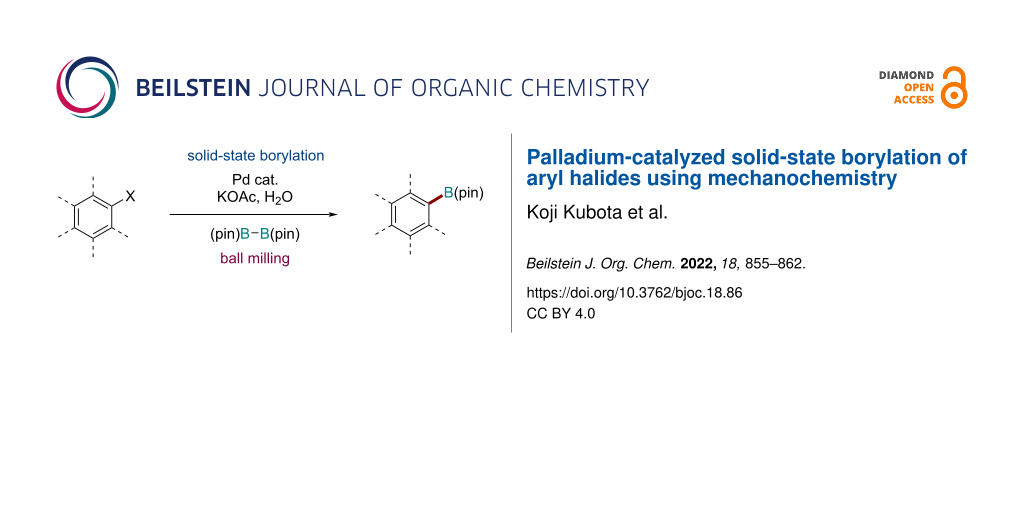
Abstract
This study describes the solid-state palladium-catalyzed cross-coupling between aryl halides and bis(pinacolato)diboron using ball milling. The reactions were completed within 10 min for most aryl halides to afford a variety of synthetically useful arylboronates in high yields. Notably, all experimental operations could be performed in air, and did not require the use of large amounts of dry and degassed organic solvents. The utility of this method was further demonstrated by gram-scale synthesis under solvent-free, mechanochemical conditions.
Graphical Abstract
Introduction
Arylboronic acid and its derivatives are indispensable reagents in modern synthetic chemistry because they have been frequently used for the preparation of many bioactive molecules, natural products, and functional organic materials, typically through Suzuki–Miyaura coupling [1-7]. The palladium-catalyzed boryl substitution of aryl halides with boron reagents, termed Miyaura–Ishiyama borylation, is an efficient method for synthesizing arylboronates with high functional group compatibility [8-14]. To date, many palladium-based catalytic systems in solution for the borylation of aryl halides have been reported [8-14]. However, these solution-based reactions usually require long reaction times and significant amounts of dry and degassed organic solvents. Additionally, to avoid the deterioration of reactivity due to moisture and oxygen, conventional protocols require synthesis techniques that involve the use of high-vacuum Schlenk lines and/or glove boxes, which are costly and require special training to handle. Thus, the development of an operationally simple, solvent-free palladium-catalyzed borylation process applicable for a wide range of aryl halides would greatly improve the practicality of the desired arylboronic acid derivatives.
Nechaev et al. reported the first solvent-free protocol for the palladium-catalyzed Miyaura–Ishiyama borylation of aryl halides in a test tube [15]. They found that a Pd(dba)2/DPEphos catalytic system was effective for aryl bromides, and aryl chlorides reacted more efficiently when XPhos was used as the ligand [15]. Although their achievements are remarkable, this protocol is only applicable to liquid substrates, which can serve as reactants and solvents (neat liquid conditions). In addition, a long reaction time (12 h) is required to complete the reaction [15]. A general and efficient solvent-free borylation protocol that can be applied to liquid as well as solid aryl halides remains undeveloped.
Recently, mechanochemical synthesis using ball milling has attracted considerable attention as an efficient solvent-free synthetic technique [16-33]. Notably, the strong mechanical agitation provided by ball milling enables efficient solid-state organic transformations. Thus far, mechanochemical palladium-catalyzed cross-coupling reactions such as Suzuki–Miyaura [34-47], Buchwald–Hartwig [48-52], Sonogashira [53-56], Negishi [57], Mizoroki–Heck [58-60], and C–S bond-forming [61] reactions have been developed. Our group has also been interested in this class of mechanochemical transformations, particularly in the development of cross-coupling reactions that proceed in the solid state [43-50]. These mechanochemical cross-coupling reactions often show much faster reaction kinetics than those under conventional solution-based conditions because of the high concentration; further, the experimental operations can be carried out in air. Considering these achievements, including our recent success in solid-state cross-coupling chemistry, we envisioned that this mechanochemical strategy could be applied to the palladium-catalyzed borylation of aryl halides [8-14]. The development of a solid-state borylation protocol could provide a practical solution to the many issues associated with conventional solution-based protocols (Scheme 1).
Scheme 1: Development of the first solid-state palladium-catalyzed borylation protocol of aryl halides using mechanochemistry.
Scheme 1: Development of the first solid-state palladium-catalyzed borylation protocol of aryl halides using ...
