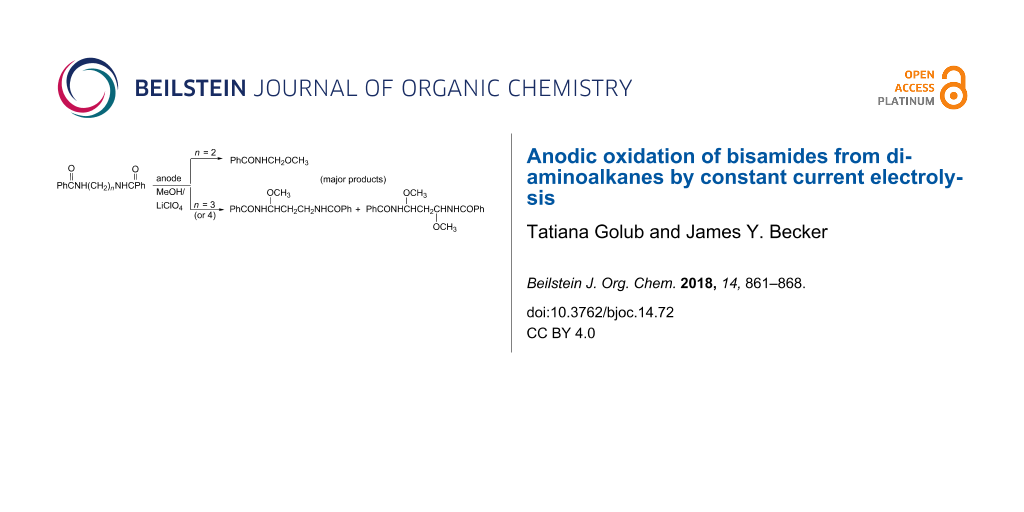
Abstract
In general, bisamides derived from diamines and involving 3 and 4 methylene groups as spacers between the two amide functionalities behave similar to monoamides upon anodic oxidation in methanol/LiClO4 because both types undergo majorly mono- and dimethoxylations at the α-position to the N atom. However, in cases where the spacer contains two methylene groups only the anodic process leads mostly to CH2–CH2 bond cleavage to afford products of type RCONHCH2OCH3. Moreover, upon replacing LiClO4 with Et4NBF4 an additional fragmentation type of product was generated from the latter amides, namely RCONHCHO. Also, the anodic process was found to be more efficient with C felt as the anode, and in a mixture of 1:1 methanol/acetonitrile co-solvents.
Graphical Abstract
Introduction
It is well known that the anodic oxidation of amides involving a hydrogen atom at the α-position to the N atom could undergo alkoxylation, carboxylation and hydroxylation at this position [1-5] (Scheme 1). It has been found that anodic methoxylation of amides (and carbamates) can be utilized to form new carbon–carbon bonds [6,7] (Scheme 2). Furthermore, this anodic route could also be important from a synthetic point of view, affording ring-expansion [8-10] and annulation of rings [1,11,12] (Scheme 3).
Scheme 2: Anodic oxidation of an amide in the presence of alkene.
Scheme 2: Anodic oxidation of an amide in the presence of alkene.
Scheme 3: Intramolecular cyclization via anodic oxidation of an eneamide.
Scheme 3: Intramolecular cyclization via anodic oxidation of an eneamide.
Interestingly, in the case of anodic oxidation of aromatic amides of type Ph2CHCONHAr, where no hydrogen atom is present at the α-position to the N atom, they undergo three types of bond cleavages (instead of the common substitution) [13] (Scheme 4).
Scheme 4: Anodic bond cleavages in amides of type Ph2CHCONHAr.
Scheme 4: Anodic bond cleavages in amides of type Ph2CHCONHAr.
Previously we investigated [14] the effect of the ring size (5-, 6-, and 7-membered) and the nature of the supporting electrolyte on the anodic methoxylation of N-acylazacycloalkanes at the α-position to the nitrogen. The outcome revealed the formation of four types of products of which two involve saturated and two unsaturated cyclic amides (Scheme 5).
Scheme 5: Type of products obtained (n = 0, 1, 2).
Scheme 5: Type of products obtained (n = 0, 1, 2).
The selectivity of the anodic process was found to be highly dependent on the electrolyte used, and to a lesser extent, on the substrate concentration. Notably the importance of the former parameter in electrolysis has been well documented [15,16].
