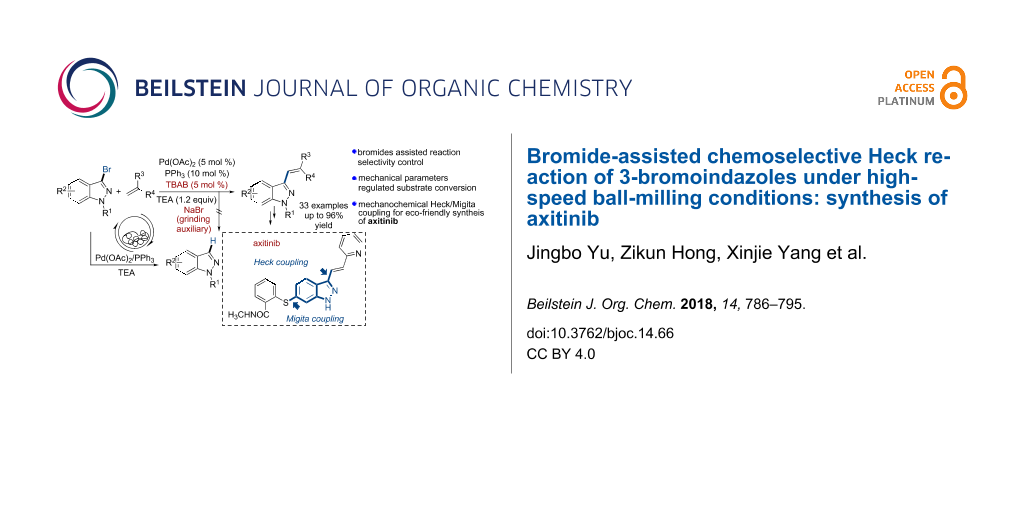
Abstract
A mechanically-activated chemoselective Heck coupling for the synthesis of 3-vinylindazoles has been developed with the aid of catalytic amounts of TBAB and NaBr as both dehalogenation restrainer and grinding auxiliary. After tuning of the chemical conditions and mechanical parameters, a series of non-activated 3-bromoindazoles and a broad scope of olefins worked well to give the corresponding coupling products in good to excellent yields. A further application of this protocol was performed in a two-step mechanochemical Heck/Migita cross coupling, which provided a highly efficient route for the synthesis of axitinib.
Graphical Abstract
Introduction
The palladium-catalyzed vinylation of alkenes in the presence of a base, known as the Heck reaction (Mizoroki–Heck reaction), is one of the most important transition-metal-catalyzed reactions [1,2], which has shown itself as a powerful synthetic tool in both academic and industrial practice [3-7]. The transformation has been enrolled as key steps for numerous synthetic routes, including the recent President Green Chemistry Award winner route of letermovir [8].
Hitherto, highly effective systems had been developed for the aryliodines that participated in Heck reactions with turn-over numbers of >1000 [9,10]. However, the couplings of bromo and chloro derivatives with unactivated alkenes still remain challenging. Though aryl bromides are always interesting substrates for industrial applications [11,12], possessing the characteristics of lower cost, easier to obtain and stable to store, they face the problem of dehalogenations especially under metal-catalyzed reactions [8,13-18], affecting the reaction yield and selectivity. Currently, the Heck reaction is usually carried out by adding an excess of phase-transfer catalyst such as tetrabutylammonium bromide (TBAB) or tetrabutylammonium iodide (TBAI) to increase the reaction yield under both solvent-heating [19-23] and solvent-free conditions [24-27]. Despite two proposals for the role of quaternary ammonium salts NR4+X−, (1) Pd(0) stablizer and (2) phase transfer were suggested [28-30], their effects and functions remain unrevealed. Hence, we want to get insight to the reaction pathway and the actual functions of NR4+X− for better inhibiting the dehalogenation of aryl bromides, wherever possible. Herein, 3-bromoindazoles were chosen as model substrates not only for their low activity and easy dehalogenation properties, but also for their potential applications in the synthesis of natural products and pharmaceuticals, such as gamendazole [31,32], YC-1 [33,34] and axitinib [35-38] (Scheme 1).
Scheme 1: Representative pharmaceutically useful indazoles.
Scheme 1: Representative pharmaceutically useful indazoles.
Mechanochemistry as a burgeoning technique to promote solvent-free reactions has led to remarkable advances [39-42], particularly for cross-coupling reactions [43-45], involving Heck coupling with the aid of stoichiometric amounts of TBAB [24-27]. However, for inert and liable to dehalogenation bromo-heteroarenes, no desired response had been obtained yet. Thus, this work was going to establish a mild and chemoselective olefination of 3-bromoindazoles under ball-milling conditions (Scheme 2).
Scheme 2: Model Heck reaction of 3-bromo-N-methyl-1H-indazole (1a) and n-butyl acrylate (2a). (173 stainless-steel balls (dMB = 6 mm, ФMB = 0.245) were used. dMB = milling ball diameter. ФMB = milling ball filling degree.)
Scheme 2: Model Heck reaction of 3-bromo-N-methyl-1H-indazole (1a) and n-butyl acrylate (2a). (173 stainless-...
Results and Discussion
Optimisation of chemical conditons
At the commencement of the investigation, the cross-coupling between 3-bromo-1-methyl-1H-indazole (1a) and n-butyl acrylate (2a) was chosen as model reaction (Scheme 2). The initial attempts using our previous established conditions [24,25], gave only moderate conversion with a low yield of 53%. Noticeably, considerable amounts of 1-methyl-1H-indazole (4a) were obtained, which implicated the existence of a significant debromination process. As expected, the situation was more badly when the reaction was conducted under classic solvent-heating conditions (see Scheme S1 in Supporting Information File 1). Subsequently, the optimization was carried out to improve the performance of the reaction. Palladium catalysts and ligands were firstly screened, which showed Pd(OAc)2/PPh3 as the most efficient catalyst system (Table 1, entry 5). The catalyst loading could be reduced to 5 mol % without depriving of the product yield (Table 1, entry 23). Other Pd catalysts and phosphorous ligands displayed little effects on the reaction selectivity and product yield (Table 1, entries 1–4 and entries 6–9). As expected, without any catalyst and ligand, the reaction cannot proceed (Table 1, entry 10). For the investigated bases, triethylamine (TEA) exhibited the best result though it was reported to donate hydrides to arylpalladium species and lead to dehalogenation [46]. While different from literature report [47], no improvement was found in the reaction selectivity when replacing TEA by 1,4-diazabicyclo[2.2.2]octane (DABCO) (Table 1, entry 11). Besides, other organic or inorganic bases gave poor results (Table 1, entries 12–18).