Results and Discussion
Initially, we conducted an optimization study on the mechanochemical cross-coupling between 2-bromo-6-methoxynaphthalene (1a, 0.3 mmol) and bis(pinacolato)diboron (2, 1.2 equiv) in the presence of Pd(OAc)2 (2 mol %), KOAc (3.0 equiv), and H2O (60 μL) as a liquid additive [43-45] (Table 1). The reactions were conducted in a Retch MM400 ball mill in a stainless-steel milling jar (1.5 mL) at 30 Hz using one stainless-steel ball (diameter: 5 mm) for 10 min. We used a commercially available temperature-controllable heat gun that was placed directly above the ball-milling jar to control the reaction temperature [45]. Reactions were performed using a heat gun with a preset temperature of 100 °C, and the internal temperature of the reaction mixture (60 °C) was assessed by thermography immediately after opening the milling jar (see Supporting Information File 1 for details). First, we tested various phosphine ligands [62] for this reaction (Table 1, entries 1–8). Interestingly, we found that tri-tert-butylphosphonium tetrafluoroborate (t-Bu3P·HBF4) provided the borylation product 3a in excellent yield (92%, Table 1, entry 1), with a small amount of the protonation product 4a (5%, entry 1). When the reaction was stopped after 5 min, only a 26% yield of 3a was obtained (Table 1, entry 2). The use of 1,1'-bis(diphenylphosphino)ferrocene (dppf), which is the optimal ligand under the original conditions for the solution-based protocol [8], provided only trace amounts of 3a (1%, Table 1, entry 3). Reactions with DPEphos and XPhos, which are the optimal ligands for neat liquid conditions reported by Nechaev [15], also yielded only poor results (Table 1, entries 4 and 5). Other monophosphine ligands, such as SPhos, PCy3, and PAd3, did not improve the yield of 3a (Table 1, entries 6–8). The amount of H2O added was important for the efficiency of the reaction. Decreasing the amount of H2O to 40 μL led to a lower yield of 3a (68%; Table 1, entry 9). The reaction without H2O afforded 3a in only 8% yield (Table 1, entry 10). Finally, we investigated the effect of the reaction temperature. The reaction at a higher temperature (110 °C) also provided 3a in a good yield (90%; Table 1, entry 11), but when the reaction was carried out at 30 °C, the yield was very low (6%; Table 1, entry 12). A longer reaction time (90 min) at 30 °C afforded 3a in a moderate yield (41%; Table 1, entry 13). Note that no homocoupling product of 1a was formed, or only trace amounts (<1%) were formed in all cases.
Table 1: Optimization of the reaction conditions.a
|
|
||||||
| Entry | Ligand | Amount of H2O (μL) | Internal temp (°C) | Time (min) | Yield of 3a (%)b | Yield of 4a (%)b |
| 1 | t-Bu3P·HBF4 | 60 | 60 | 10 | 92 | 5 |
| 2 | t-Bu3P·HBF4 | 60 | 60 | 5 | 26 | 1 |
| 3 | dppf | 60 | 60 | 10 | 1 | <1 |
| 4 | DPEphos | 60 | 60 | 10 | <1 | <1 |
| 5 | XPhos | 60 | 60 | 10 | 5 | <1 |
| 6 | SPhos | 60 | 60 | 10 | 2 | <1 |
| 7 | PCy3 | 60 | 60 | 10 | 5 | <1 |
| 8 | PAd3 | 60 | 60 | 10 | 6 | <1 |
| 9 | t-Bu3P·HBF4 | 40 | 60 | 10 | 68 | 3 |
| 10 | t-Bu3P·HBF4 | 0 | 60 | 10 | 8 | 1 |
| 11 | t-Bu3P·HBF4 | 60 | 110 | 10 | 90 | 6 |
| 12 | t-Bu3P·HBF4 | 60 | 30 | 10 | 6 | 1 |
| 13 | t-Bu3P·HBF4 | 60 | 30 | 90 | 41 | 2 |
aReaction conditions: a mixture of 1a (0.30 mmol), 2 (0.36 mmol), KOAc (0.9 mmol), Pd(OAc)2 (0.006 mmol), ligand (0.009 mmol), and H2O was milled in a 1.5 mL stainless-steel jar at 30 Hz with one stainless-steel ball that was 5 mm in diameter. bDetermined by GC analysis using an internal standard.
Under the optimized reaction conditions (Table 1, entry 1), we explored the substrate scope of aryl bromides for the solid-state borylation (Scheme 2). The reactions of aryl bromides bearing electron-donating and electron-withdrawing groups at the para position (1b–d) proceeded smoothly to afford the corresponding arylboronates (3b–d) in good to high yields. 4-Bromobiphenyl (1e) also underwent borylation efficiently to form the borylation product (3e) in excellent yield. We found that substrates bearing relatively large conjugated structures (1f−m) tended to show low reactivity under the optimized conditions at 60 °C, but the reactions proceeded smoothly at 110 °C. For example, the bromo-substituted triphenylamine (1f), pyrene (1g and 1h), fluoranthene (1i), biphenyl (1j), triphenylene (1k), tetraphenylethylene (1l), and anthracene (1m) reacted with diboron 2 to form the desired products (3f–m) in high yields.
Scheme 2: Substrate scope of solid aryl bromides. Reaction conditions: a mixture of 1 (0.30 mmol), 2 (0.36 mmol), KOAc (0.9 mmol), Pd(OAc)2 (0.006 mmol), t-Bu3·HBF4 (0.009 mmol), and H2O (60 μL) was milled in a 1.5 mL stainless-steel jar at 30 Hz using one stainless-steel ball that was 5 mm in diameter. The isolated yields are shown. The NMR yields are shown in parentheses.
Scheme 2: Substrate scope of solid aryl bromides. Reaction conditions: a mixture of 1 (0.30 mmol), 2 (0.36 mm...
Next, the substrate scope of liquid aryl bromides was investigated (Scheme 3). We found that the present mechanochemical conditions were applicable to the solid substrate and various liquid substrates (1n–p), and the desired borylation products (3n–p) were obtained in good yields.
Scheme 3: Substrate scope of liquid aryl bromides. Reaction conditions: a mixture of 1 (0.30 mmol), 2 (0.36 mmol), KOAc (0.9 mmol), Pd(OAc)2 (0.006 mmol), t-Bu3·HBF4 (0.009 mmol), and H2O (60 μL) was milled in a 1.5 mL stainless-steel jar at 30 Hz using one stainless-steel ball that was 5 mm in diameter. The isolated yields are shown. The NMR yields are shown in parentheses.
Scheme 3: Substrate scope of liquid aryl bromides. Reaction conditions: a mixture of 1 (0.30 mmol), 2 (0.36 m...