More recently the effect of the functional group attached to the N atom in various piperidine derivatives (Scheme 6) on the anodic oxidation of 'cyclic amides' was explored in methanol under different experimental conditions [17]. The results indicate that this type of amides mostly undergo mono- and dimethoxylation at the α and α'-positions to the N atom. It was also found that the relative ratio among products was strongly dependent on the nature of the supporting electrolyte, the anode material and the substituent group attached to the N atom.
Scheme 6: Synthesized cyclic N-acyl and N-sulfonyl piperidines for electrolysis.
Scheme 6: Synthesized cyclic N-acyl and N-sulfonyl piperidines for electrolysis.
Amides and polyamides have been found as key units in many biologically active and pharmaceutical compounds. For instance, symmetrical and unsymmetrical bisamides derived from diamines are significant components as structural subunits for the construction of peptidomimetric frameworks [18] and as lubricants [19]. To the best of our knowledge nothing has been known so far about electrochemical properties of α,ω-bisamides derived from α,ω-diaminoalkanes. However, notably that the bisamide 3,5-diaza-2,6-heptanedione was obtained from N-methylacetamide by electrolysis on a Pt anode in water [20].
The present work describes the electrochemical behavior of eight synthesized bisamides (from diamines, Scheme 7) and the outcome from their preparative electrolysis (at constant current) under different experimental conditions, as a function of the length of spacer between the two amide functionalities.
Scheme 7: Type of bisamides (derived from diamines) studied (n = 2, 3, 4).
Scheme 7: Type of bisamides (derived from diamines) studied (n = 2, 3, 4).
Results and Discussion
The electrochemical properties of the bisamides described in Scheme 6 were studied by cyclic voltammetry and all of their redox potentials were found to be irreversible in acetonitrile. Their first oxidation potentials (in the range of 2.1–2.35 V vs Ag/AgCl) are summarized in Table 1. The first and second columns indicate that the longer the spacer the higher the oxidation potential. Also it is not surprising that the derivative with EWG (IV) is more difficult to oxidize than that with EDG (III). All bisamides derivatives exhibit one irreversible cathodic wave (not shown, at −2.2 to −2.4 V).
Table 1: Oxidation potentials of α,ω-bisamides (Scheme 7) measured by cyclic voltammetrya.
| entry | Ep(ox) (V) | |||
|---|---|---|---|---|
| I | II | III | IV | |
| n = 2 | 2.10 | 2.13 | 2.17 | 2.33 |
| n = 3 | 2.23 | 2.28 | ||
| n = 4 | 2.27 | 2.35 | ||
aIn CH3CN/0.1 M LiClO4; potentials are quoted versus Ag/AgCl reference electrode. Working electrode: glassy carbon disk (1.5 mm in diameter). Auxiliary electrode: a Pt wire.
Constant current electrolysis (CCE) at a current density of 20 mA/cm2 was carried out for all of the above synthesized bisamides under various experimental conditions, using different supporting electrolytes, anodes, and electricity consumption. Bisamide II (n = 3, will be designated as II-3 hereafter) was arbitrarily chosen as a model compound for initial electrochemical studies. The spectrum of products obtained is described in Scheme 8. Except for the expected monomethoxylated II-3a and dimethoxylated II-3b products, fragmentation products (II-3c, 3d, 3e) were observed too.
Scheme 8: Type of products obtained from anodic oxidation of II-3.
Scheme 8: Type of products obtained from anodic oxidation of II-3.
Table 2 below summarizes the type of products and their relative ratios obtained from initial electrochemical oxidation of II-3 under various experimental conditions. It appears that the selectivity and efficiency of the anodic process depends on both the anode material and electricity consumption (F/mol). Thus the oxidation of II-3 on a C anode (Table 2, entries 1 and 2) is quite selective providing mostly mono-II-3a and dimethoxy- II-3b products in addition to ≈10% of methyl benzoate (II-3d) as a fragment. Notably that 20–30% of unreacted substrate remains. An increase in electricity consumption (entries 2 vs 1) promotes the formation of the dimethoxy product II-3b over the monomethoxy one II-3a.