Table 1: Optimisation of the reaction conditions for the olefination of 3-bromoindazoles.a
|
|
||||
| Entry | Catalyst (mol %) | Ligand (mol %) | Base (equiv) | Yield (%) 3aa/4a |
| 1 | Pd(OAc)2 (10) | Xantphos (20) | TEA (2) | 43/24 |
| 2 | Pd(OAc)2 (10) | dppf (20) | TEA (2) | 44/21 |
| 3 | Pd(OAc)2 (10) | dppe (20) | TEA (2) | 45/19 |
| 4 | Pd(OAc)2 (10) | P(o-tol)3 (20) | TEA (2) | 32/22 |
| 5 | Pd(OAc)2 (10) | PPh3 (20) | TEA (2) | 53/18 |
| 6 | PdCl2 (10) | PPh3 (20) | TEA (2) | 47/18 |
| 7 | PdCl2(dppf) (10) | — | TEA (2) | 47/20 |
| 8 | Pd2(dba)3 (10) | — | TEA (2) | 37/17 |
| 9 | Pd(PPh3)4 (10) | — | TEA (2) | 48/18 |
| 10 | — | — | TEA (2) | 0/30 |
| 11 | Pd(OAc)2 (10) | PPh3 (20) | DABCO (2) | 51/18 |
| 12 | Pd(OAc)2 (10) | PPh3 (20) | DIPEA (2) | 35/23 |
| 13 | Pd(OAc)2 (10) | PPh3 (20) | DCHA (2) | 35/18 |
| 14 | Pd(OAc)2 (10) | PPh3 (20) | DBU (2) | 16/16 |
| 15 | Pd(OAc)2 (10) | PPh3 (20) | t-BuOK (2) | 40/21 |
| 16 | Pd(OAc)2 (10) | PPh3 (20) | K2CO3 (2) | 30/19 |
| 17 | Pd(OAc)2 (10) | PPh3 (20) | NaOH (2) | 30/24 |
| 18 | Pd(OAc)2 (10) | PPh3 (20) | NaHCO3 (2) | 21/16 |
| 19 | Pd(OAc)2 (10) | PPh3 (20) | TEA (1.5) | 53/19 |
| 20 | Pd(OAc)2 (10) | PPh3 (20) | TEA (1.2) | 53/19 |
| 21 | Pd(OAc)2 (10) | PPh3 (20) | TEA (1.0) | 45/23 |
| 22 | Pd(OAc)2 (20) | PPh3 (40) | TEA (1.2) | 54/20 |
| 23 | Pd(OAc)2 (5) | PPh3 (10) | TEA (1.2) | 53/19 |
| 24 | Pd(OAc)2 (2.5) | PPh3 (5) | TEA (1.2) | 36/24 |
aRaction conditions: 1a (1.5 mmol), 2a (2.25 mmol), Pd catalyst, ligand, base, TBAB (3 mmol), and silica gel (5.0 g) were placed in an 80 mL stainless steel vessel along with 173 stainless-steel balls (dMB = 6 mm, ФMB = 0.245), milling at 800 rpm for 90 min. TEA = triethylamine. DABCO = 1,4-diazabicyclo[2.2.2]octane. DIPEA = N,N-diisopropylethylamine. DCHA = dicyclohexylamine. DBU = 1,8-diazabicyclo[5.4.0]undec-7-ene.
Next, the additives were investigated in the coupling reactions (Figure 1, see detailed results in Supporting Information File 1, Table S1). Consistent to previous reports [3,24-27,48-52], TBAB helped to improve the product yield in this reaction, since without additive under the standard conditions, only 14% of target product 3aa was obtained along with 38% of the dehalogenation counterpart 4a (Table S1, entry 2). However, TBAI or tetrabutylammonium chloride (TBAC) did not provide a satisfactory result (Table S1, entries 3 and 4). It was interesting to find that using bromide salts (LiBr, NaBr, KBr and TBAB) as additives, not only the conversion rate of 1a was increased, but also the side-product 4a was suppressed, suggesting the bromide ion plays an important role in ameliorating the reaction selectivity (Table S1, entries 9–11). Further investigating the alkyl chain (R) of NR4+Br− showed that the medium-length butyl was most efficient (Table S1, entries 5–7). In order to see whether the quaternary ammonium salt had the function of phase-transfer, other kinds of phase-transfer catalysts (SDS = sodium dodecyl sulfate) were also examined, however, this gave rise to a negative effect (Table S1, entry 8). To our delight, when we replaced the grinding auxiliary by sodium bromide, a moderate product yield (69%) and an excellent selectivity (trace of 4a) were achieved (Table S1, entry 12). In this way, the amount of TBAB could even be reduced to 5 mol % (Table S1, entry 14).
![[1860-5397-14-66-1]](/bjoc/content/figures/1860-5397-14-66-1.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 1: Investigation of additives in the Heck reaction: 1a (1.5 mmol), 2a (2.25 mmol), Pd(OAc)2 (5 mol %), PPh3 (10 mol %), TEA (1.8 mmol), additive (3.0 mmol), and silica gel (5.0 g) were placed in an 80 mL stainless steel vessel along with 173 stainless-steel balls (dMB = 6 mm, ФMB = 0.245), milling at 800 rpm for 90 min.
Figure 1: Investigation of additives in the Heck reaction: 1a (1.5 mmol), 2a (2.25 mmol), Pd(OAc)2 (5 mol %),...
Influence of grinding auxiliary
In the process of ball milling, the grinding auxiliary was always found to be an efficient transfer medium between energy and reactant [53-55]. Thus, the effect of the grinding auxiliaries was also investigated. The results were shown in Table 2. Compared to using silica gel as grinding auxiliary, the reaction under neat conditions gave only 10% yield of 3aa, but the dehalogenation was depressed (Table 2, entry 1), which showed silica gel played an important role of promoting the dehalogenations. When using a halogen salt (NaBr, KBr, NaCl) as grinding auxiliary, NaBr displayed the most efficiency (Table 2, entries 3–6), and lowering the amount of NaBr did not significantly influence the product yield (Table 2, entry 4). Other solid auxiliaries such as γ-Al2O3 and sand afforded a low yield of 3aa with poor selectivity (Table 2, entries 7 and 8).
Table 2: Examination of the influence of the grinding auxiliary on the reaction outcomea.
| Entry | Grinding auxiliary | Weight (g) | Yield (%) 3aa/4a |
|---|---|---|---|
| 1 | — | — | 10/trace |
| 2 | silica gel | 5.0 | 53/19 |
| 3 | NaBr | 10.0 | 69/trace |
| 4 | NaBr | 5.0 | 65/trace |
| 5 | KBr | 5.0 | 63/trace |
| 6 | NaCl | 5.0 | 27/5 |
| 7 | γ-Al2O3 (neutral) | 5.0 | 20/6 |
| 8 | sand | 5.0 | 30/10 |
aInfluence of the grinding auxiliary on the Heck reaction: 1a (1.5 mmol), 2a (2.25 mmol), Pd(OAc)2 (5 mol %), PPh3 (10 mol %), TEA (1.8 mmol), TBAB (5 mol %), and grinding auxiliary were placed in an 80 mL stainless steel vessel along with 173 stainless-steel balls (dMB = 6 mm, ФMB = 0.245), milling at 800 rpm for 90 min.
Reaction pathway investigation
The control experiments were further conducted to disclose the reaction pathway (Scheme 3). In the case of taking TEA as sole reagent in the reaction, the substrate 1a was converted to 4a in 27% yield (conditions a), while the dehalogenation was exacerbated in the presence of Pd(OAc)2/PPh3 (conditions b). This phenomenon was in agreement with previous reports [14-16], that Pd-catalyzed homocoupling of aryl halides under alkaline heating conditions was often accompanied by dehalogenation as side reaction. When using NaBr as a grinding auxiliary instead of silica gel, the dehalogenation of 1a was greatly inhibited (conditions c), but still gave 4a in 30% without the presence of olefins.
Scheme 3: The control experiments. aTEA (1.8 mmol), silica gel (5.0 g), bPd(OAc)2 (5 mol %), PPh3 (10 mol %), TEA (1.8 mmol), silica gel (5.0 g), cPd(OAc)2 (5 mol %), PPh3 (10 mol %), TEA (1.8 mmol), NaBr (10.0 g).