We also investigated the solid-state borylation reactions of aryl iodides and chlorides (Scheme 4). The reaction of 4-iodo-N,N-diphenylaniline (1q) under the optimized conditions at 130 °C for 30 min proceeded smoothly and provided the desired product 3f in high yield (84%). Also, the reaction of (4-chlorophenyl)(phenyl)methanone (1r) afforded the corresponding product 3q in good yield.
Scheme 4: Reactions of solid aryl iodide and chloride. Reaction conditions: a mixture of 1 (0.30 mmol), 2 (0.36 mmol), KOAc (0.9 mmol), Pd(OAc)2 (0.006 mmol), t-Bu3·HBF4 (0.009 mmol), and H2O (60 μL) was milled in a 1.5 mL stainless-steel jar at 30 Hz using one stainless-steel ball that was 5 mm in diameter. The isolated yields are shown. The NMR yields are shown in parentheses.
Scheme 4: Reactions of solid aryl iodide and chloride. Reaction conditions: a mixture of 1 (0.30 mmol), 2 (0....
To demonstrate the practical utility of the developed protocol, the solid-state borylation reaction was investigated on a gram scale (Scheme 5). We carried out the reaction of 1a at 5 mmol in the presence of the optimized catalytic system using a 25 mL stainless-steel jar and four stainless-steel balls (diameter: 10 mm) at 25 Hz and a preset heat-gun temperature of 150 °C. The gram-scale borylation proceeded efficiently and afforded the borylation product 3a in high yield, which was comparable to the yield obtained at the 0.3 mmol scale. We confirmed by thermography that the internal temperature after the reaction was approximately 100 °C.
Scheme 5: Solid-state borylation of aryl halides on a gram scale.
Scheme 5: Solid-state borylation of aryl halides on a gram scale.
Conclusion
In summary, we have developed the first protocol for the solid-state palladium-catalyzed borylation of aryl halides. This mechanochemical protocol allows the synthesis of various arylboronates in high yields within a short reaction time (within 10 min). Notably, the borylation reactions can be conducted without the use of organic solvents, and all synthetic operations can be carried out in air without the requirement of Schlenk-line techniques or glovebox operations. Therefore, the present solid-state approach is a practical and sustainable method to complement conventional solution-based protocols. The application of this method to the solid-state borylation of insoluble substrates for the synthesis of new arylboronates that are difficult to prepare by other means is currently under investigation.
Supporting Information
| Supporting Information File 1: Experimental procedures, experimental set-ups, characterization data, and NMR spectra. | ||
| Format: PDF | Size: 4.3 MB | Download |
Funding
This work was financially supported by the Japan Society for the Promotion of Science (JSPS) via KAKENHI grants 22H00318 and 21H01926; by the JST via CREST grant JPMJCR19R1; FOREST grant JPMJFR201I; and by the Institute for Chemical Reaction Design and Discovery (ICReDD) established by the World Premier International Research Initiative (WPI), MEXT, Japan.
References
-
Hall, D. G., Ed. Boronic Acids: Preparation and Application in Organic Synthesis, Medicine and Materials, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2011. doi:10.1002/9783527639328
Return to citation in text: [1] -
Diner, C.; Szabó, K. J. J. Am. Chem. Soc. 2017, 139, 2–14. doi:10.1021/jacs.6b10017
Return to citation in text: [1] -
Diaz, D. B.; Yudin, A. K. Nat. Chem. 2017, 9, 731–742. doi:10.1038/nchem.2814
Return to citation in text: [1] -
Touchet, S.; Carreaux, F.; Carboni, B.; Bouillon, A.; Boucher, J.-L. Chem. Soc. Rev. 2011, 40, 3895–3914. doi:10.1039/c0cs00154f
Return to citation in text: [1] -
Smoum, R.; Rubinstein, A.; Dembitsky, V. M.; Srebnik, M. Chem. Rev. 2012, 112, 4156–4220. doi:10.1021/cr608202m
Return to citation in text: [1] -
Mellerup, S. K.; Wang, S. Trends Chem. 2019, 1, 77–89. doi:10.1016/j.trechm.2019.01.003
Return to citation in text: [1] -
Budiman, Y. P.; Westcott, S. A.; Radius, U.; Marder, T. B. Adv. Synth. Catal. 2021, 363, 2224–2255. doi:10.1002/adsc.202001291
