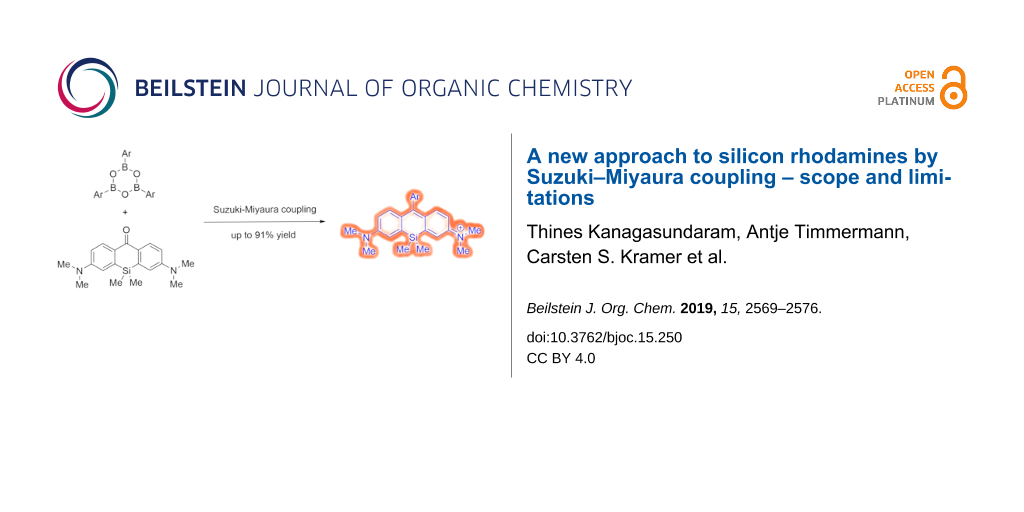
Abstract
Background: Silicon rhodamines are of particular interest because of their advantageous dye properties (fluorescence- and biostability, quantum efficiency, tolerance to photobleaching). Therefore, silicon rhodamines find frequent application in STED (stimulated emission depletion) microscopy, as sensor molecules for, e.g., ions and as fluorophores for the optical imaging of tumors. Different strategies were already employed for their synthesis. Because of just three known literature examples in which Suzuki–Miyaura cross couplings gave access to silicon rhodamines in poor to moderate yields, we wanted to improve these first valuable experimental results.
Results: The preparation of the xanthene triflate was enhanced and several boron sources were screened to find the optimal coupling partner. After optimization of the palladium catalyst, different substituted boroxines were assessed to explore the scope of the Pd-catalyzed cross-coupling reaction.
Conclusions: A number of silicon rhodamines were synthesized under the optimized conditions in up to 91% yield without the necessity of HPLC purification. Moreover, silicon rhodamines functionalized with free acid moieties are directly accessible in contrast to previously described methods.

Graphical Abstract
Introduction
Silicon rhodamines are versatile fluorescent dyes that found extensive use in super-resolution microscopy [1-8] and as probes for targeting various biomolecules [9-12] or sensors for metal ions [13-17], pH [15], voltage [18] or metabolites [19-22]. Since our group is interested in synthesizing new tumor tracers for intraoperative imaging of cancerous lesions, we were interested in silicon rhodamines due to their fluorescence properties in the biological window (650 nm to 1350 nm). While clinically approved fluorescence dyes like ICG (indocyanine green, Mw = 775 g/mol) have a high molecular weight and could therefore alter pharmacokinetic or -dynamic properties of the tumor tracers, silicon rhodamines are relatively small and already examined as fluorophores for the optical imaging of tumors. Using silicon rhodamine SiR700 a more enhanced tumor-to-background ratio in optical imaging could be achieved compared to the cyanine based dyes Cy5.5 and Alexa Fluor® 680 [23]. Moreover, silicon rhodamines demonstrated in in vivo imaging experiments excellent fluorescence properties and biostabilities [23] as well as exhibited high quantum efficiencies with high tolerance to photobleaching [24]. A silicon rhodamine antibody conjugate could also be successfully applied for optical imaging of a xenograft tumor (human malignant meningioma) in a mouse model [24]. Again, in direct comparison with the cyanine dye Cy5.5, the silicon rhodamine conjugate showed no fading indicating that silicon rhodamine dyes are more suitable for long time observation than cyanine-based fluorophores [24].
Different synthetic approaches were established to form the silicon rhodamine framework 1 (Scheme 1). While the group of Wu used a copper(II) bromide-catalyzed solvent-free condensation of a diarylsilane 2 with various benzaldehydes 3 [25], Sparr and Fischer added the double Grignard reagent 4 to methyl esters 5 [26]. A similar approach was established by Lavis, herein electrophiles (anhydrides or esters) were added to lithium or magnesium organyls 4 [27]. Johnsson and co-workers could establish dye formation by addition of aryllithium 7 to the silicon xanthone 6 [8]. A related strategy, adding lithium compound 7 to a preformed tricyclic system 8, was used by Nagano et al. to synthesize the Ge and Sn rhodamine analogues [14].