Scheme 3: The control experiments. aTEA (1.8 mmol), silica gel (5.0 g), bPd(OAc)2 (5 mol %), PPh3 (10 mol %),...
Based on the above research, the reaction pathway for this Heck reaction under mechanical ball milling conditions was proposed. As shown in path a, (Scheme 4). The reaction proceeded through Heck cross coupling, where a catalytic amount of TBAB was enough to stabilize the Pd(0). However, the oxidative addition intermediate I was unstable so that it was prone to produce 4a, as depicted in path b, particularly in the absence of the coupling counterpart 2. Besides, alkaline and high energy input conditions promoted the dehalogenation as shown in path c, while the addition of bromine salts helped diminishing this effect dramatically [56].
Adjusting of mechanical parameters
Having identified the optimal chemical conditions for the reaction selectivity on the basis of the reaction pathway, we then focussed on the milling parameters such as rotation speed (νrot), ball milling time (t), milling ball filling degree (ФMB), and milling ball diameter (dMB), which usually play important roles in mechanochemistry processes [57-60]. First, the combined effect between ball-milling time and rotation speed was screened systematically (Figure 2). The results show a sharp increase of the product yield when elevating the rotation speed from 600 to 800 rpm, and a progressive increase of the yield by prolonging the reaction time from 60 to 90 min. Further increasing the rotation speed or prolonging the reaction time did not help to improve the product yield but promoted the occurrence of 4a (see Table S2 in Supporting Information File 1), which mainly due to the redundant energy input spurred the dehalogenation.
![[1860-5397-14-66-2]](/bjoc/content/figures/1860-5397-14-66-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: Influence of milling time and rotation speed on the Heck reaction: 1a (1.5 mmol), 2a (2.25 mmol), Pd(OAc)2 (5 mol %), PPh3 (10 mol %), TEA (1.8 mmol), TBAB (5 mol %), and NaBr (10.0 g) were placed in 80 mL stainless steel vessel with stainless-steel balls (dMB = 6 mm, ФMB = 0.245).
Figure 2: Influence of milling time and rotation speed on the Heck reaction: 1a (1.5 mmol), 2a (2.25 mmol), P...
Next, the combination of filling degree (ФMB) and size of the milling balls (dMB) was investigated for further improving the product yield. As seen in Figure 3, the yield of 3aa elevated sharply as the filling degree increased to around 0.3–0.35 for all types of the milling balls, and then decreased gradually due to milling vessel space limitations [24,61,62]. A maximum product yield (93%) was obtained by using 6 mm diameter milling balls (ФMB = 0.293), indicating small-size milling balls more beneficial for the reaction, which was in accordance with the previous studies reported by us [24] and others [57,63,64].
![[1860-5397-14-66-3]](/bjoc/content/figures/1860-5397-14-66-3.png?scale=1.6&max-width=1024&background=FFFFFF)
Figure 3: Influence of the milling ball filling degree with different size on the Heck reaction: 1a (1.5 mmol), 2a (2.25 mmol), Pd(OAc)2 (5 mol %), PPh3 (10 mol %), TEA (1.8 mmol), TBAB (5 mol %), and NaBr (10.0 g) were placed in 80 mL stainless steel vessel milling, milling at 800 rpm for 90 min.
Figure 3: Influence of the milling ball filling degree with different size on the Heck reaction: 1a (1.5 mmol...
Substrate scopes
After a comprehensive study of the reaction pathway and the reaction conditions, an excellent product yield (93%) and selectivity (trace of 4a) can be achieved. We then turned our efforts toward the investigation of the scope and limitations of the developed method with respect to a broad range of indazoles and olefins (Scheme 5). Pleasingly, neutral, electron-rich and electron-poor indazoles were perfectly tolerated in this reaction, affording the corresponding target product 3ba–ka in high yields (88–96%) and excellent selectivity (the dehalogenated side-product 4 was not detected in most cases). Among which, strong electron-withdrawing substrates required long reaction times to achieve the desired results (3ca, 3ga, 3pn). It was suprising to find that substrate 1d showed excellent site-selectivity under 700 rpm, giving 6-bromo-substituted product 3da in 94% yield. Indazoles with N-Me, THP and Bn groups afforded good to excellent yields in the coupling reaction with n-butyl acrylate. However, the N-Boc substrate readily underwent removal of the protecting group [65], and resulted in the coupling product 3oa (70%). Besides, N-unsubstituted indazole 1n could also be tolerated in this reaction giving 3na (52%) and 3nf (49%) in moderate yield by using 1,8-bis(dimethylamino)naphthalene as a base. To our delight, 3-chloroindazole could also be activated in this reaction, giving the corresponding coupling products 3aa and 3af in considerable yields. The scope of the reaction with respect to the olefins was also extensively investigated and encompasses acrylates 2a, acrylamides 2b and 2c, styrenes 2f–m and 2-vinylpyridine (2n). In addition, even non-activated allylbenzene 2o and disubstituted olefins 2d and 2e could participate in the reaction to deliver 3ao (67%), 3ad (26%) and 3ae (27%). Finally, the steric hindrance of styrene was examined. Larger steric hindrance (2m) led to lower yield (58%) as compared with 2k (89%) and 2l (88%).
Scheme 5: Examination of the substrate scope. Reaction conditions: 1 (1.5 mmol), 2 (2.25 mmol), Pd(OAc)2 (5 mol %), PPh3 (10 mol %), TEA (1.8 mmol), TBAB (5 mol %), and NaBr (10.0 g) were placed in an 80 mL stainless steel vessel along with 207 stainless-steel balls (dMB = 6 mm, ФMB = 0.293), milling at 800 rpm for 90 min. aN-Boc-3-bromoindazole was used as substrate. b150 min. c700 rpm. d3-chloro-1-methyl-1H-indazole was used as substrate, νrot = 900 rpm. e1,8-Bis(dimethylamino)naphthalene was used as base.
Scheme 5: Examination of the substrate scope. Reaction conditions: 1 (1.5 mmol), 2 (2.25 mmol), Pd(OAc)2 (5 m...
Application in API synthesis
To demonstrate the broad utility of this method, a concise synthesis of axitinib, eutherapeutic drug for the treatment of renal cell carcinoma, was undertaken (Scheme 6). The reaction started from commercially available 6-bromo-1H-indazole (5), bromination of the 3-position and N-protection gave 3,6-dibromo-1-(tetrahydro-2H-pyran-2-yl)-1H-indazole (3q) in 90% yield. Next, sequential two-step Heck coupling and Migita coupling under ball milling conditions selectively afforded THP-axitinib 7. Finally, deprotection of 7 with p-TsOH gave axitinib in a total yield of 44% (41% [66]). Comparing to previous synthetic procedures [35,36], this mechanochemical protocol provided a solvent-free, highly efficient and tractable alternative, and the residual Pd content in the axitinib was determined to be no more than 2 ppm by ICP analysis.
Scheme 6: Synthesis of axitinib by mechanochemical Heck–Migita coupling. Reagents and conditions: (i) NBS, NaOH, silica gel (4.0 g), 173 stainless-steel balls (dMB = 6 mm, ФMB = 0.245), 200 rpm, 30 min; (ii) CH3SO3H, dihydropyran, silica gel (4.0 g), 173 stainless-steel balls (dMB = 6 mm, ФMB = 0.245), 200 rpm, 45 min; (iii) compound 2n, Pd(OAc)2, PPh3, TBAB, TEA, NaBr (10.0 g), 207 stainless-steel balls (dMB = 6 mm, ФMB = 0.293), 700 rpm, 90 min; (iv) compound 6, Pd2(dba)3, Xantphos, Cs2CO3, silica gel (4.0 g), 207 stainless-steel balls (dMB = 6 mm, ФMB = 0.293), 750 rpm, 50 min; (v) p-TsOH, silica gel (4.0 g), 207 stainless-steel balls (dMB = 6 mm, ФMB = 0.293), 500 rpm, 45 min.