Table 2: The effects of anode material and electricity consumption on the results of anodic oxidation of substrate II-3 in MeOH/LiClO4a.
| entry | F/mol | anode material | products | unreacted substrate | ||||
|---|---|---|---|---|---|---|---|---|
| II-3a | II-3b | II-3c | methyl benzoate (II-3d) | benzoic acid (II-3e) | ||||
| 1 | 5 | C | 40 | 18 | – | 12 | – | 30 |
| 2 | 10 | C | 27 | 40 | 5 | 9 | – | 19 |
| 3 | 5 | Pt | 26 | 8 | – | 21 | 10 | 35 |
| 4 | 10 | Pt | 20 | 40 | 5 | 5 | 20 | 10 |
| 5b | 10 | GC | 43 | 12 | – | 5 | 3 | 36 |
| 6c | 10 | PbO2 | – | – | – | – | – | 90 |
| 7d | 10 | DSA | – | – | – | – | – | ≈100 |
aYield of products was determined by 1H NMR relative integration. bGC = glassy carbon. cUnidentified products (≈10%). dDSA = dimensionally stable anode, coated with oxides of Ru and Ir.
In comparison to the above results, oxidation of II-3 on a Pt anode (Table 2, entries 3 and 4) affords similar products but with less selectivity because of the formation of an additional fragmentation product, benzoic acid (II-3e) in 10–20% yield. Other anodes were tested as well (Table 2, entries 5–7) at an electricity consumption of 10 F/mol. It appears that a GC anode favors the formation of the monomethoxy product II-3a whereas the anodes of PbO2 and DSA are the worst because most of the substrate remained unreacted. Apparently at these two anodes the oxidation of the solvent methanol prevails.
Based on the results in Table 2 in which a C anode afforded better selectivity and efficiency compared to the other anodes studied, all other substrates outlined in Scheme 7 were oxidized at this anode and under the same conditions (namely, in methanol/LiClO4, at 20 mA/cm2, and with electricity consumption of 10 F/mol). The results are shown in Table 3. It appears that except for entries 5 and 6, the amounts of unreacted starting materials are considerably high (60–70%), indicating that the reaction is far from being efficient under these conditions, presumably because of favorable oxidation of the solvent methanol. Another reason for this observation could stem partially from the limited solubility of some of the substrates in this solvent (e.g., II-2, III-2 and IV-2). The yield of total products is in the range of 30–80%, depending on the nature of the substituent attached to the carbonyl moiety, roughly in the order of: Ph > CH3 > p-MeOC6H4, p-NO2C6H4. In terms of type of products, they are mostly analogous to those described in Scheme 8 for II-3. However, it is obvious that in the case of anodic oxidation of substrates with n = 2, an additional new fragmented product (type f) was formed due to CH2–CH2 bond cleavage, which is exclusive for this type of bisamides (Scheme 9).
Scheme 9: Anodic splitting of C–C bond in bisamides in the presence of LiClO4 electrolyte.
Scheme 9: Anodic splitting of C–C bond in bisamides in the presence of LiClO4 electrolyte.
Table 3: Results of anodic oxidation of all substrates on a C rod anode in MeOH/LiClO4. (10 F/mol; 20 mA/cm2)a.
| entry | substrate | monomethoxy type 'a' | dimethoxy type 'b' | methoxylated amide type 'c' | methyl benzoate 'd' | RCONHCH2OCH3 'f' | unreacted substrate |
|---|---|---|---|---|---|---|---|
| 1 | I-2 (n = 2) | 10 | – | – | – | 25 | 65 |
| 2 | I-3 (n = 3) | 30 | 10 | – | – | – | 60 |
| 3 | I-4 (n = 4) | 5 | 30 | – | – | – | 65 |
| 4 | II-2 | 9 | – | – | 5 | 23 | 63 |
| 5 | II-3 | 27 | 40 | 5 | 9 | – | 19 |
| 6 | II-4 | 26 | 27 | – | 17 | – | 30 |
| 7 | III-2 | 15 | – | – | – | 15 | 70 |
| 8b | IV-2 | – | – | – | – | 10 | 65 |
aRelative yields of all products were determined by 1H NMR integration. Analogous type of products (a–d) are described in Scheme 7. bPhCONH2 was obtained in 10% yield along with 25% of unidentified products.
Obviously the fact that considerable amounts of starting materials were left unreacted (in most cases, except for Table 3, entries 5 and 6) has been dissatisfying and therefore, prompted us to change some parameters. At first, the LiClO4 electrolyte was replaced by Et4NBF4 and the results are shown in Table 4. Clearly the bisamides with n = 2 afforded now, in addition to f, new fragmented products, aldehydes of type g (Scheme 10).
