D-glucosamine (9) could be obtained in 74% yield. The need of using higher amounts of catalyst in this reaction could be attributed to the possible coordination of AuBr3 with the amide.
Having successfully accomplished the gold(III)-catalyzed azido glycosidation of per-O-acetates, we next turned our attention to per-O-benzoylated sugars. Gratifyingly, using 12 mol % AuBr3, the easily accessible per-O-benzoylated mannopyranose and glucopyranose (Table 2, entries 1 and 2) were readily converted into the corresponding 2,3,4,6-tetra-O-benzoyl-α-D-mannopyranosyl azide (10) and 2,3,4,6-tetra-O-benzoyl-β-D-glucopyranosyl azide (11) in excellent yields within 3 h reaction at room temperature. It is noteworthy that the present method can be successfully applied to perbenzoylated sugars with a slightly higher catalyst loading given the fact that they these sugars are more deactivated than the corresponding acetates. Conversely, the reaction of 1,2,3,4-tetra-O-benzoyl-L-rhamnopyranoside (Table 2, entry 3) proceeded within 1 h at room temperature giving 2,3,4-tri-O-benzoyl-α-L-rhamnopyranosyl azide (12) in 71% yield. Furthermore, C5-O-TBDPS-protected perbenzoylated arabinofuranose (Table 2, entry 4) afforded the desired azide 13 in 70% yield along with some amounts of desilylated product. Furthermore, azidation of perbenzoylated maltose and lactose (Table 2, entries 5 and 6) did not proceed at room temperature and required heating at 55 °C for 2 h to provide the desired products β-D-maltopyranosyl azide and β-D-lactopyranosyl azide 14 and 15 in 91% and 84% yields, respectively. We found these results very intriguing as the rate of N-glycosylation of benzoylated glycosyl donors which is usually considered low, could be achieved using a catalytic amount of the mildly Lewis acidic AuBr3 and an excellent azide source, trimethylsilyl azide.
Table 2: Scope of AuBr3-catalyzed azido glycosylation of perbenzoylated sugars.
|
|
||||
| Entrya | Substrate | Product |
Time (h)/
temp |
α:β ratio/
yieldb |
|---|---|---|---|---|
| 1 |
|
10 |
3/rt |
α:β
49:1 α:90% |
| 2 |
|
11 |
3/rt |
α:β
1:49 β:88% |
| 3 |
|
12 |
1/rt |
α:β
9:1/ α:71% |
| 4 |
|
13 |
1/rt |
α only
70% |
| 5 |
|
14 |
2/55 °C |
β only
91% |
| 6 |
|
15 |
2.5/55 °C |
β only
84% |
aAll reactions were carried out on a 300 mg scale using 12 mol % AuBr3 and 3 equiv TMSN3 in 4 mL of CH2Cl2; rt: room temperature; bisolated purified yield.
Further, we checked the possibility of O-glycosylation and C-glycosylation of peracetylated sugars with 10 mol % AuBr3, but the starting materials remained unaffected. Finally, the potential of the gold(III)-catalyzed azidation for large scale applications was demonstrated by performing a gram-scale synthesis on glucose peracetate giving product 2 in 90% yield.
Conclusion
In summary, a facile methodology demonstrating the ability of Au(III) in catalyzing the azidation of deactivated sugars was shown. The reaction proceeds in the absence of molecular sieves without forming lactols as byproducts. This operationally simple protocol enables the synthesis of various N-glycoconjugates offering a wide range of applications and further demonstrates the value of gold catalysis in carbohydrate chemistry.
Experimental
General experimental methods: Chemicals and materials were obtained from commercial sources and used without further purification unless otherwise noted. 1H and 13C NMR spectra were recorded on a 400 MHz and 100 MHz spectrometer, respectively using CDCl3 as the solvent. Chemical shifts (δ) are given in ppm. For perbenzoate compounds 10–15, tetramethyl silane was used as internal standard. Electrospray ionization (ESI) was used for high resolution mass spectrometry (HRMS). An FTIR spectrometer was used for recording IR spectra and only major peaks are reported in cm−1. Optical rotations were measured on a polarimeter using sodium light (D line at 589 nm). Column chromatography was performed on silica gel (120–200 mesh) using mixtures of ethyl acetate and hexane as the eluents.