Scheme 6: Synthesis of axitinib by mechanochemical Heck–Migita coupling. Reagents and conditions: (i) NBS, Na...
Conclusion
In conclusion, a solvent-free, chemoselective Heck cross-coupling for the synthesis of 3-vinylindazoles has been developed by sophisticated tuning of the chemical and mechanical parameters under ball-milling conditions. The reaction pathway was comprehensively studied, revealing the bromide salts to play a dual role by not only suppressing the dehalogenation of 3-bromoindazoles but also assisting grinding, while a catalytic amount of TBAB was sufficient to stabilize Pd(0) and to promote the cross coupling. A series of non-activated indazoles and a broad scope of olefins were tolerated in the reaction giving high yields and excellent selectivity. Further application of this protocol was conducted in a total mechanosynthesis of axitinib in short reaction time and high efficiency. With this system, we hope to expand the pharmaceutical synthetic toolbox in mechanochemistry.
Supporting Information
| Supporting Information File 1: Reaction optimization studies, details of experimental procedures, characterization and copies of 1H and 13C NMR spectra of prepared compounds. | ||
| Format: PDF | Size: 3.7 MB | Download |
References
-
Heck, R. F.; Nolley, J. P. J. Org. Chem. 1972, 37, 2320–2322. doi:10.1021/jo00979a024
Return to citation in text: [1] -
Mizoroki, T.; Mori, K.; Ozaki, A. Bull. Chem. Soc. Jpn. 1971, 44, 581. doi:10.1246/bcsj.44.581
Return to citation in text: [1] -
Mc Cartney, D.; Guiry, P. J. Chem. Soc. Rev. 2011, 40, 5122–5150. doi:10.1039/c1cs15101k
Return to citation in text: [1] [2] -
Beletskaya, I. P.; Cheprakov, A. V. Chem. Rev. 2000, 100, 3009–3066. doi:10.1021/cr9903048
Return to citation in text: [1] -
Lucks, S.; Brunner, H. Org. Process Res. Dev. 2017, 21, 1835–1842. doi:10.1021/acs.oprd.7b00279
Return to citation in text: [1] -
Jensen, R. K.; Thykier, N.; Enevoldsen, M. V.; Lindhardt, A. T. Org. Process Res. Dev. 2017, 21, 370–376. doi:10.1021/acs.oprd.6b00441
Return to citation in text: [1] -
Hansen, M. M.; Kallman, N. J.; Koenig, T. M.; Linder, R. J.; Richey, R. N.; Rizzo, J. R.; Ward, J. A.; Yu, H.; Zhang, T. Y.; Mitchell, D. Org. Process Res. Dev. 2017, 21, 208–217. doi:10.1021/acs.oprd.6b00368
Return to citation in text: [1] -
Humphrey, G. R.; Dalby, S. M.; Andreani, T.; Xiang, B.; Luzung, M. R.; Song, Z. J.; Shevlin, M.; Christensen, M.; Belyk, K. M.; Tschaen, D. M. Org. Process Res. Dev. 2016, 20, 1097–1103. doi:10.1021/acs.oprd.6b00076
Return to citation in text: [1] [2] -
Bangar, P. G.; Jawalkar, P. R.; Dumbre, S. R.; Patil, D. J.; Iyer, S. Appl. Organomet. Chem. 2018, 32, e4159. doi:10.1002/aoc.4159
Return to citation in text: [1] -
Beletskaya, I. P.; Chuchuryukin, A. V.; van Koten, G.; Dijkstra, H. P.; van Klink, G. P. M.; Kashin, A. N.; Nefedov, S. E.; Eremenko, I. L. Russ. J. Org. Chem. 2003, 39, 1268–1281. doi:10.1023/B:RUJO.0000010214.72250.5a
Return to citation in text: [1] -
Zapf, A.; Beller, M. Top. Catal. 2002, 19, 101–109. doi:10.1023/A:1013889401432
Return to citation in text: [1] -
Bader, R. R.; Baumeister, P.; Blaser, H. U. Chimia 1996, 50, 99–105.
Return to citation in text: [1] -
Cyr, P.; Deng, S. T.; Hawkins, J. M.; Price, K. E. Org. Lett. 2013, 15, 4342–4345. doi:10.1021/ol4018134
Return to citation in text: [1] -
Cunha, S.; Oliveira, C. C.; Sabino, J. R. J. Braz. Chem. Soc. 2011, 22, 598–603. doi:10.1590/S0103-50532011000300026
Return to citation in text: [1] [2] -
Zawisza, A. M.; Muzart, J. Tetrahedron Lett. 2007, 48, 6738–6742. doi:10.1016/j.tetlet.2007.07.077
Return to citation in text: [1] [2] -
Chen, J.; Zhang, Y.; Yang, L.; Zhang, X.; Liu, J.; Li, L.; Zhang, H. Tetrahedron 2007, 63, 4266–4270. doi:10.1016/j.tet.2007.03.061
Return to citation in text: [1] [2] -
Nakao, R.; Rhee, H.; Uozumi, Y. Org. Lett. 2005, 7, 163–165. doi:10.1021/ol047670k
Return to citation in text: [1] -
Jedinák, L.; Zátopková, R.; Zemánková, H.; Šustková, A.; Cankař, P. J. Org. Chem. 2017, 82, 157–169. doi:10.1021/acs.joc.6b02306
Return to citation in text: [1] -
Zhang, L.; Jiang, Z.; Dong, C.; Xue, X.; Qiu, R.; Tang, W.; Li, H.; Xiao, J.; Xu, L. ChemCatChem 2014, 6, 311–318. doi:10.1002/cctc.201300755
Return to citation in text: [1] -
Ataei, A.; Nadri, S.; Rafiee, E.; Jamali, S.; Joshaghani, M. J. Mol. Catal. A: Chem. 2013, 366, 30–35. doi:10.1016/j.molcata.2012.08.025
Return to citation in text: [1] -
Calò, V.; Nacci, A.; Monopoli, A. J. Mol. Catal. A: Chem. 2004, 214, 45–56. doi:10.1016/j.molcata.2003.12.028
Return to citation in text: [1] -
Handy, S. T.; Okello, M. Tetrahedron Lett. 2003, 44, 8395–8397. doi:10.1016/j.tetlet.2003.09.120
Return to citation in text: [1] -
Mo, J.; Xiao, J. Angew. Chem., Int. Ed. 2006, 45, 4152–4157. doi:10.1002/anie.200600799
Return to citation in text: [1] -
Shi, W.; Yu, J.; Jiang, Z.; Shao, Q.; Su, W. Beilstein J. Org. Chem. 2017, 13, 1661–1668. doi:10.3762/bjoc.13.160
Return to citation in text: [1] [2] [3] [4] [5] [6] -
Zhu, X.; Liu, J.; Chen, T.; Su, W. Appl. Organomet. Chem. 2012, 26, 145–147. doi:10.1002/aoc.2827
Return to citation in text: [1] [2] [3] [4] -
Tullberg, E.; Schacher, F.; Peters, D.; Frejd, T. Synthesis 2006, 1183–1189. doi:10.1055/s-2006-926371
Return to citation in text: [1] [2] [3] -