Return to citation in text: [1] -
Ishiyama, T.; Murata, M.; Miyaura, N. J. Org. Chem. 1995, 60, 7508–7510. doi:10.1021/jo00128a024
Return to citation in text: [1] [2] [3] [4] -
Ishiyama, T.; Itoh, Y.; Kitano, T.; Miyaura, N. Tetrahedron Lett. 1997, 38, 3447–3450. doi:10.1016/s0040-4039(97)00642-4
Return to citation in text: [1] [2] [3] -
Ishiyama, T.; Ishida, K.; Miyaura, N. Tetrahedron 2001, 57, 9813–9816. doi:10.1016/s0040-4020(01)00998-x
Return to citation in text: [1] [2] [3] -
Chow, W. K.; Yuen, O. Y.; Choy, P. Y.; So, C. M.; Lau, C. P.; Wong, W. T.; Kwong, F. Y. RSC Adv. 2013, 3, 12518–12539. doi:10.1039/c3ra22905j
Return to citation in text: [1] [2] [3] -
Barroso, S.; Joksch, M.; Puylaert, P.; Tin, S.; Bell, S. J.; Donnellan, L.; Duguid, S.; Muir, C.; Zhao, P.; Farina, V.; Tran, D. N.; de Vries, J. G. J. Org. Chem. 2021, 86, 103–109. doi:10.1021/acs.joc.0c01758
Return to citation in text: [1] [2] [3] -
Murata, M.; Oyama, T.; Watanabe, S.; Masuda, Y. J. Org. Chem. 2000, 65, 164–168. doi:10.1021/jo991337q
Return to citation in text: [1] [2] [3] -
Kubota, K.; Iwamoto, H.; Ito, H. Org. Biomol. Chem. 2017, 15, 285–300. doi:10.1039/c6ob02369j
Return to citation in text: [1] [2] [3] -
Dzhevakov, P. B.; Topchiy, M. A.; Zharkova, D. A.; Morozov, O. S.; Asachenko, A. F.; Nechaev, M. S. Adv. Synth. Catal. 2016, 358, 977–983. doi:10.1002/adsc.201500844
Return to citation in text: [1] [2] [3] [4] -
James, S. L.; Adams, C. J.; Bolm, C.; Braga, D.; Collier, P.; Friščić, T.; Grepioni, F.; Harris, K. D. M.; Hyett, G.; Jones, W.; Krebs, A.; Mack, J.; Maini, L.; Orpen, A. G.; Parkin, I. P.; Shearouse, W. C.; Steed, J. W.; Waddell, D. C. Chem. Soc. Rev. 2012, 41, 413–447. doi:10.1039/c1cs15171a
Return to citation in text: [1] -
Bowmaker, G. A. Chem. Commun. 2013, 49, 334–348. doi:10.1039/c2cc35694e
Return to citation in text: [1] -
Wang, G.-W. Chem. Soc. Rev. 2013, 42, 7668–7700. doi:10.1039/c3cs35526h
Return to citation in text: [1] -
Hernández, J. G.; Bolm, C. J. Org. Chem. 2017, 82, 4007–4019. doi:10.1021/acs.joc.6b02887
Return to citation in text: [1] -
Stolle, A.; Szuppa, T.; Leonhardt, S. E. S.; Ondruschka, B. Chem. Soc. Rev. 2011, 40, 2317–2329. doi:10.1039/c0cs00195c
Return to citation in text: [1] -
Kubota, K.; Ito, H. Trends Chem. 2020, 2, 1066–1081. doi:10.1016/j.trechm.2020.09.006
Return to citation in text: [1] -
Howard, J. L.; Cao, Q.; Browne, D. L. Chem. Sci. 2018, 9, 3080–3094. doi:10.1039/c7sc05371a
Return to citation in text: [1] -
Braga, D.; Maini, L.; Grepioni, F. Chem. Soc. Rev. 2013, 42, 7638–7648. doi:10.1039/c3cs60014a
Return to citation in text: [1] -
Achar, T. K.; Bose, A.; Mal, P. Beilstein J. Org. Chem. 2017, 13, 1907–1931. doi:10.3762/bjoc.13.186
Return to citation in text: [1] -
Do, J.-L.; Friščić, T. ACS Cent. Sci. 2017, 3, 13–19. doi:10.1021/acscentsci.6b00277
Return to citation in text: [1] -
Métro, T.-X.; Martinez, J.; Lamaty, F. ACS Sustainable Chem. Eng. 2017, 5, 9599–9602. doi:10.1021/acssuschemeng.7b03260
Return to citation in text: [1] -
Eguaogie, O.; Vyle, J. S.; Conlon, P. F.; Gîlea, M. A.; Liang, Y. Beilstein J. Org. Chem. 2018, 14, 955–970. doi:10.3762/bjoc.14.81
Return to citation in text: [1] -
Bolm, C.; Hernández, J. G. Angew. Chem., Int. Ed. 2019, 58, 3285–3299. doi:10.1002/anie.201810902
Return to citation in text: [1] -
Friščić, T.; Mottillo, C.; Titi, H. M. Angew. Chem., Int. Ed. 2020, 59, 1018–1029. doi:10.1002/anie.201906755
Return to citation in text: [1] -
Andersen, J.; Mack, J. Green Chem. 2018, 20, 1435–1443. doi:10.1039/c7gc03797j
Return to citation in text: [1] -
Porcheddu, A.; Colacino, E.; De Luca, L.; Delogu, F. ACS Catal. 2020, 10, 8344–8394. doi:10.1021/acscatal.0c00142
Return to citation in text: [1] -
Leitch, J. A.; Browne, D. L. Chem. – Eur. J. 2021, 27, 9721–9726. doi:10.1002/chem.202100348
Return to citation in text: [1] -
Kaupp, G. CrystEngComm 2009, 11, 388–403. doi:10.1039/b810822f
Return to citation in text: [1] -