General procedure for the anomeric azidation: To a solution of peracetylated or perbenzoylated sugars (300 mg) in 4 mL of dry DCM at room temperature, TMSN3 (3 equiv) was added followed by the addition of AuBr3 (amounts of the catalyst are given in Table 1 and Table 2). The reaction mixture was stirred either at room temperature or heated to 55–60 °C as mentioned in the Table 1 and Table 2. Then, the reaction was quenched by adding triethylamine (20 μL). The mixture was concentrated in vacuo and the crude product was purified by column chromatography. Alternatively, the reaction can be quenched by adding sodium bicarbonate solution followed by extraction with DCM (2 × 20 mL). The combined organic layers were washed with water, brine and dried over Na2SO4 and concentrated to dryness. The residue was purified by column chromatography on silica gel using petroleum ether (bp 60–70 °C) and EtOAc.
Supporting Information
| Supporting Information File 1: Plausible catalytic cycle, experimental data and copies of 1H and 13C NMR spectra of glycosyl azides 1–15 were provided. | ||
| Format: PDF | Size: 3.2 MB | Download |
References
-
Li, X.; Zhu, J. Eur. J. Org. Chem. 2016, 4724–4767. doi:10.1002/ejoc.201600484
Return to citation in text: [1] -
Dorel, R.; Echavarren, A. M. Chem. Rev. 2015, 115, 9028–9072. doi:10.1021/cr500691k
Return to citation in text: [1] -
Hashmi, A. S. K.; Hutchings, G. J. Angew. Chem., Int. Ed. 2006, 45, 7896–7936. doi:10.1002/anie.200602454
Return to citation in text: [1] -
Fürstner, A.; Davies, P. W. Angew. Chem., Int. Ed. 2007, 46, 3410–3449. doi:10.1002/anie.200604335
Return to citation in text: [1] -
Hashmi, A. S. K. Chem. Rev. 2007, 107, 3180–3211. doi:10.1021/cr000436x
Return to citation in text: [1] -
Morita, N.; Yasuda, A.; Shibata, M.; Ban, S.; Hashimoto, Y.; Okamoto, I.; Tamura, O. Org. Lett. 2015, 17, 2668–2671. doi:10.1021/acs.orglett.5b01046
Return to citation in text: [1] -
Hotha, S.; Kashyap, S. J. Am. Chem. Soc. 2006, 128, 9620–9621. doi:10.1021/ja062425c
Return to citation in text: [1] -
Li, Y.; Yang, Y.; Yu, B. Tetrahedron Lett. 2008, 49, 3604–3608. doi:10.1016/j.tetlet.2008.04.017
Return to citation in text: [1] -
Mamidyala, S. K.; Finn, M. G. J. Org. Chem. 2009, 74, 8417–8420. doi:10.1021/jo901857x
Return to citation in text: [1] -
Kayastha, A. K.; Hotha, S. Chem. Commun. 2012, 48, 7161–7163. doi:10.1039/C2CC32649C
Return to citation in text: [1] -
Sureshkumar, G.; Hotha, S. Tetrahedron Lett. 2007, 48, 6564–6568. doi:10.1016/j.tetlet.2007.07.015
Return to citation in text: [1] -
Li, Y.; Yang, X.; Liu, Y.; Zhu, C.; Yang, Y.; Yu, B. Chem. – Eur. J. 2010, 16, 1871–1882. doi:10.1002/chem.200902548
Return to citation in text: [1] -
Tang, Y.; Li, J.; Zhu, Y.; Li, Y.; Yu, B. J. Am. Chem. Soc. 2013, 135, 18396–18405. doi:10.1021/ja4064316
Return to citation in text: [1] -
Zhu, Y.; Yu, B. Chem. – Eur. J. 2015, 21, 8771–8780. doi:10.1002/chem.201500648
Return to citation in text: [1] -
Adhikari, S.; Baryal, K. N.; Zhu, D.; Li, X.; Zhu, J. ACS Catal. 2013, 3, 57–60. doi:10.1021/cs300670k