Tullberg, E.; Peters, D.; Frejd, T. J. Organomet. Chem. 2004, 689, 3778–3781. doi:10.1016/j.jorganchem.2004.06.045
Return to citation in text: [1] [2] [3] -
Jeffery, T. J. Chem. Soc., Chem. Commun. 1984, 1287–1289. doi:10.1039/C39840001287
Return to citation in text: [1] -
Grasa, G. A.; Singh, R.; Stevens, E. D.; Nolan, S. P. J. Organomet. Chem. 2003, 687, 269–279. doi:10.1016/S0022-328X(03)00375-9
Return to citation in text: [1] -
Reetz, M. T.; Westermann, E. Angew. Chem., Int. Ed. 2000, 39, 165–168. doi:10.1002/(SICI)1521-3773(20000103)39:1<165::AID-ANIE165>3.0.CO;2-B
Return to citation in text: [1] -
Veerareddy, A.; Surendrareddy, G.; Dubey, P. K. Synth. Commun. 2013, 43, 2236–2241. doi:10.1080/00397911.2012.696306
Return to citation in text: [1] -
Tash, J. S.; Chakrasali, R.; Jakkaraj, S. R.; Hughes, J.; Smith, S. K.; Hornbaker, K.; Heckert, L. L.; Ozturk, S. B.; Hadden, M. K.; Kinzy, T. G.; Blagg, B. S. J.; Georg, G. I. Biol. Reprod. 2008, 78, 1139–1152. doi:10.1095/biolreprod.107.062679
Return to citation in text: [1] -
Xiao, J.; Jin, C.; Liu, Z.; Guo, S.; Zhang, X.; Zhou, X.; Wu, X. Org. Biomol. Chem. 2015, 13, 7257–7264. doi:10.1039/C5OB00710K
Return to citation in text: [1] -
Takeuchi, A.; Hori, M.; Sato, S.; Ban, H. S.; Kuchimaru, T.; Kizaka-Kondoh, S.; Yamori, T.; Nakamura, H. M. Chem. Commun. 2012, 3, 1455–1461. doi:10.1039/c2md20134h
Return to citation in text: [1] -
Zhai, L.-H.; Guo, L.-H.; Luo, Y.-H.; Ling, Y.; Sun, B.-W. Org. Process Res. Dev. 2015, 19, 849–857. doi:10.1021/acs.oprd.5b00123
Return to citation in text: [1] [2] -
Chekal, B. P.; Guinness, S. M.; Lillie, B. M.; McLaughlin, R. W.; Palmer, C. W.; Post, R. J.; Sieser, J. E.; Singer, R. A.; Sluggett, G. W.; Vaidyanathan, R.; Withbroe, G. J. Org. Process Res. Dev. 2014, 18, 266–274. doi:10.1021/op400088k
Return to citation in text: [1] [2] -
Laufer, R.; Forrest, B.; Li, S.-W.; Liu, Y.; Sampson, P.; Edwards, L.; Lang, Y.; Awrey, D. E.; Mao, G.; Plotnikova, O.; Leung, G.; Hodgson, R.; Beletskaya, I.; Mason, J. M.; Luo, X.; Wei, X.; Yao, Y.; Feher, M.; Ban, F.; Kiarash, R.; Green, E.; Mak, T. W.; Pan, G.; Pauls, H. W. J. Med. Chem. 2013, 56, 6069–6087. doi:10.1021/jm400380m
Return to citation in text: [1] -
Shen, C.; Shen, H.; Yang, M.; Xia, C.; Zhang, P. Green Chem. 2015, 17, 225–230. doi:10.1039/C4GC01606H
Return to citation in text: [1] -
Hernández, J. G.; Bolm, C. J. Org. Chem. 2017, 82, 4007–4019. doi:10.1021/acs.joc.6b02887
Return to citation in text: [1] -
Do, J.-L.; Friščić, T. ACS Cent. Sci. 2017, 3, 13–19. doi:10.1021/acscentsci.6b00277
Return to citation in text: [1] -
Achar, T. K.; Bose, A.; Mal, P. Beilstein J. Org. Chem. 2017, 13, 1907–1931. doi:10.3762/bjoc.13.186
Return to citation in text: [1] -
Wang, G.-W. Chem. Soc. Rev. 2013, 42, 7668–7700. doi:10.1039/c3cs35526h
Return to citation in text: [1] -
Hernández, J. G. Chem. – Eur. J. 2017, 23, 17157–17165. doi:10.1002/chem.201703605
Return to citation in text: [1] -
Jacob, K.; Schmidt, R.; Stolle, A. Carbon–Carbon Bond Forming by Ball Milling. In Ball Mills Ball Milling Towards Green Synthesis: Applications, Projects, Challenges; Stolle, A.; Ranu, B. C., Eds.; Royal Society of Chemistry: Cambridge, U.K., 2015; pp 34–57.
Return to citation in text: [1] -
Declerck, V.; Colacino, E.; Bantreil, X.; Martinez, J.; Lamaty, F. Chem. Commun. 2012, 48, 11778–11780. doi:10.1039/c2cc36286d
Return to citation in text: [1] -
Saa, J. M.; Dopico, M.; Martorell, G.; Garcia-Raso, A. J. Org. Chem. 1990, 55, 991–995. doi:10.1021/jo00290a033
Return to citation in text: [1] -
Qin, L.; Hirao, H.; Zhou, J. Chem. Commun. 2013, 49, 10236–10238. doi:10.1039/c3cc45911j
Return to citation in text: [1] -
Hattori, T.; Ueda, S.; Takakura, R.; Sawama, Y.; Monguchi, Y.; Sajiki, H. Chem. – Eur. J. 2017, 23, 8196–8202. doi:10.1002/chem.201606048
Return to citation in text: [1] -
Luong, T. T. H.; Touchet, S.; Alami, M.; Messaoudi, S. Adv. Synth. Catal. 2017, 359, 1320–1330. doi:10.1002/adsc.201601382
Return to citation in text: [1] -
Nishikata, T.; Noda, Y.; Fujimoto, R.; Sakashita, T. J. Am. Chem. Soc. 2013, 135, 16372–16375. doi:10.1021/ja409661n
Return to citation in text: [1] -
Tang, B.-X.; Fang, X.-N.; Kuang, R.-Y.; Hu, R.-H.; Wang, J.-W.; Li, P.; Li, X.-h. Synthesis 2013, 45, 2971–2976. doi:10.1055/s-0033-1339650
Return to citation in text: [1] -
Caló, V.; Nacci, A.; Monopoli, A.; Laera, S.; Cioffi, N. J. Org. Chem. 2003, 68, 2929–2933. doi:10.1021/jo026877t
Return to citation in text: [1] -
Stolle, A.; Szuppa, T.; Leonhardt, S. E. S.; Ondruschka, B. Chem. Soc. Rev. 2011, 40, 2317–2329. doi:10.1039/c0cs00195c
Return to citation in text: [1] -
Hermann, G. N.; Becker, P.; Bolm, C. Angew. Chem., Int. Ed. 2015, 54, 7414–7417. doi:10.1002/anie.201502536
Return to citation in text: [1] -
Hernández, J. G.; Turberg, M.; Schiffers, I.; Bolm, C. Chem. – Eur. J. 2016, 22, 14513–14517. doi:10.1002/chem.201603057
Return to citation in text: [1] -
When the reaction was performed under solvent-heating conditions: 1a (1.5 mmol), 2a (2.25 mmol), Pd(OAc)2 (0.075 mmol), PPh3 (0.15 mmol), TEA (1.8 mmol), TBAB (0.075 mmol) and NaBr (3.0 mmol) in DMF (4 mL) at 100 °C for 10 h, the reaction selectivity could also be increased (3aa/4a = 97:3), but with lower yield (86%) of 3aa.