Schneider, F.; Ondruschka, B. ChemSusChem 2008, 1, 622–625. doi:10.1002/cssc.200800086
Return to citation in text: [1] -
Cravotto, G.; Garella, D.; Tagliapietra, S.; Stolle, A.; Schüßler, S.; Leonhardt, S. E. S.; Ondruschka, B. New J. Chem. 2012, 36, 1304–1307. doi:10.1039/c2nj40064b
Return to citation in text: [1] -
Nielsen, S. F.; Peters, D.; Axelsson, O. Synth. Commun. 2000, 30, 3501–3509. doi:10.1080/00397910008087262
Return to citation in text: [1] -
Klingensmith, L. M.; Leadbeater, N. E. Tetrahedron Lett. 2003, 44, 765–768. doi:10.1016/s0040-4039(02)02645-x
Return to citation in text: [1] -
Bernhardt, F.; Trotzki, R.; Szuppa, T.; Stolle, A.; Ondruschka, B. Beilstein J. Org. Chem. 2010, 6, No. 7. doi:10.3762/bjoc.6.7
Return to citation in text: [1] -
Schneider, F.; Szuppa, T.; Stolle, A.; Ondruschka, B.; Hopf, H. Green Chem. 2009, 11, 1894–1899. doi:10.1039/b915744c
Return to citation in text: [1] -
Schneider, F.; Stolle, A.; Ondruschka, B.; Hopf, H. Org. Process Res. Dev. 2009, 13, 44–48. doi:10.1021/op800148y
Return to citation in text: [1] -
Jiang, Z.-J.; Li, Z.-H.; Yu, J.-B.; Su, W.-K. J. Org. Chem. 2016, 81, 10049–10055. doi:10.1021/acs.joc.6b01938
Return to citation in text: [1] -
Báti, G.; Csókás, D.; Yong, T.; Tam, S. M.; Shi, R. R. S.; Webster, R. D.; Pápai, I.; García, F.; Stuparu, M. C. Angew. Chem., Int. Ed. 2020, 59, 21620–21626. doi:10.1002/anie.202007815
Return to citation in text: [1] -
Seo, T.; Ishiyama, T.; Kubota, K.; Ito, H. Chem. Sci. 2019, 10, 8202–8210. doi:10.1039/c9sc02185j
Return to citation in text: [1] [2] [3] -
Seo, T.; Kubota, K.; Ito, H. J. Am. Chem. Soc. 2020, 142, 9884–9889. doi:10.1021/jacs.0c01739
Return to citation in text: [1] [2] [3] -
Seo, T.; Toyoshima, N.; Kubota, K.; Ito, H. J. Am. Chem. Soc. 2021, 143, 6165–6175. doi:10.1021/jacs.1c00906
Return to citation in text: [1] [2] [3] [4] -
Takahashi, R.; Seo, T.; Kubota, K.; Ito, H. ACS Catal. 2021, 11, 14803–14810. doi:10.1021/acscatal.1c03731
Return to citation in text: [1] [2] -
Kubota, K.; Kondo, K.; Seo, T.; Ito, H. Synlett 2022, 33, 898–902. doi:10.1055/a-1748-3797
Return to citation in text: [1] [2] -
Kubota, K.; Seo, T.; Koide, K.; Hasegawa, Y.; Ito, H. Nat. Commun. 2019, 10, No. 111. doi:10.1038/s41467-018-08017-9
Return to citation in text: [1] [2] -
Kubota, K.; Takahashi, R.; Uesugi, M.; Ito, H. ACS Sustainable Chem. Eng. 2020, 8, 16577–16582. doi:10.1021/acssuschemeng.0c05834
Return to citation in text: [1] [2] -
Kubota, K.; Endo, T.; Uesugi, M.; Hayashi, Y.; Ito, H. ChemSusChem 2022, 15, e202102132. doi:10.1002/cssc.202102132
Return to citation in text: [1] [2] -
Shao, Q.-L.; Jiang, Z.-J.; Su, W.-K. Tetrahedron Lett. 2018, 59, 2277–2280. doi:10.1016/j.tetlet.2018.04.078
Return to citation in text: [1] -
Cao, Q.; Nicholson, W. I.; Jones, A. C.; Browne, D. L. Org. Biomol. Chem. 2019, 17, 1722–1726. doi:10.1039/c8ob01781f
Return to citation in text: [1] -
Fulmer, D. A.; Shearouse, W. C.; Medonza, S. T.; Mack, J. Green Chem. 2009, 11, 1821–1825. doi:10.1039/b915669k
Return to citation in text: [1] -
Liang, Y.; Xie, Y.-X.; Li, J.-H. J. Org. Chem. 2006, 71, 379–381. doi:10.1021/jo051882t
Return to citation in text: [1] -
Thorwirth, R.; Stolle, A.; Ondruschka, B. Green Chem. 2010, 12, 985–991. doi:10.1039/c000674b
Return to citation in text: [1] -
Gao, Y.; Feng, C.; Seo, T.; Kubota, K.; Ito, H. Chem. Sci. 2022, 13, 430–438. doi:10.1039/d1sc05257h
Return to citation in text: [1] -
Cao, Q.; Howard, J. L.; Wheatley, E.; Browne, D. L. Angew. Chem., Int. Ed. 2018, 57, 11339–11343. doi:10.1002/anie.201806480
Return to citation in text: [1] -
Declerck, V.; Colacino, E.; Bantreil, X.; Martinez, J.; Lamaty, F. Chem. Commun. 2012, 48, 11778–11780. doi:10.1039/c2cc36286d
Return to citation in text: [1] -
Tullberg, E.; Schacher, F.; Peters, D.; Frejd, T. Synthesis 2006, 1183–1189. doi:10.1055/s-2006-926371
Return to citation in text: [1] -
Tullberg, E.; Peters, D.; Frejd, T. J. Organomet. Chem. 2004, 689, 3778–3781. doi:10.1016/j.jorganchem.2004.06.045
Return to citation in text: [1] -