Scheme 10: Anodic splitting of C–C bond in bisamides in the presence of Et4NBF4 electrolyte.
Scheme 10: Anodic splitting of C–C bond in bisamides in the presence of Et4NBF4 electrolyte.
In addition, the relative yield of the fragmented products (f and g, both derived exclusively from bisamides with n = 2) increased considerably in the presence of this electrolyte. However, again, the amounts of unreacted starting materials were still significant in some cases.
Table 4: Results of preparative electrolysis of selected bisamides in MeOH/Et4NBF4 (at C rod anode, 10F, 20 mA/cm2)a.
| entry | substrate | monomethoxy type 'a' | dimethoxy type 'b' | RCONHCH2OCH3 'f' | RCONHC(O)H 'g' | unreacted substrate |
|---|---|---|---|---|---|---|
| 1 | I-2 | 10 | – | 51 | 26 | 13 |
| 2 | I-3 | 10 | 10 | – | – | 80 |
| 3 | I-4 | 13 | – | – | – | 87 |
| 4 | II-2 | – | – | 69 | 17 | 14 |
| 5 | III-2 (p-OMe) | – | – | 23 | – | 77 |
| 6 | IV-2 (p-NO2) | – | – | 51 | 31 | 18 |
aYields were determined by 1H NMR relative integration. Analogous type of products a,b are described in Scheme 7.
The pronounced difference between the results obtained by the two electrolytes, namely affording 'f' with LiClO4, and 'g' in addition to 'f' with Et4NBF4, could stem from the different composition of the solution at the electrode surface (caused by different solvation of the electrolyte anion) than that of the bulk of the solution. Such a phenomenon was discussed in the literature previously [15,16,21]. For instance, Nyberg [16] already demonstrated the effect of ClO4− vs BF4− in the anodic oxidation of hexamethylbenzene in aqueous acetonitrile, and proposed that tetraflouroborate anion preferentially brought water into the anode surface giving high yield of ArCH2OH (compared to ArCH2NHCOMe with perchlorate). Therefore also in our case, the electro generated carbocation intermediate formed (RCONHCH2+) could meet with methanol (to form 'f') or water (to form 'g') preferentially at the electrode surface, dictating the ratio between products 'f' and 'g'.
In order to increase the solubility of the substrates with a limited one a mixture of MeOH/MeCN (1:1) was used, and in parallel, the C rod anode was replaced with a C felt (that has a considerable larger surface area) in attempts to improve both efficiency and selectivity. The results are described in Table 5 and they show a pronounced difference compared to the ones in Table 4 because product yields are higher now (Table 5, entries 1, 4 and 5 show almost completion) even after consuming only 5 F/mol. In addition, although the spectrum of major products is similar in both tables, the weight of monomethoxylated products (51–56%, entries 2 and 5) and fragmented ones (of type f, 85–95%, entries 1 and 4) increased at the expense of consumed starting material (in all entries except for entry 8).
Table 5: Resultsa of anodic oxidation of bisamides on a C felt anode in MeOH/MeCN (1:1)/LiClO4; 20 mA/cm2; 5 F/mol).
| entry | substrate | monomethoxy type 'a' | dimethoxy type 'b' |
ester type
'd' |
benzoic acid
'e' |
RCONHCH2OCH3
'f' |
unreacted substrate |
|---|---|---|---|---|---|---|---|
| 1 | I-2 | 5 | – | – | – | 95 | – |
| 2 | I-3 | 56 | 7 | – | – | –b | 34 |
| 3 | I-4 | 20 | 30 | – | – | – | 50 |
| 4 | II-2 | – | – | 15 | – | 85 | – |
| 5 | II-3 | 51 | 24 | 8 | 5 | (h)c | 5 |
| 6 | II-4 | 19 | 22 | 16 | – | – | 43 |
| 7 | III-2 | 10 | – | 5 | – | 25 | 60 |
| 8 | IV-2 | – | – | 5 | 5 | 5 | 85 |
aYield are determined by 1H NMR relative integration. Analogous type of products (type a, b, d, e) are described in Scheme 7. bAldehyde (3%): MeCONHCH2CH2CHO (from, I-3) [22]. cUnsaturated bisamide (7%): PhCONHCH2CH=CHNHCOPh (II-3h).