Return to citation in text: [1] -
Schmidt, R.; Burmeister, C. F.; Baláž, M.; Kwade, A.; Stolle, A. Org. Process Res. Dev. 2015, 19, 427–436. doi:10.1021/op5003787
Return to citation in text: [1] [2] -
Paveglio, G. C.; Longhi, K.; Moreira, D. N.; München, T. S.; Tier, A. Z.; Gindri, I. M.; Bender, C. R.; Frizzo, C. P.; Zanatta, N.; Bonacorso, H. G.; Martins, M. A. P. ACS Sustainable Chem. Eng. 2014, 2, 1895–1901. doi:10.1021/sc5002353
Return to citation in text: [1] -
Jicsinszky, L.; Tuza, K.; Cravotto, G.; Porcheddu, A.; Delogu, F.; Colacino, E. Beilstein J. Org. Chem. 2017, 13, 1893–1899. doi:10.3762/bjoc.13.184
Return to citation in text: [1] -
Julien, P. A.; Malvestiti, I.; Friščić, T. Beilstein J. Org. Chem. 2017, 13, 2160–2168. doi:10.3762/bjoc.13.216
Return to citation in text: [1] -
Yu, J.-B.; Peng, G.; Jiang, Z.-J.; Hong, Z.-K.; Su, W.-K. Eur. J. Org. Chem. 2016, 5340–5344. doi:10.1002/ejoc.201600987
Return to citation in text: [1] -
Yu, J.-B.; Zhang, Y.; Jiang, Z.-J.; Su, W.-K. J. Org. Chem. 2016, 81, 11514–11520. doi:10.1021/acs.joc.6b02197
Return to citation in text: [1] -
Stolle, A.; Schmidt, R.; Jacob, K. Faraday Discuss. 2014, 170, 267–286. doi:10.1039/C3FD00144J
Return to citation in text: [1] -
Stolle, A. Technical Implications of Organic Syntheses in Ball Mills. In Ball Mills Ball Milling Towards Green Synthesis: Applications, Projects, Challenges; Stolle, A.; Ranu, B. C., Eds.; Royal Society of Chemistry: Cambridge, U.K., 2015; pp 241–276.
Return to citation in text: [1] -
Konnert, L.; Gauliard, A.; Lamaty, F.; Martinez, J.; Colacino, E. ACS Sustainable Chem. Eng. 2013, 1, 1186–1191. doi:10.1021/sc4001115
Return to citation in text: [1] -
The results of scaling-up experiment.
Return to citation in text: [1]
| 35. | Zhai, L.-H.; Guo, L.-H.; Luo, Y.-H.; Ling, Y.; Sun, B.-W. Org. Process Res. Dev. 2015, 19, 849–857. doi:10.1021/acs.oprd.5b00123 |
| 36. | Chekal, B. P.; Guinness, S. M.; Lillie, B. M.; McLaughlin, R. W.; Palmer, C. W.; Post, R. J.; Sieser, J. E.; Singer, R. A.; Sluggett, G. W.; Vaidyanathan, R.; Withbroe, G. J. Org. Process Res. Dev. 2014, 18, 266–274. doi:10.1021/op400088k |
| 1. | Heck, R. F.; Nolley, J. P. J. Org. Chem. 1972, 37, 2320–2322. doi:10.1021/jo00979a024 |
| 2. | Mizoroki, T.; Mori, K.; Ozaki, A. Bull. Chem. Soc. Jpn. 1971, 44, 581. doi:10.1246/bcsj.44.581 |
| 11. | Zapf, A.; Beller, M. Top. Catal. 2002, 19, 101–109. doi:10.1023/A:1013889401432 |
| 12. | Bader, R. R.; Baumeister, P.; Blaser, H. U. Chimia 1996, 50, 99–105. |
| 24. | Shi, W.; Yu, J.; Jiang, Z.; Shao, Q.; Su, W. Beilstein J. Org. Chem. 2017, 13, 1661–1668. doi:10.3762/bjoc.13.160 |
| 25. | Zhu, X.; Liu, J.; Chen, T.; Su, W. Appl. Organomet. Chem. 2012, 26, 145–147. doi:10.1002/aoc.2827 |
| 26. | Tullberg, E.; Schacher, F.; Peters, D.; Frejd, T. Synthesis 2006, 1183–1189. doi:10.1055/s-2006-926371 |
| 27. | Tullberg, E.; Peters, D.; Frejd, T. J. Organomet. Chem. 2004, 689, 3778–3781. doi:10.1016/j.jorganchem.2004.06.045 |
| 9. | Bangar, P. G.; Jawalkar, P. R.; Dumbre, S. R.; Patil, D. J.; Iyer, S. Appl. Organomet. Chem. 2018, 32, e4159. doi:10.1002/aoc.4159 |
| 10. | Beletskaya, I. P.; Chuchuryukin, A. V.; van Koten, G.; Dijkstra, H. P.; van Klink, G. P. M.; Kashin, A. N.; Nefedov, S. E.; Eremenko, I. L. Russ. J. Org. Chem. 2003, 39, 1268–1281. doi:10.1023/B:RUJO.0000010214.72250.5a |
| 24. | Shi, W.; Yu, J.; Jiang, Z.; Shao, Q.; Su, W. Beilstein J. Org. Chem. 2017, 13, 1661–1668. doi:10.3762/bjoc.13.160 |
| 25. | Zhu, X.; Liu, J.; Chen, T.; Su, W. Appl. Organomet. Chem. 2012, 26, 145–147. doi:10.1002/aoc.2827 |
| 8. | Humphrey, G. R.; Dalby, S. M.; Andreani, T.; Xiang, B.; Luzung, M. R.; Song, Z. J.; Shevlin, M.; Christensen, M.; Belyk, K. M.; Tschaen, D. M. Org. Process Res. Dev. 2016, 20, 1097–1103. doi:10.1021/acs.oprd.6b00076 |
| 39. | Hernández, J. G.; Bolm, C. J. Org. Chem. 2017, 82, 4007–4019. doi:10.1021/acs.joc.6b02887 |
| 40. | Do, J.-L.; Friščić, T. ACS Cent. Sci. 2017, 3, 13–19. doi:10.1021/acscentsci.6b00277 |
| 41. | Achar, T. K.; Bose, A.; Mal, P. Beilstein J. Org. Chem. 2017, 13, 1907–1931. doi:10.3762/bjoc.13.186 |
| 42. | Wang, G.-W. Chem. Soc. Rev. 2013, 42, 7668–7700. doi:10.1039/c3cs35526h |
| 3. | Mc Cartney, D.; Guiry, P. J. Chem. Soc. Rev. 2011, 40, 5122–5150. doi:10.1039/c1cs15101k |
| 4. | Beletskaya, I. P.; Cheprakov, A. V. Chem. Rev. 2000, 100, 3009–3066. doi:10.1021/cr9903048 |
| 5. | Lucks, S.; Brunner, H. Org. Process Res. Dev. 2017, 21, 1835–1842. doi:10.1021/acs.oprd.7b00279 |
| 6. | Jensen, R. K.; Thykier, N.; Enevoldsen, M. V.; Lindhardt, A. T. Org. Process Res. Dev. 2017, 21, 370–376. doi:10.1021/acs.oprd.6b00441 |