Jones, A. C.; Nicholson, W. I.; Smallman, H. R.; Browne, D. L. Org. Lett. 2020, 22, 7433–7438. doi:10.1021/acs.orglett.0c02418
Return to citation in text: [1] -
Tang, W.; Keshipeddy, S.; Zhang, Y.; Wei, X.; Savoie, J.; Patel, N. D.; Yee, N. K.; Senanayake, C. H. Org. Lett. 2011, 13, 1366–1369. doi:10.1021/ol2000556
Return to citation in text: [1]
| 8. | Ishiyama, T.; Murata, M.; Miyaura, N. J. Org. Chem. 1995, 60, 7508–7510. doi:10.1021/jo00128a024 |
| 45. | Seo, T.; Toyoshima, N.; Kubota, K.; Ito, H. J. Am. Chem. Soc. 2021, 143, 6165–6175. doi:10.1021/jacs.1c00906 |
| 62. | Tang, W.; Keshipeddy, S.; Zhang, Y.; Wei, X.; Savoie, J.; Patel, N. D.; Yee, N. K.; Senanayake, C. H. Org. Lett. 2011, 13, 1366–1369. doi:10.1021/ol2000556 |
| 1. | Hall, D. G., Ed. Boronic Acids: Preparation and Application in Organic Synthesis, Medicine and Materials, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2011. doi:10.1002/9783527639328 |
| 2. | Diner, C.; Szabó, K. J. J. Am. Chem. Soc. 2017, 139, 2–14. doi:10.1021/jacs.6b10017 |
| 3. | Diaz, D. B.; Yudin, A. K. Nat. Chem. 2017, 9, 731–742. doi:10.1038/nchem.2814 |
| 4. | Touchet, S.; Carreaux, F.; Carboni, B.; Bouillon, A.; Boucher, J.-L. Chem. Soc. Rev. 2011, 40, 3895–3914. doi:10.1039/c0cs00154f |
| 5. | Smoum, R.; Rubinstein, A.; Dembitsky, V. M.; Srebnik, M. Chem. Rev. 2012, 112, 4156–4220. doi:10.1021/cr608202m |
| 6. | Mellerup, S. K.; Wang, S. Trends Chem. 2019, 1, 77–89. doi:10.1016/j.trechm.2019.01.003 |
| 7. | Budiman, Y. P.; Westcott, S. A.; Radius, U.; Marder, T. B. Adv. Synth. Catal. 2021, 363, 2224–2255. doi:10.1002/adsc.202001291 |
| 15. | Dzhevakov, P. B.; Topchiy, M. A.; Zharkova, D. A.; Morozov, O. S.; Asachenko, A. F.; Nechaev, M. S. Adv. Synth. Catal. 2016, 358, 977–983. doi:10.1002/adsc.201500844 |
| 8. | Ishiyama, T.; Murata, M.; Miyaura, N. J. Org. Chem. 1995, 60, 7508–7510. doi:10.1021/jo00128a024 |
| 9. | Ishiyama, T.; Itoh, Y.; Kitano, T.; Miyaura, N. Tetrahedron Lett. 1997, 38, 3447–3450. doi:10.1016/s0040-4039(97)00642-4 |
| 10. | Ishiyama, T.; Ishida, K.; Miyaura, N. Tetrahedron 2001, 57, 9813–9816. doi:10.1016/s0040-4020(01)00998-x |
| 11. | Chow, W. K.; Yuen, O. Y.; Choy, P. Y.; So, C. M.; Lau, C. P.; Wong, W. T.; Kwong, F. Y. RSC Adv. 2013, 3, 12518–12539. doi:10.1039/c3ra22905j |
| 12. | Barroso, S.; Joksch, M.; Puylaert, P.; Tin, S.; Bell, S. J.; Donnellan, L.; Duguid, S.; Muir, C.; Zhao, P.; Farina, V.; Tran, D. N.; de Vries, J. G. J. Org. Chem. 2021, 86, 103–109. doi:10.1021/acs.joc.0c01758 |
| 13. | Murata, M.; Oyama, T.; Watanabe, S.; Masuda, Y. J. Org. Chem. 2000, 65, 164–168. doi:10.1021/jo991337q |
| 14. | Kubota, K.; Iwamoto, H.; Ito, H. Org. Biomol. Chem. 2017, 15, 285–300. doi:10.1039/c6ob02369j |
| 15. | Dzhevakov, P. B.; Topchiy, M. A.; Zharkova, D. A.; Morozov, O. S.; Asachenko, A. F.; Nechaev, M. S. Adv. Synth. Catal. 2016, 358, 977–983. doi:10.1002/adsc.201500844 |
| 43. | Seo, T.; Ishiyama, T.; Kubota, K.; Ito, H. Chem. Sci. 2019, 10, 8202–8210. doi:10.1039/c9sc02185j |
| 44. | Seo, T.; Kubota, K.; Ito, H. J. Am. Chem. Soc. 2020, 142, 9884–9889. doi:10.1021/jacs.0c01739 |
| 45. | Seo, T.; Toyoshima, N.; Kubota, K.; Ito, H. J. Am. Chem. Soc. 2021, 143, 6165–6175. doi:10.1021/jacs.1c00906 |
| 8. | Ishiyama, T.; Murata, M.; Miyaura, N. J. Org. Chem. 1995, 60, 7508–7510. doi:10.1021/jo00128a024 |
| 9. | Ishiyama, T.; Itoh, Y.; Kitano, T.; Miyaura, N. Tetrahedron Lett. 1997, 38, 3447–3450. doi:10.1016/s0040-4039(97)00642-4 |
| 10. | Ishiyama, T.; Ishida, K.; Miyaura, N. Tetrahedron 2001, 57, 9813–9816. doi:10.1016/s0040-4020(01)00998-x |
| 11. | Chow, W. K.; Yuen, O. Y.; Choy, P. Y.; So, C. M.; Lau, C. P.; Wong, W. T.; Kwong, F. Y. RSC Adv. 2013, 3, 12518–12539. doi:10.1039/c3ra22905j |
| 12. | Barroso, S.; Joksch, M.; Puylaert, P.; Tin, S.; Bell, S. J.; Donnellan, L.; Duguid, S.; Muir, C.; Zhao, P.; Farina, V.; Tran, D. N.; de Vries, J. G. J. Org. Chem. 2021, 86, 103–109. doi:10.1021/acs.joc.0c01758 |
| 13. | Murata, M.; Oyama, T.; Watanabe, S.; Masuda, Y. J. Org. Chem. 2000, 65, 164–168. doi:10.1021/jo991337q |