Previously (Table 3 vs Table 4) we observed a marked difference in results upon replacing LiClO4 with Et4NBF4. Whereas in the former case mono- and dimethoxylated products were predominant, fragmentation products of type f and g became major in the latter case. Based on these observations a further attempt to improve the results outlined in Table 5 was conducted by employing similar conditions except for using Et4NBF4 (instead of LiClO4) this time. Some selected substrates from Table 5 that left a considerable amount of unreacted starting material, namely I-4, II-4, III-2 and IV-2, were chosen to be reoxidized under these modified conditions. The outcome described in Table 6 indicates that this approach was useful for one substrate only, II-4.
Table 6: Results of anodic oxidation of selected substrates on C felt anode in MeOH/MeCN (1:1)/Et4NBF4; 20 mA/cm2; 5 F/mol.
| entry | substrate | monomethoxy type 'a' |
dimethoxy type
'b' |
ester type
'd' |
fragmented
products |
unreacted substrate |
|---|---|---|---|---|---|---|
| 1 | I-4 | 10 | – | – |
MeCONHCHO
(I-4g, 5%) |
85 |
| 2 | II-4 | 12 | 74 | 8 | – | 6 |
| 3 | III-2 (p-OMe) | – | – | 15 |
ArCONHCH2OCH3
(III-2f, 10%) |
75 |
| 4 | IV-2 (p-NO2) | – | – | 31 | – | 69 |
Mechanism
A mechanism of formation of mono- and dimethoxylated amides is well-documented in the published literature [1-6,14,17,23]. It is generally accepted that the initial electron transfer forms an iminium cation radical followed by deprotonation and further oxidation to generate an iminium ion/carbocation that undergoes methoxylation in methanol, as described in Scheme 11.
Scheme 11: A suggested mechanism for anodic methoxylation of amides.
Scheme 11: A suggested mechanism for anodic methoxylation of amides.
Plausible mechanisms for the formation of various fragmentation products are described in Scheme 12.
Scheme 12: Mechanisms of formation of fragmentation products.
Scheme 12: Mechanisms of formation of fragmentation products.
Actually whenever benzoic acid or methyl benzoate were formed (top of Scheme 12), the corresponding aldehyde from the other part of the molecule was detected too and fully characterized.
Conclusion
In general, bisamides derived from diamines and involving 3 and 4 methylene groups as spacers between the two amide functionalities behave similary to monoamides upon anodic oxidation in methanol/LiCiO4 because both types undergo majorly mono- and dimethoxylations at the α-position to the N atom. However, in cases where the spacer contains two methylene groups only the anodic process leads mostly to CH2–CH2 bond cleavage to afford products of type RCONHCH2OCH3. Moreover, upon replacing LiClO4 with Et4NBF4 an additional fragmentation type of product was generated from the latter amides, namely RCONHCHO. Also, the anodic process was found to be more efficient with C felt as the anode, and in a mixture of 1:1 methanol/acetonitrile co-solvents.
Experimental
Materials
Reagents, electrolytes and solvents (all analytical grade) were supplied by various vendors, as mentioned in [23].
Preparation of bisamides I–III
All types of bisamides were prepared according to our own procedure by reacting the corresponding diamines (commercially available) with acetic anhydride, or benzoyl chloride or 4-methoxybenzoyl chloride. In a typical experiment, 30 mmol of a diamine (ethylenediamide, 1,3-diaminopropane or 1,4-diaminobutane) were introduced into a 500 mL Erlenmeyer flask with 50 mL of DCM and 20 mL of saturated aqueous bicarbonate solution. Then 70 mmol of acetic anhydride (or benzoyl chloride or 4-methoxybenzoyl chloride) were added dropwise by a separatory funnel to the diamine solution while stirring by a magnetic stirrer. Then the reaction mixture was filtered under vacuum and the solid residue was recrystallized from a mixture of ethyl acetate and water (9:1). The resulting white precipitate (except for the yellowish one derived from p-nitrobenzene derivative) was dried, weighed and verified by NMR spectra. The isolated yields of the bisamides were around 72–86%.
General methods
Instruments used in this study for 1H NMR and 13C NMR measurements, mass and IR spectra, high-resolution mass analyses, and cyclic voltammetry were described in [23].
Thin-layer chromatography (TLC) was carried out on aluminum sheets coated with aluminum oxide 60 F254 and silica gel 60 F254. Retention time was evaluated by UV (for amides with benzene ring) or by using a general purpose stain of cerium molybdate [containing a mixture of Ce(NH4)2(NO3)6 - (NH4)6Mo7·4H2O in H2SO4] for amides of type I. Preparative TLC was carried out by using 20 × 20 cm of glass plates coated with silica gel 60 F254. Evaporation of solvents was performed at reduced pressure using a rotary evaporator.