| 7. | Hansen, M. M.; Kallman, N. J.; Koenig, T. M.; Linder, R. J.; Richey, R. N.; Rizzo, J. R.; Ward, J. A.; Yu, H.; Zhang, T. Y.; Mitchell, D. Org. Process Res. Dev. 2017, 21, 208–217. doi:10.1021/acs.oprd.6b00368 |
| 43. | Hernández, J. G. Chem. – Eur. J. 2017, 23, 17157–17165. doi:10.1002/chem.201703605 |
| 44. | Jacob, K.; Schmidt, R.; Stolle, A. Carbon–Carbon Bond Forming by Ball Milling. In Ball Mills Ball Milling Towards Green Synthesis: Applications, Projects, Challenges; Stolle, A.; Ranu, B. C., Eds.; Royal Society of Chemistry: Cambridge, U.K., 2015; pp 34–57. |
| 45. | Declerck, V.; Colacino, E.; Bantreil, X.; Martinez, J.; Lamaty, F. Chem. Commun. 2012, 48, 11778–11780. doi:10.1039/c2cc36286d |
| 28. | Jeffery, T. J. Chem. Soc., Chem. Commun. 1984, 1287–1289. doi:10.1039/C39840001287 |
| 29. | Grasa, G. A.; Singh, R.; Stevens, E. D.; Nolan, S. P. J. Organomet. Chem. 2003, 687, 269–279. doi:10.1016/S0022-328X(03)00375-9 |
| 30. | Reetz, M. T.; Westermann, E. Angew. Chem., Int. Ed. 2000, 39, 165–168. doi:10.1002/(SICI)1521-3773(20000103)39:1<165::AID-ANIE165>3.0.CO;2-B |
| 33. | Xiao, J.; Jin, C.; Liu, Z.; Guo, S.; Zhang, X.; Zhou, X.; Wu, X. Org. Biomol. Chem. 2015, 13, 7257–7264. doi:10.1039/C5OB00710K |
| 34. | Takeuchi, A.; Hori, M.; Sato, S.; Ban, H. S.; Kuchimaru, T.; Kizaka-Kondoh, S.; Yamori, T.; Nakamura, H. M. Chem. Commun. 2012, 3, 1455–1461. doi:10.1039/c2md20134h |
| 24. | Shi, W.; Yu, J.; Jiang, Z.; Shao, Q.; Su, W. Beilstein J. Org. Chem. 2017, 13, 1661–1668. doi:10.3762/bjoc.13.160 |
| 25. | Zhu, X.; Liu, J.; Chen, T.; Su, W. Appl. Organomet. Chem. 2012, 26, 145–147. doi:10.1002/aoc.2827 |
| 26. | Tullberg, E.; Schacher, F.; Peters, D.; Frejd, T. Synthesis 2006, 1183–1189. doi:10.1055/s-2006-926371 |
| 27. | Tullberg, E.; Peters, D.; Frejd, T. J. Organomet. Chem. 2004, 689, 3778–3781. doi:10.1016/j.jorganchem.2004.06.045 |
| 35. | Zhai, L.-H.; Guo, L.-H.; Luo, Y.-H.; Ling, Y.; Sun, B.-W. Org. Process Res. Dev. 2015, 19, 849–857. doi:10.1021/acs.oprd.5b00123 |
| 36. | Chekal, B. P.; Guinness, S. M.; Lillie, B. M.; McLaughlin, R. W.; Palmer, C. W.; Post, R. J.; Sieser, J. E.; Singer, R. A.; Sluggett, G. W.; Vaidyanathan, R.; Withbroe, G. J. Org. Process Res. Dev. 2014, 18, 266–274. doi:10.1021/op400088k |
| 37. | Laufer, R.; Forrest, B.; Li, S.-W.; Liu, Y.; Sampson, P.; Edwards, L.; Lang, Y.; Awrey, D. E.; Mao, G.; Plotnikova, O.; Leung, G.; Hodgson, R.; Beletskaya, I.; Mason, J. M.; Luo, X.; Wei, X.; Yao, Y.; Feher, M.; Ban, F.; Kiarash, R.; Green, E.; Mak, T. W.; Pan, G.; Pauls, H. W. J. Med. Chem. 2013, 56, 6069–6087. doi:10.1021/jm400380m |
| 38. | Shen, C.; Shen, H.; Yang, M.; Xia, C.; Zhang, P. Green Chem. 2015, 17, 225–230. doi:10.1039/C4GC01606H |
| 19. | Zhang, L.; Jiang, Z.; Dong, C.; Xue, X.; Qiu, R.; Tang, W.; Li, H.; Xiao, J.; Xu, L. ChemCatChem 2014, 6, 311–318. doi:10.1002/cctc.201300755 |
| 20. | Ataei, A.; Nadri, S.; Rafiee, E.; Jamali, S.; Joshaghani, M. J. Mol. Catal. A: Chem. 2013, 366, 30–35. doi:10.1016/j.molcata.2012.08.025 |
| 21. | Calò, V.; Nacci, A.; Monopoli, A. J. Mol. Catal. A: Chem. 2004, 214, 45–56. doi:10.1016/j.molcata.2003.12.028 |
| 22. | Handy, S. T.; Okello, M. Tetrahedron Lett. 2003, 44, 8395–8397. doi:10.1016/j.tetlet.2003.09.120 |
| 23. | Mo, J.; Xiao, J. Angew. Chem., Int. Ed. 2006, 45, 4152–4157. doi:10.1002/anie.200600799 |
| 8. | Humphrey, G. R.; Dalby, S. M.; Andreani, T.; Xiang, B.; Luzung, M. R.; Song, Z. J.; Shevlin, M.; Christensen, M.; Belyk, K. M.; Tschaen, D. M. Org. Process Res. Dev. 2016, 20, 1097–1103. doi:10.1021/acs.oprd.6b00076 |
| 13. | Cyr, P.; Deng, S. T.; Hawkins, J. M.; Price, K. E. Org. Lett. 2013, 15, 4342–4345. doi:10.1021/ol4018134 |
| 14. | Cunha, S.; Oliveira, C. C.; Sabino, J. R. J. Braz. Chem. Soc. 2011, 22, 598–603. doi:10.1590/S0103-50532011000300026 |
| 15. | Zawisza, A. M.; Muzart, J. Tetrahedron Lett. 2007, 48, 6738–6742. doi:10.1016/j.tetlet.2007.07.077 |
| 16. | Chen, J.; Zhang, Y.; Yang, L.; Zhang, X.; Liu, J.; Li, L.; Zhang, H. Tetrahedron 2007, 63, 4266–4270. doi:10.1016/j.tet.2007.03.061 |
| 17. | Nakao, R.; Rhee, H.; Uozumi, Y. Org. Lett. 2005, 7, 163–165. doi:10.1021/ol047670k |
| 18. | Jedinák, L.; Zátopková, R.; Zemánková, H.; Šustková, A.; Cankař, P. J. Org. Chem. 2017, 82, 157–169. doi:10.1021/acs.joc.6b02306 |
| 31. | Veerareddy, A.; Surendrareddy, G.; Dubey, P. K. Synth. Commun. 2013, 43, 2236–2241. doi:10.1080/00397911.2012.696306 |
| 32. | Tash, J. S.; Chakrasali, R.; Jakkaraj, S. R.; Hughes, J.; Smith, S. K.; Hornbaker, K.; Heckert, L. L.; Ozturk, S. B.; Hadden, M. K.; Kinzy, T. G.; Blagg, B. S. J.; Georg, G. I. Biol. Reprod. 2008, 78, 1139–1152. doi:10.1095/biolreprod.107.062679 |