Scheme 1: Different synthetic approaches to silicon rhodamine dyes.
Scheme 1: Different synthetic approaches to silicon rhodamine dyes.
In a recent publication, Urano et al. synthesized the rhodamines 13–15 by coupling the triflate of xanthone 12 with boroxines 9b–11b (Scheme 2) [22,28]. Hereby, the boroxines 9b–11b were accessible by thermal dehydration of the corresponding boronic acids 9a–11a. With this procedure product 13 was obtained in only 6% yield, which is presumably due to a competing coupling reaction of the boroxine moiety of 9b with the chlorine atom of 9b or sterical reasons (the chlorine in 2’-position might lead to repulsion during the cross-coupling reaction). The reaction of the triflate with cyano-substituted phenylboroxines 10b and 11b led to silicon rhodamine dyes 14 and 15 in poor yields of 23 and 19%, respectively. The reaction conditions applied for the cross coupling of the triflate were similar to those published by Calitree and Detty for the coupling of the triflates derived from the O, S, Se, and Te-xanthones 16 with various phenylboroxines (bearing nitro, carboxylic acid, methyl and methoxy substituents) [29]. Here yields of 53–79% were obtained (for O and S analogues; 85–99% yields based on recovered starting material (brsm)). Since the yields reported by Urano for the Si-analogous Suzuki reactions were much lower (6–23%) [22], we wanted to examine if the aforementioned substrates were outliers and a cross-coupling reaction could be a valuable approach to silicon rhodamines. Thus, we aimed at the optimization of coupling conditions as well as evaluation of the best boron compounds for coupling. Since carboxylic acid-substituted dyes like compound 17 (X = Si, R = COOH) can be easily coupled to tumor binding vectors, we wanted to investigate if these dyes are also accessible by Suzuki–Miyaura coupling.
Scheme 2: Previous work from Calitree [29] and Urano [22,28] on the Suzuki–Miyaura coupling of triflates, derived from xanthones 12 and 16, with boroxines.
Scheme 2: Previous work from Calitree [29] and Urano [22,28] on the Suzuki–Miyaura coupling of triflates, derived from x...
Results and Discussion
Optimization of reaction conditions
At first we investigated the effects of different catalysts and boron compounds on the synthesis of silicon rhodamine 22 via Suzuki–Miyaura cross coupling (Scheme 3, Table 1). Triflate 21 was obtained without further purification from 12 by addition of triflic anhydride in dry acetonitrile. Boroxine 18b was formed by heating of boronic acid (18a) at 110 °C because it was shown by Calitree and Detty that free boronic acid leads to the destruction of the triflate, resulting in the corresponding xanthone [29]. Applying standard conditions on xanthone 12 by treatment with triflic anhydride in dry acetonitrile and subsequent addition of base, catalyst and boroxine 18b yielded the desired fluorophore 22 in 41% yield together with unreacted xanthone 12 (Table 1, entry 1). Since the initial triflate formation to 21 was unreliable and often incomplete, leading to lower yields, Comins reagent was investigated as an alternative triflation reagent. Notably, the use of Comins reagent showed no transformation from the yellow xanthone 12 to the deep blue triflate 21 at all (Table 1, entry 2). Exchange of anhydrous acetonitrile by anhydrous dichloromethane, which was removed in vacuo prior to coupling, provided triflate 21 as a blue salt without xanthone residues, hereby the yield could be slightly enhanced but still the conditions of the coupling reaction led to some back reaction of 21 to 12 (Table 1, entry 3). While the use of PdCl2(PPh3)2 was successful in the synthesis of chalcogenorhodamine dyes [29], the usage of that catalyst gave just low yields when applied in the synthesis of the silicon analogues (Scheme 2) [22]. Although Pd(PPh3)4 was not found to be an effective catalyst for the synthesis of rhodamine and rosamine dyes as well as for their selenium or tellurium analogous [29], the usage of that Pd(0) catalyst showed yields comparable with those obtained with PdCl2(PPh3)2 (Table 1, entry 4). The exchange of sodium carbonate with cesium carbonate resulted in no reaction at all (Table 1, entry 5). Whereby usage of potassium phenyltrifluoroborate (19) resulted in a yield comparable to boroxine 18b (Table 1, entry 6), usage of pinacol ester 20 showed no reaction in the cross-coupling reaction (Table 1, entry 7). Although described optimizations of the reaction conditions could lead to the silicon rhodamine 22 in moderate yields, an inseparable impurity of the cationic fluorophore was detected. After identifying this impurity as the tetraphenylphosphonium cation, we exchanged the triphenylphosphine ligand of the catalyst with dppf (1,1'-bis(diphenylphosphino)ferrocene). Remarkably, not only the yield was increased with PdCl2(dppf) from 49% to 67%, even the dye 22 was obtained with high purity after column chromatography without the necessity of further HPLC purification (Table 1, entry 8).