Constant current electrolysis
Constant current electrolysis at preparative scale was performed at constant currents using a PAR Potentiostat/Galvanostat Model 273A, and a beaker-type undivided cell equipped with a C rod, C felt, PbO2, GC, DSA or a Pt foil (immersed area of ≈5 cm2) as the anode, and a Pt foil as the cathode. In a typical electrolysis α,ω-bisamides (1 mmol) were dissolved in methanol (or 1:1 methanol/acetonitrile, 25 mL) containing 0.1 M supporting electrolytes. Electrolysis took place at room temperature with a current density of 20 mA/cm2 and was terminated after the desired consumption of electricity was passed (Tables 2–5). Then the reaction mixture was concentrated by rotary evaporator till all the solvents evaporated. The relative yield of products was determined by 1H NMR integration. Notably this procedure of analyzing a mixture of products simultaneously and successfully is based on prior separation and characterization of the individual products in the mixture. Their previous separation was carried out either by silica gel column chromatography or preparative coated glass plates, using different mixtures of ethyl acetate (20–50%)/hexane or acetone/ethyl acetate, as eluent. Also since some of the products undergo facile hydrolysis/decomposition it is suggested that the analysis will be done immediately after terminating the electrolysis.
Characterization of products
See Supporting Information File 1 for spectral data and copies of 1H and 13C NMR spectra.
Supporting Information
| Supporting Information File 1: Spectral data and copies of 1H and 13C NMR spectra. | ||
| Format: PDF | Size: 1.4 MB | Download |
References
-
Steckhan, E. Anodic oxidation of nitrogen-containing compounds. In Organic Electrochemistry, 4th ed.; Lund, H.; Hemmerich, O., Eds.; Ch. 15; Marcel Dekker, Inc.: NY, 2001; pp 570–602.
Return to citation in text: [1] [2] [3] -
Grimshaw, J. Electrochemical reactions and mechanisms in organic electrochemistry; Ch. 8; Elsevier, 2000; pp 282–290.
Return to citation in text: [1] [2] -
Torii, S. Electroorganic synthesis, Part 1: Oxidations; Ch. 5.2; Kodansha VCH, 1985; pp 171–180.
Return to citation in text: [1] [2] -
Shono, T.; Matsumura, Y.; Tsubata, K.; Sugihara, Y.; Yamane, S.; Kanazawa, T.; Aoki, T. J. Am. Chem. Soc. 1982, 104, 6697–6703. doi:10.1021/ja00388a037
Return to citation in text: [1] [2] -
Shono, T.; Matsumura, Y.; Onomura, O.; Yamamoto, Y. Tetrahedron Lett. 1987, 28, 4073–4074. doi:10.1016/S0040-4039(01)83864-8
Return to citation in text: [1] [2] -
Genies, M. Bull. Soc. Chim. Fr. 1975, 389–392.
Return to citation in text: [1] [2] -
Shono, T.; Hamaguchi, H.; Matsumura, Y. J. Am. Chem. Soc. 1975, 97, 4264–4268. doi:10.1021/ja00848a020
Return to citation in text: [1] -
Wong, P. L.; Moeller, K. D. J. Am. Chem. Soc. 1993, 115, 11434–11445. doi:10.1021/ja00077a048
Return to citation in text: [1] -
Moeller, K. D.; Rutledge, L. D. J. Org. Chem. 1992, 57, 6360–6363. doi:10.1021/jo00049a060
Return to citation in text: [1] -
Moeller, K. D.; Rothfus, S. L.; Wong, P. L. Tetrahedron 1991, 47, 583–592. doi:10.1016/S0040-4020(01)87048-4
Return to citation in text: [1] -
Okita, M.; Wakamatsu, T.; Mori, M.; Ban, Y. Heterocycles 1980, 14, 1089–1092. doi:10.3987/R-1980-08-1089
Return to citation in text: [1] -
Okita, M.; Wakamatsu, T.; Ban, Y. Heterocycles 1983, 20, 401–404. doi:10.3987/R-1983-03-0401
Return to citation in text: [1] -
Golub, T.; Becker, J. Y. Org. Biomol. Chem. 2012, 10, 3906–3912. doi:10.1039/c2ob06878h
Return to citation in text: [1] -
Golub, T.; Becker, J. Y. J. Electrochem. Soc. 2013, 160, G3123–G3127. doi:10.1149/2.017307jes
Return to citation in text: [1] [2] -
Eberson, L.; Olofsson, B. Acta Chem. Scand. 1969, 23, 2355–2366. doi:10.3891/acta.chem.scand.23-2355
Return to citation in text: [1] [2] -
Nyberg, K. J. Chem. Soc. D 1969, 774–775. doi:10.1039/c29690000774
Return to citation in text: [1] [2] [3] -
Golub, T.; Becker, J. Y. Electrochim. Acta 2015, 173, 408–415. doi:10.1016/j.electacta.2015.04.133
Return to citation in text: [1] [2] -
Harichandran, G.; Amalraj, S. D.; Shanmugan, P. Indian J. Chem. 2011, 50B, 77–82.