| 14. | Kubota, K.; Iwamoto, H.; Ito, H. Org. Biomol. Chem. 2017, 15, 285–300. doi:10.1039/c6ob02369j |
| 61. | Jones, A. C.; Nicholson, W. I.; Smallman, H. R.; Browne, D. L. Org. Lett. 2020, 22, 7433–7438. doi:10.1021/acs.orglett.0c02418 |
| 8. | Ishiyama, T.; Murata, M.; Miyaura, N. J. Org. Chem. 1995, 60, 7508–7510. doi:10.1021/jo00128a024 |
| 9. | Ishiyama, T.; Itoh, Y.; Kitano, T.; Miyaura, N. Tetrahedron Lett. 1997, 38, 3447–3450. doi:10.1016/s0040-4039(97)00642-4 |
| 10. | Ishiyama, T.; Ishida, K.; Miyaura, N. Tetrahedron 2001, 57, 9813–9816. doi:10.1016/s0040-4020(01)00998-x |
| 11. | Chow, W. K.; Yuen, O. Y.; Choy, P. Y.; So, C. M.; Lau, C. P.; Wong, W. T.; Kwong, F. Y. RSC Adv. 2013, 3, 12518–12539. doi:10.1039/c3ra22905j |
| 12. | Barroso, S.; Joksch, M.; Puylaert, P.; Tin, S.; Bell, S. J.; Donnellan, L.; Duguid, S.; Muir, C.; Zhao, P.; Farina, V.; Tran, D. N.; de Vries, J. G. J. Org. Chem. 2021, 86, 103–109. doi:10.1021/acs.joc.0c01758 |
| 13. | Murata, M.; Oyama, T.; Watanabe, S.; Masuda, Y. J. Org. Chem. 2000, 65, 164–168. doi:10.1021/jo991337q |
| 14. | Kubota, K.; Iwamoto, H.; Ito, H. Org. Biomol. Chem. 2017, 15, 285–300. doi:10.1039/c6ob02369j |
| 43. | Seo, T.; Ishiyama, T.; Kubota, K.; Ito, H. Chem. Sci. 2019, 10, 8202–8210. doi:10.1039/c9sc02185j |
| 44. | Seo, T.; Kubota, K.; Ito, H. J. Am. Chem. Soc. 2020, 142, 9884–9889. doi:10.1021/jacs.0c01739 |
| 45. | Seo, T.; Toyoshima, N.; Kubota, K.; Ito, H. J. Am. Chem. Soc. 2021, 143, 6165–6175. doi:10.1021/jacs.1c00906 |
| 46. | Takahashi, R.; Seo, T.; Kubota, K.; Ito, H. ACS Catal. 2021, 11, 14803–14810. doi:10.1021/acscatal.1c03731 |
| 47. | Kubota, K.; Kondo, K.; Seo, T.; Ito, H. Synlett 2022, 33, 898–902. doi:10.1055/a-1748-3797 |
| 48. | Kubota, K.; Seo, T.; Koide, K.; Hasegawa, Y.; Ito, H. Nat. Commun. 2019, 10, No. 111. doi:10.1038/s41467-018-08017-9 |
| 49. | Kubota, K.; Takahashi, R.; Uesugi, M.; Ito, H. ACS Sustainable Chem. Eng. 2020, 8, 16577–16582. doi:10.1021/acssuschemeng.0c05834 |
| 50. | Kubota, K.; Endo, T.; Uesugi, M.; Hayashi, Y.; Ito, H. ChemSusChem 2022, 15, e202102132. doi:10.1002/cssc.202102132 |
| 48. | Kubota, K.; Seo, T.; Koide, K.; Hasegawa, Y.; Ito, H. Nat. Commun. 2019, 10, No. 111. doi:10.1038/s41467-018-08017-9 |
| 49. | Kubota, K.; Takahashi, R.; Uesugi, M.; Ito, H. ACS Sustainable Chem. Eng. 2020, 8, 16577–16582. doi:10.1021/acssuschemeng.0c05834 |
| 50. | Kubota, K.; Endo, T.; Uesugi, M.; Hayashi, Y.; Ito, H. ChemSusChem 2022, 15, e202102132. doi:10.1002/cssc.202102132 |
| 51. | Shao, Q.-L.; Jiang, Z.-J.; Su, W.-K. Tetrahedron Lett. 2018, 59, 2277–2280. doi:10.1016/j.tetlet.2018.04.078 |
| 52. | Cao, Q.; Nicholson, W. I.; Jones, A. C.; Browne, D. L. Org. Biomol. Chem. 2019, 17, 1722–1726. doi:10.1039/c8ob01781f |
| 57. | Cao, Q.; Howard, J. L.; Wheatley, E.; Browne, D. L. Angew. Chem., Int. Ed. 2018, 57, 11339–11343. doi:10.1002/anie.201806480 |
| 34. | Schneider, F.; Ondruschka, B. ChemSusChem 2008, 1, 622–625. doi:10.1002/cssc.200800086 |
| 35. | Cravotto, G.; Garella, D.; Tagliapietra, S.; Stolle, A.; Schüßler, S.; Leonhardt, S. E. S.; Ondruschka, B. New J. Chem. 2012, 36, 1304–1307. doi:10.1039/c2nj40064b |
| 36. | Nielsen, S. F.; Peters, D.; Axelsson, O. Synth. Commun. 2000, 30, 3501–3509. doi:10.1080/00397910008087262 |
| 37. | Klingensmith, L. M.; Leadbeater, N. E. Tetrahedron Lett. 2003, 44, 765–768. doi:10.1016/s0040-4039(02)02645-x |
| 38. | Bernhardt, F.; Trotzki, R.; Szuppa, T.; Stolle, A.; Ondruschka, B. Beilstein J. Org. Chem. 2010, 6, No. 7. doi:10.3762/bjoc.6.7 |
| 39. | Schneider, F.; Szuppa, T.; Stolle, A.; Ondruschka, B.; Hopf, H. Green Chem. 2009, 11, 1894–1899. doi:10.1039/b915744c |
| 40. | Schneider, F.; Stolle, A.; Ondruschka, B.; Hopf, H. Org. Process Res. Dev. 2009, 13, 44–48. doi:10.1021/op800148y |
| 41. | Jiang, Z.-J.; Li, Z.-H.; Yu, J.-B.; Su, W.-K. J. Org. Chem. 2016, 81, 10049–10055. doi:10.1021/acs.joc.6b01938 |