Return to citation in text: [1] -
Koppolu, S. R.; Niddana, R.; Balamurugan, R. Org. Biomol. Chem. 2015, 13, 5094–5097. doi:10.1039/C5OB00248F
Return to citation in text: [1] -
Roy, R.; Rajasekaran, P.; Mallick, A.; Vankar, Y. D. Eur. J. Org. Chem. 2014, 5564–5573. doi:10.1002/ejoc.201402606
Return to citation in text: [1] [2] [3] -
Götze, S.; Fitzner, R.; Kunz, H. Synlett 2009, 3346–3348. doi:10.1055/s-0029-1218356
Return to citation in text: [1] -
Vidadala, S. R.; Hotha, S. Chem. Commun. 2009, 2505–2507. doi:10.1039/B822526E
Return to citation in text: [1] -
Vibhute, A. M.; Dhaka, A.; Athiyarath, V.; Sureshan, K. M. Chem. Sci. 2016, 7, 4259–4263. doi:10.1039/C6SC00633G
Return to citation in text: [1] -
Peng, P.; Schmidt, R. R. J. Am. Chem. Soc. 2015, 137, 12653–12659. doi:10.1021/jacs.5b07895
Return to citation in text: [1] -
Roy, R.; Palanivel, A. K.; Mallick, A.; Vankar, Y. D. Eur. J. Org. Chem. 2015, 4000–4005. doi:10.1002/ejoc.201500137
Return to citation in text: [1] -
Zhang, Q.; Sun, J.; Zhu, Y.; Zhang, F.; Yu, B. Angew. Chem., Int. Ed. 2011, 50, 4933–4936. doi:10.1002/anie.201100514
Return to citation in text: [1] -
Rao, B. V.; Manmode, S.; Hotha, S. J. Org. Chem. 2015, 80, 1499–1505. doi:10.1021/jo502413z
Return to citation in text: [1] -
Mishra, B.; Neralkar, M.; Hotha, S. Angew. Chem., Int. Ed. 2016, 55, 7786–7791. doi:10.1002/anie.201511695
Return to citation in text: [1] -
Kayastha, A. K.; Hotha, S. Beilstein J. Org. Chem. 2013, 9, 2147–2155. doi:10.3762/bjoc.9.252
Return to citation in text: [1] -
Mallick, A.; Mallikharjunarao, Y.; Rajasekaran, P.; Roy, R.; Vankar, Y. D. Eur. J. Org. Chem. 2016, 579–588. doi:10.1002/ejoc.201501245
Return to citation in text: [1] -
Tomabechi, Y.; Squire, M. A.; Fairbanks, A. J. Org. Biomol. Chem. 2014, 12, 942–955. doi:10.1039/C3OB42104J
Return to citation in text: [1] -
Bianchi, A.; Bernardi, A. J. Org. Chem. 2006, 71, 4565–4577. doi:10.1021/jo060409s
Return to citation in text: [1] -
Doores, K. J.; Mimura, Y.; Dwek, R. A.; Rudd, P. M.; Eliott, T.; Davis, B. G. Chem. Commun. 2006, 1401–1403. doi:10.1039/B515472C
Return to citation in text: [1] -
Dedola, S.; Nepogodiev, S. A.; Field, R. A. Org. Biomol. Chem. 2007, 5, 1006–1017. doi:10.1039/B618048P
Return to citation in text: [1] -
Kasuya, M. C. Z.; Wang, L. X.; Lee, Y. C.; Mitsuki, M.; Nakajima, H.; Miura, Y.; Sato, T.; Hatanaka, K.; Yamagata, S.; Yamagata, T. Carbohydr. Res. 2000, 329, 755–763. doi:10.1016/S0008-6215(00)00238-X
Return to citation in text: [1] -
Pal, A. P. J.; Vankar, Y. D. Tetrahedron Lett. 2010, 51, 2519–2524. doi:10.1016/j.tetlet.2010.03.003
Return to citation in text: [1] -
Pal, A. P. J.; Kadigachalam, P.; Mallick, A.; Doddi, V. R.; Vankar, Y. D. Org. Biomol. Chem. 2011, 9, 809–819. doi:10.1039/C0OB00555J
Return to citation in text: [1] -
Lim, D.; Brimble, M. A.; Kowalczyk, R.; Watson, A. J. A.; Fairbanks, A. J. Angew. Chem., Int. Ed. 2014, 53, 11907–11911. doi:10.1002/anie.201406694
Return to citation in text: [1] -