Return to citation in text: [1] -
Williams, J. B.; Geick, K. S.; Falter, J. A. J. Vinyl Addit. Technol. 1997, 3, 216–219. doi:10.1002/vnl.10194
Return to citation in text: [1] -
Couch, D. E. Electrochim. Acta 1964, 9, 327–336. doi:10.1016/0013-4686(64)80040-2
Return to citation in text: [1] -
Mayeda, E. A.; Miller, L. L. Tetrahedron 1972, 28, 3375–3380. doi:10.1016/0040-4020(72)88098-0
Return to citation in text: [1] -
Kalisiak, J.; Trauger, S. A.; Kalisiak, E.; Morita, H.; Fokin, V. V.; Adams, M. W. W.; Sharpless, K. B.; Siuzdak, G. J. Am. Chem. Soc. 2009, 131, 378–386. doi:10.1021/ja808172n
Return to citation in text: [1] -
Golub, T.; Becker, J. Y. Electrochim. Acta 2016, 205, 207–214. doi:10.1016/j.electacta.2016.04.074
Return to citation in text: [1] [2] [3]
| 23. | Golub, T.; Becker, J. Y. Electrochim. Acta 2016, 205, 207–214. doi:10.1016/j.electacta.2016.04.074 |
| 1. | Steckhan, E. Anodic oxidation of nitrogen-containing compounds. In Organic Electrochemistry, 4th ed.; Lund, H.; Hemmerich, O., Eds.; Ch. 15; Marcel Dekker, Inc.: NY, 2001; pp 570–602. |
| 2. | Grimshaw, J. Electrochemical reactions and mechanisms in organic electrochemistry; Ch. 8; Elsevier, 2000; pp 282–290. |
| 3. | Torii, S. Electroorganic synthesis, Part 1: Oxidations; Ch. 5.2; Kodansha VCH, 1985; pp 171–180. |
| 4. | Shono, T.; Matsumura, Y.; Tsubata, K.; Sugihara, Y.; Yamane, S.; Kanazawa, T.; Aoki, T. J. Am. Chem. Soc. 1982, 104, 6697–6703. doi:10.1021/ja00388a037 |
| 5. | Shono, T.; Matsumura, Y.; Onomura, O.; Yamamoto, Y. Tetrahedron Lett. 1987, 28, 4073–4074. doi:10.1016/S0040-4039(01)83864-8 |
| 13. | Golub, T.; Becker, J. Y. Org. Biomol. Chem. 2012, 10, 3906–3912. doi:10.1039/c2ob06878h |
| 1. | Steckhan, E. Anodic oxidation of nitrogen-containing compounds. In Organic Electrochemistry, 4th ed.; Lund, H.; Hemmerich, O., Eds.; Ch. 15; Marcel Dekker, Inc.: NY, 2001; pp 570–602. |
| 2. | Grimshaw, J. Electrochemical reactions and mechanisms in organic electrochemistry; Ch. 8; Elsevier, 2000; pp 282–290. |
| 3. | Torii, S. Electroorganic synthesis, Part 1: Oxidations; Ch. 5.2; Kodansha VCH, 1985; pp 171–180. |
| 4. | Shono, T.; Matsumura, Y.; Tsubata, K.; Sugihara, Y.; Yamane, S.; Kanazawa, T.; Aoki, T. J. Am. Chem. Soc. 1982, 104, 6697–6703. doi:10.1021/ja00388a037 |
| 5. | Shono, T.; Matsumura, Y.; Onomura, O.; Yamamoto, Y. Tetrahedron Lett. 1987, 28, 4073–4074. doi:10.1016/S0040-4039(01)83864-8 |
| 6. | Genies, M. Bull. Soc. Chim. Fr. 1975, 389–392. |
| 14. | Golub, T.; Becker, J. Y. J. Electrochem. Soc. 2013, 160, G3123–G3127. doi:10.1149/2.017307jes |