| 42. | Báti, G.; Csókás, D.; Yong, T.; Tam, S. M.; Shi, R. R. S.; Webster, R. D.; Pápai, I.; García, F.; Stuparu, M. C. Angew. Chem., Int. Ed. 2020, 59, 21620–21626. doi:10.1002/anie.202007815 |
| 43. | Seo, T.; Ishiyama, T.; Kubota, K.; Ito, H. Chem. Sci. 2019, 10, 8202–8210. doi:10.1039/c9sc02185j |
| 44. | Seo, T.; Kubota, K.; Ito, H. J. Am. Chem. Soc. 2020, 142, 9884–9889. doi:10.1021/jacs.0c01739 |
| 45. | Seo, T.; Toyoshima, N.; Kubota, K.; Ito, H. J. Am. Chem. Soc. 2021, 143, 6165–6175. doi:10.1021/jacs.1c00906 |
| 46. | Takahashi, R.; Seo, T.; Kubota, K.; Ito, H. ACS Catal. 2021, 11, 14803–14810. doi:10.1021/acscatal.1c03731 |
| 47. | Kubota, K.; Kondo, K.; Seo, T.; Ito, H. Synlett 2022, 33, 898–902. doi:10.1055/a-1748-3797 |
| 58. | Declerck, V.; Colacino, E.; Bantreil, X.; Martinez, J.; Lamaty, F. Chem. Commun. 2012, 48, 11778–11780. doi:10.1039/c2cc36286d |
| 59. | Tullberg, E.; Schacher, F.; Peters, D.; Frejd, T. Synthesis 2006, 1183–1189. doi:10.1055/s-2006-926371 |
| 60. | Tullberg, E.; Peters, D.; Frejd, T. J. Organomet. Chem. 2004, 689, 3778–3781. doi:10.1016/j.jorganchem.2004.06.045 |
| 16. | James, S. L.; Adams, C. J.; Bolm, C.; Braga, D.; Collier, P.; Friščić, T.; Grepioni, F.; Harris, K. D. M.; Hyett, G.; Jones, W.; Krebs, A.; Mack, J.; Maini, L.; Orpen, A. G.; Parkin, I. P.; Shearouse, W. C.; Steed, J. W.; Waddell, D. C. Chem. Soc. Rev. 2012, 41, 413–447. doi:10.1039/c1cs15171a |
| 17. | Bowmaker, G. A. Chem. Commun. 2013, 49, 334–348. doi:10.1039/c2cc35694e |
| 18. | Wang, G.-W. Chem. Soc. Rev. 2013, 42, 7668–7700. doi:10.1039/c3cs35526h |
| 19. | Hernández, J. G.; Bolm, C. J. Org. Chem. 2017, 82, 4007–4019. doi:10.1021/acs.joc.6b02887 |
| 20. | Stolle, A.; Szuppa, T.; Leonhardt, S. E. S.; Ondruschka, B. Chem. Soc. Rev. 2011, 40, 2317–2329. doi:10.1039/c0cs00195c |
| 21. | Kubota, K.; Ito, H. Trends Chem. 2020, 2, 1066–1081. doi:10.1016/j.trechm.2020.09.006 |
| 22. | Howard, J. L.; Cao, Q.; Browne, D. L. Chem. Sci. 2018, 9, 3080–3094. doi:10.1039/c7sc05371a |
| 23. | Braga, D.; Maini, L.; Grepioni, F. Chem. Soc. Rev. 2013, 42, 7638–7648. doi:10.1039/c3cs60014a |
| 24. | Achar, T. K.; Bose, A.; Mal, P. Beilstein J. Org. Chem. 2017, 13, 1907–1931. doi:10.3762/bjoc.13.186 |
| 25. | Do, J.-L.; Friščić, T. ACS Cent. Sci. 2017, 3, 13–19. doi:10.1021/acscentsci.6b00277 |
| 26. | Métro, T.-X.; Martinez, J.; Lamaty, F. ACS Sustainable Chem. Eng. 2017, 5, 9599–9602. doi:10.1021/acssuschemeng.7b03260 |
| 27. | Eguaogie, O.; Vyle, J. S.; Conlon, P. F.; Gîlea, M. A.; Liang, Y. Beilstein J. Org. Chem. 2018, 14, 955–970. doi:10.3762/bjoc.14.81 |
| 28. | Bolm, C.; Hernández, J. G. Angew. Chem., Int. Ed. 2019, 58, 3285–3299. doi:10.1002/anie.201810902 |
| 29. | Friščić, T.; Mottillo, C.; Titi, H. M. Angew. Chem., Int. Ed. 2020, 59, 1018–1029. doi:10.1002/anie.201906755 |
| 30. | Andersen, J.; Mack, J. Green Chem. 2018, 20, 1435–1443. doi:10.1039/c7gc03797j |
| 31. | Porcheddu, A.; Colacino, E.; De Luca, L.; Delogu, F. ACS Catal. 2020, 10, 8344–8394. doi:10.1021/acscatal.0c00142 |
| 32. | Leitch, J. A.; Browne, D. L. Chem. – Eur. J. 2021, 27, 9721–9726. doi:10.1002/chem.202100348 |
| 33. | Kaupp, G. CrystEngComm 2009, 11, 388–403. doi:10.1039/b810822f |
| 15. | Dzhevakov, P. B.; Topchiy, M. A.; Zharkova, D. A.; Morozov, O. S.; Asachenko, A. F.; Nechaev, M. S. Adv. Synth. Catal. 2016, 358, 977–983. doi:10.1002/adsc.201500844 |
| 15. | Dzhevakov, P. B.; Topchiy, M. A.; Zharkova, D. A.; Morozov, O. S.; Asachenko, A. F.; Nechaev, M. S. Adv. Synth. Catal. 2016, 358, 977–983. doi:10.1002/adsc.201500844 |
| 53. | Fulmer, D. A.; Shearouse, W. C.; Medonza, S. T.; Mack, J. Green Chem. 2009, 11, 1821–1825. doi:10.1039/b915669k |
| 54. | Liang, Y.; Xie, Y.-X.; Li, J.-H. J. Org. Chem. 2006, 71, 379–381. doi:10.1021/jo051882t |
| 55. | Thorwirth, R.; Stolle, A.; Ondruschka, B. Green Chem. 2010, 12, 985–991. doi:10.1039/c000674b |
| 56. | Gao, Y.; Feng, C.; Seo, T.; Kubota, K.; Ito, H. Chem. Sci. 2022, 13, 430–438. doi:10.1039/d1sc05257h |
© 2022 Kubota et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.