| 3. | Mc Cartney, D.; Guiry, P. J. Chem. Soc. Rev. 2011, 40, 5122–5150. doi:10.1039/c1cs15101k |
| 24. | Shi, W.; Yu, J.; Jiang, Z.; Shao, Q.; Su, W. Beilstein J. Org. Chem. 2017, 13, 1661–1668. doi:10.3762/bjoc.13.160 |
| 25. | Zhu, X.; Liu, J.; Chen, T.; Su, W. Appl. Organomet. Chem. 2012, 26, 145–147. doi:10.1002/aoc.2827 |
| 26. | Tullberg, E.; Schacher, F.; Peters, D.; Frejd, T. Synthesis 2006, 1183–1189. doi:10.1055/s-2006-926371 |
| 27. | Tullberg, E.; Peters, D.; Frejd, T. J. Organomet. Chem. 2004, 689, 3778–3781. doi:10.1016/j.jorganchem.2004.06.045 |
| 48. | Hattori, T.; Ueda, S.; Takakura, R.; Sawama, Y.; Monguchi, Y.; Sajiki, H. Chem. – Eur. J. 2017, 23, 8196–8202. doi:10.1002/chem.201606048 |
| 49. | Luong, T. T. H.; Touchet, S.; Alami, M.; Messaoudi, S. Adv. Synth. Catal. 2017, 359, 1320–1330. doi:10.1002/adsc.201601382 |
| 50. | Nishikata, T.; Noda, Y.; Fujimoto, R.; Sakashita, T. J. Am. Chem. Soc. 2013, 135, 16372–16375. doi:10.1021/ja409661n |
| 51. | Tang, B.-X.; Fang, X.-N.; Kuang, R.-Y.; Hu, R.-H.; Wang, J.-W.; Li, P.; Li, X.-h. Synthesis 2013, 45, 2971–2976. doi:10.1055/s-0033-1339650 |
| 52. | Caló, V.; Nacci, A.; Monopoli, A.; Laera, S.; Cioffi, N. J. Org. Chem. 2003, 68, 2929–2933. doi:10.1021/jo026877t |
| 46. | Saa, J. M.; Dopico, M.; Martorell, G.; Garcia-Raso, A. J. Org. Chem. 1990, 55, 991–995. doi:10.1021/jo00290a033 |
| 47. | Qin, L.; Hirao, H.; Zhou, J. Chem. Commun. 2013, 49, 10236–10238. doi:10.1039/c3cc45911j |
| 57. | Schmidt, R.; Burmeister, C. F.; Baláž, M.; Kwade, A.; Stolle, A. Org. Process Res. Dev. 2015, 19, 427–436. doi:10.1021/op5003787 |
| 63. | Stolle, A.; Schmidt, R.; Jacob, K. Faraday Discuss. 2014, 170, 267–286. doi:10.1039/C3FD00144J |
| 64. | Stolle, A. Technical Implications of Organic Syntheses in Ball Mills. In Ball Mills Ball Milling Towards Green Synthesis: Applications, Projects, Challenges; Stolle, A.; Ranu, B. C., Eds.; Royal Society of Chemistry: Cambridge, U.K., 2015; pp 241–276. |
| 65. | Konnert, L.; Gauliard, A.; Lamaty, F.; Martinez, J.; Colacino, E. ACS Sustainable Chem. Eng. 2013, 1, 1186–1191. doi:10.1021/sc4001115 |
| 24. | Shi, W.; Yu, J.; Jiang, Z.; Shao, Q.; Su, W. Beilstein J. Org. Chem. 2017, 13, 1661–1668. doi:10.3762/bjoc.13.160 |
| 61. | Yu, J.-B.; Peng, G.; Jiang, Z.-J.; Hong, Z.-K.; Su, W.-K. Eur. J. Org. Chem. 2016, 5340–5344. doi:10.1002/ejoc.201600987 |
| 62. | Yu, J.-B.; Zhang, Y.; Jiang, Z.-J.; Su, W.-K. J. Org. Chem. 2016, 81, 11514–11520. doi:10.1021/acs.joc.6b02197 |
| 24. | Shi, W.; Yu, J.; Jiang, Z.; Shao, Q.; Su, W. Beilstein J. Org. Chem. 2017, 13, 1661–1668. doi:10.3762/bjoc.13.160 |
| 56. | When the reaction was performed under solvent-heating conditions: 1a (1.5 mmol), 2a (2.25 mmol), Pd(OAc)2 (0.075 mmol), PPh3 (0.15 mmol), TEA (1.8 mmol), TBAB (0.075 mmol) and NaBr (3.0 mmol) in DMF (4 mL) at 100 °C for 10 h, the reaction selectivity could also be increased (3aa/4a = 97:3), but with lower yield (86%) of 3aa. |
| 57. | Schmidt, R.; Burmeister, C. F.; Baláž, M.; Kwade, A.; Stolle, A. Org. Process Res. Dev. 2015, 19, 427–436. doi:10.1021/op5003787 |
| 58. | Paveglio, G. C.; Longhi, K.; Moreira, D. N.; München, T. S.; Tier, A. Z.; Gindri, I. M.; Bender, C. R.; Frizzo, C. P.; Zanatta, N.; Bonacorso, H. G.; Martins, M. A. P. ACS Sustainable Chem. Eng. 2014, 2, 1895–1901. doi:10.1021/sc5002353 |
| 59. | Jicsinszky, L.; Tuza, K.; Cravotto, G.; Porcheddu, A.; Delogu, F.; Colacino, E. Beilstein J. Org. Chem. 2017, 13, 1893–1899. doi:10.3762/bjoc.13.184 |
| 60. | Julien, P. A.; Malvestiti, I.; Friščić, T. Beilstein J. Org. Chem. 2017, 13, 2160–2168. doi:10.3762/bjoc.13.216 |
| 53. | Stolle, A.; Szuppa, T.; Leonhardt, S. E. S.; Ondruschka, B. Chem. Soc. Rev. 2011, 40, 2317–2329. doi:10.1039/c0cs00195c |
| 54. | Hermann, G. N.; Becker, P.; Bolm, C. Angew. Chem., Int. Ed. 2015, 54, 7414–7417. doi:10.1002/anie.201502536 |
| 55. | Hernández, J. G.; Turberg, M.; Schiffers, I.; Bolm, C. Chem. – Eur. J. 2016, 22, 14513–14517. doi:10.1002/chem.201603057 |
| 14. | Cunha, S.; Oliveira, C. C.; Sabino, J. R. J. Braz. Chem. Soc. 2011, 22, 598–603. doi:10.1590/S0103-50532011000300026 |
| 15. | Zawisza, A. M.; Muzart, J. Tetrahedron Lett. 2007, 48, 6738–6742. doi:10.1016/j.tetlet.2007.07.077 |
| 16. | Chen, J.; Zhang, Y.; Yang, L.; Zhang, X.; Liu, J.; Li, L.; Zhang, H. Tetrahedron 2007, 63, 4266–4270. doi:10.1016/j.tet.2007.03.061 |
© 2018 Yu et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (https://www.beilstein-journals.org/bjoc)