| 17. | Golub, T.; Becker, J. Y. Electrochim. Acta 2015, 173, 408–415. doi:10.1016/j.electacta.2015.04.133 |
| 23. | Golub, T.; Becker, J. Y. Electrochim. Acta 2016, 205, 207–214. doi:10.1016/j.electacta.2016.04.074 |
| 1. | Steckhan, E. Anodic oxidation of nitrogen-containing compounds. In Organic Electrochemistry, 4th ed.; Lund, H.; Hemmerich, O., Eds.; Ch. 15; Marcel Dekker, Inc.: NY, 2001; pp 570–602. |
| 11. | Okita, M.; Wakamatsu, T.; Mori, M.; Ban, Y. Heterocycles 1980, 14, 1089–1092. doi:10.3987/R-1980-08-1089 |
| 12. | Okita, M.; Wakamatsu, T.; Ban, Y. Heterocycles 1983, 20, 401–404. doi:10.3987/R-1983-03-0401 |
| 23. | Golub, T.; Becker, J. Y. Electrochim. Acta 2016, 205, 207–214. doi:10.1016/j.electacta.2016.04.074 |
| 8. | Wong, P. L.; Moeller, K. D. J. Am. Chem. Soc. 1993, 115, 11434–11445. doi:10.1021/ja00077a048 |
| 9. | Moeller, K. D.; Rutledge, L. D. J. Org. Chem. 1992, 57, 6360–6363. doi:10.1021/jo00049a060 |
| 10. | Moeller, K. D.; Rothfus, S. L.; Wong, P. L. Tetrahedron 1991, 47, 583–592. doi:10.1016/S0040-4020(01)87048-4 |
| 6. | Genies, M. Bull. Soc. Chim. Fr. 1975, 389–392. |
| 7. | Shono, T.; Hamaguchi, H.; Matsumura, Y. J. Am. Chem. Soc. 1975, 97, 4264–4268. doi:10.1021/ja00848a020 |
| 22. | Kalisiak, J.; Trauger, S. A.; Kalisiak, E.; Morita, H.; Fokin, V. V.; Adams, M. W. W.; Sharpless, K. B.; Siuzdak, G. J. Am. Chem. Soc. 2009, 131, 378–386. doi:10.1021/ja808172n |
| 18. | Harichandran, G.; Amalraj, S. D.; Shanmugan, P. Indian J. Chem. 2011, 50B, 77–82. |
| 20. | Couch, D. E. Electrochim. Acta 1964, 9, 327–336. doi:10.1016/0013-4686(64)80040-2 |
| 17. | Golub, T.; Becker, J. Y. Electrochim. Acta 2015, 173, 408–415. doi:10.1016/j.electacta.2015.04.133 |
| 15. | Eberson, L.; Olofsson, B. Acta Chem. Scand. 1969, 23, 2355–2366. doi:10.3891/acta.chem.scand.23-2355 |
| 16. | Nyberg, K. J. Chem. Soc. D 1969, 774–775. doi:10.1039/c29690000774 |
| 21. | Mayeda, E. A.; Miller, L. L. Tetrahedron 1972, 28, 3375–3380. doi:10.1016/0040-4020(72)88098-0 |
| 15. | Eberson, L.; Olofsson, B. Acta Chem. Scand. 1969, 23, 2355–2366. doi:10.3891/acta.chem.scand.23-2355 |
| 16. | Nyberg, K. J. Chem. Soc. D 1969, 774–775. doi:10.1039/c29690000774 |
| 14. | Golub, T.; Becker, J. Y. J. Electrochem. Soc. 2013, 160, G3123–G3127. doi:10.1149/2.017307jes |
| 19. | Williams, J. B.; Geick, K. S.; Falter, J. A. J. Vinyl Addit. Technol. 1997, 3, 216–219. doi:10.1002/vnl.10194 |
© 2018 Golub and Becker; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (https://www.beilstein-journals.org/bjoc)