Meldal, M.; Tornøe, C. W. Chem. Rev. 2008, 108, 2952–3015. doi:10.1021/cr0783479
Return to citation in text: [1] -
Korytnyk, W.; Mills, J. A. J. Chem. Soc. 1959, 636–649. doi:10.1039/JR9590000636
Return to citation in text: [1] -
Martichonok, V.; Whitesides, G. M. J. Org. Chem. 1996, 61, 1702–1706. doi:10.1021/jo951711w
Return to citation in text: [1] -
Baluja, G.; Chase, B. H.; Kenner, G. W.; Todd, A. J. Chem. Soc. 1960, 4678–4681. doi:10.1039/JR9600004678
Return to citation in text: [1] -
Nolte, R. J. M.; van Zomeren, J. A. J.; Zwikker, J. W. J. Org. Chem. 1978, 43, 1972–1975. doi:10.1021/jo00404a027
Return to citation in text: [1] -
Lee, D. J.; Mandal, K.; Harris, P. W. R.; Brimble, M. A.; Kent, S. B. H. Org. Lett. 2009, 11, 5270–5273. doi:10.1021/ol902131n
Return to citation in text: [1] -
Györgydeák, Z.; Thiem, J. Carbohydr. Res. 1995, 268, 85–92.
Return to citation in text: [1] -
Tropper, F. D.; Anderson, F. O.; Braun, S.; Roy, R. Synthesis 1992, 618–620. doi:10.1055/s-1992-26175
Return to citation in text: [1] -
Soli, E. D.; Manoso, A. S.; Patterson, M. C.; DeShong, P. J. Org. Chem. 1999, 64, 3171–3177. doi:10.1021/jo982302d
Return to citation in text: [1] -
Nepogodiev, S. A.; Dedola, S.; Marmuse, L.; de Oliveira, M. T.; Field, R. A. Carbohydr. Res. 2007, 342, 529–540. doi:10.1016/j.carres.2006.09.026
Return to citation in text: [1] -
Tosin, M.; Murphy, P. V. Org. Lett. 2002, 4, 3675–3678. doi:10.1021/ol026629j
Return to citation in text: [1] -
Paulsen, H. Adv. Carbohydr. Chem. Biochem. 1971, 26, 127.
Return to citation in text: [1] -
Györgydeák, Z.; Szilágyi, L.; Paulsen, H. J. Carbohydr. Chem. 1993, 12, 139–163. doi:10.1080/07328309308021266
Return to citation in text: [1] -
Marzag, H.; Alaoui, S.; Amdouni, H.; Martin, A. R.; Bougrin, K.; Benhida, R. New J. Chem. 2015, 39, 5437–5444. doi:10.1039/C5NJ00624D
Return to citation in text: [1] -
Lepage, M. L.; Bodlenner, A.; Compain, P. Eur. J. Org. Chem. 2013, 1963–1972. doi:10.1002/ejoc.201201580
Return to citation in text: [1] -
Tanaka, T.; Nagai, H.; Noguchi, M.; Kobayashia, A.; Shoda, S.-i. Chem. Commun. 2009, 3378–3379. doi:10.1039/B905761G
Return to citation in text: [1] -
Pilgrim, W.; Murphy, P. V. J. Org. Chem. 2010, 75, 6747–6755. doi:10.1021/jo101090f
Return to citation in text: [1] -
Salunke, S. B.; Babu, N. S.; Chen, C.-T. Chem. Commun. 2011, 47, 10440–10442. doi:10.1039/C1CC13370E
Return to citation in text: [1] -
Vangala, M.; Shinde, G. P. Beilstein J. Org. Chem. 2015, 11, 2289–2296. doi:10.3762/bjoc.11.249
Return to citation in text: [1] -
Vangala, M.; Shinde, G. P. Beilstein J. Org. Chem. 2016, 12, 2086–2092. doi:10.3762/bjoc.12.197
Return to citation in text: [1] -
Vangala, M.; Dhokale, S. A.; Gawade, R. L.; Pattuparambil, R. R.; Puranik, V. G.; Dhavale, D. D. Org. Biomol. Chem. 2013, 11, 6874–6878. doi:10.1039/C3OB41462K
Return to citation in text: [1] -
The NMR spectrum of purified and separated β isomer of fucosyl azide, compound 5 shows trace amounts of the other isomer as both compounds have nearly identical Rf values.
Return to citation in text: [1]
| 57. | The NMR spectrum of purified and separated β isomer of fucosyl azide, compound 5 shows trace amounts of the other isomer as both compounds have nearly identical Rf values. |
| 17. | Roy, R.; Rajasekaran, P.; Mallick, A.; Vankar, Y. D. Eur. J. Org. Chem. 2014, 5564–5573. doi:10.1002/ejoc.201402606 |
| 28. | Tomabechi, Y.; Squire, M. A.; Fairbanks, A. J. Org. Biomol. Chem. 2014, 12, 942–955. doi:10.1039/C3OB42104J |
| 29. | Bianchi, A.; Bernardi, A. J. Org. Chem. 2006, 71, 4565–4577. doi:10.1021/jo060409s |
| 7. | Hotha, S.; Kashyap, S. J. Am. Chem. Soc. 2006, 128, 9620–9621. doi:10.1021/ja062425c |
| 8. | Li, Y.; Yang, Y.; Yu, B. Tetrahedron Lett. 2008, 49, 3604–3608. doi:10.1016/j.tetlet.2008.04.017 |
| 9. | Mamidyala, S. K.; Finn, M. G. J. Org. Chem. 2009, 74, 8417–8420. doi:10.1021/jo901857x |
| 10. | Kayastha, A. K.; Hotha, S. Chem. Commun. 2012, 48, 7161–7163. doi:10.1039/C2CC32649C |
| 11. | Sureshkumar, G.; Hotha, S. Tetrahedron Lett. 2007, 48, 6564–6568. doi:10.1016/j.tetlet.2007.07.015 |
| 12. | Li, Y.; Yang, X.; Liu, Y.; Zhu, C.; Yang, Y.; Yu, B. Chem. – Eur. J. 2010, 16, 1871–1882. doi:10.1002/chem.200902548 |
| 13. | Tang, Y.; Li, J.; Zhu, Y.; Li, Y.; Yu, B. J. Am. Chem. Soc. 2013, 135, 18396–18405. doi:10.1021/ja4064316 |
| 14. | Zhu, Y.; Yu, B. Chem. – Eur. J. 2015, 21, 8771–8780. doi:10.1002/chem.201500648 |
| 15. | Adhikari, S.; Baryal, K. N.; Zhu, D.; Li, X.; Zhu, J. ACS Catal. 2013, 3, 57–60. doi:10.1021/cs300670k |
| 16. | Koppolu, S. R.; Niddana, R.; Balamurugan, R. Org. Biomol. Chem. 2015, 13, 5094–5097. doi:10.1039/C5OB00248F |
| 30. | Doores, K. J.; Mimura, Y.; Dwek, R. A.; Rudd, P. M.; Eliott, T.; Davis, B. G. Chem. Commun. 2006, 1401–1403. doi:10.1039/B515472C |
| 31. | Dedola, S.; Nepogodiev, S. A.; Field, R. A. Org. Biomol. Chem. 2007, 5, 1006–1017. doi:10.1039/B618048P |
| 32. | Kasuya, M. C. Z.; Wang, L. X.; Lee, Y. C.; Mitsuki, M.; Nakajima, H.; Miura, Y.; Sato, T.; Hatanaka, K.; Yamagata, S.; Yamagata, T. Carbohydr. Res. 2000, 329, 755–763. doi:10.1016/S0008-6215(00)00238-X |
| 3. | Hashmi, A. S. K.; Hutchings, G. J. Angew. Chem., Int. Ed. 2006, 45, 7896–7936. doi:10.1002/anie.200602454 |
| 4. | Fürstner, A.; Davies, P. W. Angew. Chem., Int. Ed. 2007, 46, 3410–3449. doi:10.1002/anie.200604335 |
| 5. | Hashmi, A. S. K. Chem. Rev. 2007, 107, 3180–3211. doi:10.1021/cr000436x |
| 6. | Morita, N.; Yasuda, A.; Shibata, M.; Ban, S.; Hashimoto, Y.; Okamoto, I.; Tamura, O. Org. Lett. 2015, 17, 2668–2671. doi:10.1021/acs.orglett.5b01046 |
| 26. | Kayastha, A. K.; Hotha, S. Beilstein J. Org. Chem. 2013, 9, 2147–2155. doi:10.3762/bjoc.9.252 |
| 2. | Dorel, R.; Echavarren, A. M. Chem. Rev. 2015, 115, 9028–9072. doi:10.1021/cr500691k |
| 17. | Roy, R.; Rajasekaran, P.; Mallick, A.; Vankar, Y. D. Eur. J. Org. Chem. 2014, 5564–5573. doi:10.1002/ejoc.201402606 |
| 27. | Mallick, A.; Mallikharjunarao, Y.; Rajasekaran, P.; Roy, R.; Vankar, Y. D. Eur. J. Org. Chem. 2016, 579–588. doi:10.1002/ejoc.201501245 |
| 21. | Peng, P.; Schmidt, R. R. J. Am. Chem. Soc. 2015, 137, 12653–12659. doi:10.1021/jacs.5b07895 |
| 22. | Roy, R.; Palanivel, A. K.; Mallick, A.; Vankar, Y. D. Eur. J. Org. Chem. 2015, 4000–4005. doi:10.1002/ejoc.201500137 |
| 24. | Rao, B. V.; Manmode, S.; Hotha, S. J. Org. Chem. 2015, 80, 1499–1505. doi:10.1021/jo502413z |
| 25. | Mishra, B.; Neralkar, M.; Hotha, S. Angew. Chem., Int. Ed. 2016, 55, 7786–7791. doi:10.1002/anie.201511695 |
| 20. | Vibhute, A. M.; Dhaka, A.; Athiyarath, V.; Sureshan, K. M. Chem. Sci. 2016, 7, 4259–4263. doi:10.1039/C6SC00633G |
| 17. | Roy, R.; Rajasekaran, P.; Mallick, A.; Vankar, Y. D. Eur. J. Org. Chem. 2014, 5564–5573. doi:10.1002/ejoc.201402606 |
| 19. | Vidadala, S. R.; Hotha, S. Chem. Commun. 2009, 2505–2507. doi:10.1039/B822526E |
| 18. | Götze, S.; Fitzner, R.; Kunz, H. Synlett 2009, 3346–3348. doi:10.1055/s-0029-1218356 |
| 23. | Zhang, Q.; Sun, J.; Zhu, Y.; Zhang, F.; Yu, B. Angew. Chem., Int. Ed. 2011, 50, 4933–4936. doi:10.1002/anie.201100514 |
| 37. | Korytnyk, W.; Mills, J. A. J. Chem. Soc. 1959, 636–649. doi:10.1039/JR9590000636 |
| 38. | Martichonok, V.; Whitesides, G. M. J. Org. Chem. 1996, 61, 1702–1706. doi:10.1021/jo951711w |
| 39. | Baluja, G.; Chase, B. H.; Kenner, G. W.; Todd, A. J. Chem. Soc. 1960, 4678–4681. doi:10.1039/JR9600004678 |
| 40. | Nolte, R. J. M.; van Zomeren, J. A. J.; Zwikker, J. W. J. Org. Chem. 1978, 43, 1972–1975. doi:10.1021/jo00404a027 |
| 33. | Pal, A. P. J.; Vankar, Y. D. Tetrahedron Lett. 2010, 51, 2519–2524. doi:10.1016/j.tetlet.2010.03.003 |
| 34. | Pal, A. P. J.; Kadigachalam, P.; Mallick, A.; Doddi, V. R.; Vankar, Y. D. Org. Biomol. Chem. 2011, 9, 809–819. doi:10.1039/C0OB00555J |
| 35. | Lim, D.; Brimble, M. A.; Kowalczyk, R.; Watson, A. J. A.; Fairbanks, A. J. Angew. Chem., Int. Ed. 2014, 53, 11907–11911. doi:10.1002/anie.201406694 |
| 36. | Meldal, M.; Tornøe, C. W. Chem. Rev. 2008, 108, 2952–3015. doi:10.1021/cr0783479 |
| 53. | Salunke, S. B.; Babu, N. S.; Chen, C.-T. Chem. Commun. 2011, 47, 10440–10442. doi:10.1039/C1CC13370E |
| 54. | Vangala, M.; Shinde, G. P. Beilstein J. Org. Chem. 2015, 11, 2289–2296. doi:10.3762/bjoc.11.249 |
| 55. | Vangala, M.; Shinde, G. P. Beilstein J. Org. Chem. 2016, 12, 2086–2092. doi:10.3762/bjoc.12.197 |
| 56. | Vangala, M.; Dhokale, S. A.; Gawade, R. L.; Pattuparambil, R. R.; Puranik, V. G.; Dhavale, D. D. Org. Biomol. Chem. 2013, 11, 6874–6878. doi:10.1039/C3OB41462K |
| 50. | Lepage, M. L.; Bodlenner, A.; Compain, P. Eur. J. Org. Chem. 2013, 1963–1972. doi:10.1002/ejoc.201201580 |
| 51. | Tanaka, T.; Nagai, H.; Noguchi, M.; Kobayashia, A.; Shoda, S.-i. Chem. Commun. 2009, 3378–3379. doi:10.1039/B905761G |
| 52. | Pilgrim, W.; Murphy, P. V. J. Org. Chem. 2010, 75, 6747–6755. doi:10.1021/jo101090f |
| 47. | Paulsen, H. Adv. Carbohydr. Chem. Biochem. 1971, 26, 127. |
| 48. | Györgydeák, Z.; Szilágyi, L.; Paulsen, H. J. Carbohydr. Chem. 1993, 12, 139–163. doi:10.1080/07328309308021266 |
| 49. | Marzag, H.; Alaoui, S.; Amdouni, H.; Martin, A. R.; Bougrin, K.; Benhida, R. New J. Chem. 2015, 39, 5437–5444. doi:10.1039/C5NJ00624D |
| 41. | Lee, D. J.; Mandal, K.; Harris, P. W. R.; Brimble, M. A.; Kent, S. B. H. Org. Lett. 2009, 11, 5270–5273. doi:10.1021/ol902131n |
| 42. | Györgydeák, Z.; Thiem, J. Carbohydr. Res. 1995, 268, 85–92. |
| 43. | Tropper, F. D.; Anderson, F. O.; Braun, S.; Roy, R. Synthesis 1992, 618–620. doi:10.1055/s-1992-26175 |
| 44. | Soli, E. D.; Manoso, A. S.; Patterson, M. C.; DeShong, P. J. Org. Chem. 1999, 64, 3171–3177. doi:10.1021/jo982302d |
| 45. | Nepogodiev, S. A.; Dedola, S.; Marmuse, L.; de Oliveira, M. T.; Field, R. A. Carbohydr. Res. 2007, 342, 529–540. doi:10.1016/j.carres.2006.09.026 |
| 46. | Tosin, M.; Murphy, P. V. Org. Lett. 2002, 4, 3675–3678. doi:10.1021/ol026629j |
© 2018 Rajput et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (https://www.beilstein-journals.org/bjoc)