Scheme 3: Optimization of cross-coupling conditions of triflate 21, derived from Si-xanthone 12, with boron species 18b, 19 and 20 (see Table 1).
Scheme 3: Optimization of cross-coupling conditions of triflate 21, derived from Si-xanthone 12, with boron s...
Table 1: Optimization of cross-coupling conditions of triflate 21, derived from Si-xanthone 12, with boron species 18b, 19 and 20.
| Entry | Triflation | Cross coupling | Yield (22) | ||
|
Catalyst
(10 mol %) |
Boron
species (1 equiv) |
Conditions | |||
| 1 | 1.1 equiv Tf2O, MeCN, rt, 20 min | PdCl2(PPh3)2 | 18b | 3 equiv Na2CO3, MeCN, 70 °C, overnight | 41%a |
| 2 | 1 equiv Comins’ reagent (5-Cl-2-pyridyl-NTf2), MeCN, rt, 1 h | – | – | – | – |
| 3 | 1.1 equiv Tf2O, DCM, rt, 20 min, then evaporation | PdCl2(PPh3)2 | 18b | 3 equiv Na2CO3, MeCN, 70 °C, overnight |
49%a,
80%a,b |
| 4 | 1.1 equiv Tf2O, DCM, rt, 20 min, then evaporation | Pd(PPh3)4 | 18b | 3 equiv Na2CO3, MeCN, 70 °C, overnight |
39%a,
82%a,b |
| 5 | 1.1 equiv Tf2O, DCM, rt, 20 min, then evaporation | PdCl2(PPh3)2 | 18b | 3 equiv Cs2CO3, MeCN, 70 °C, overnight | n.r. |
| 6 | 1.1 equiv Tf2O, DCM, rt, 20 min, then evaporation | PdCl2(PPh3)2 | 19 | 3 equiv Na2CO3, MeCN, 70 °C, overnight | 48%a |
| 7 | 1.1 equiv Tf2O, DCM, rt, 20 min, then evaporation | PdCl2(PPh3)2 | 20 | 3 equiv Na2CO3, MeCN, 70 °C, overnight | n.r. |
| 8 | 1.1 equiv Tf2O, DCM, rt, 20 min, then evaporation | PdCl2(dppf) | 18b | 3 equiv Na2CO3, MeCN, 70 °C, overnight |
67%,
73%b |
aCorrected yield, contamination with [PPh4]+. bBased on recovered starting material (brsm) 12.
Exploration of substrate scope
Next we explored the substrate scope of the Suzuki–Miyaura coupling by screening commercially available boronic acids (Scheme 4, Table 2). Hereby, PdCl2(dppf) was also tested in order to suppress the formation of the inseparable phosphonium cation species. At first, we investigated the use of 3-boronobenzoic acid (23a) that should lead to a rhodamine suitable for coupling to a tumor vector, but boroxine 23b was converted to 23c with PdCl2(PPh3)2 in poor yields (Scheme 4 and Table 2, entry 1). However, PdCl2(dppf) performed better and led to the acid-substituted silicon rhodamine 23c in a moderate yield of 31% (56% brsm) (Table 2, entry 2). The moderate yield might be explained with the destruction of the triflate by the acid moiety of 23c. In order to prevent the destruction of the initially formed triflate 21, 4-boronobenzaldehyde (24a) was intended as a coupling substrate but yielded silicon rhodamine 24c only in traces (Table 2, entry 3). Usage of the tert-butyl-protected boronobenzoic acid 25a, or its boroxine counterpart 25b, respectively, gave fluorophore 25c suitable for later coupling reactions in reasonable yields of 43% and 53%, depending on the catalyst used (Table 2, entries 4 and 5). Again, the reaction catalyzed by PdCl2(dppf) resulted in an enhanced yield compared to catalysis with PdCl2(PPh3)2. Next we aimed at the synthesis of a silicon rhodamine bearing an acid function in 2’-position. With a less bulky methyl ester in the 2’-position of the phenylboroxine, the transmetalation and the new bond formation through reductive elimination should be less hindered, but remarkably, no reaction was observed either with the methyl ester 26b or the free acid 27b (Table 2, entries 6 and 7). Next we explored if amino-substituted silicon rhodamine 28c is accessible via Pd-catalysis. The resulting rhodamine 28c could be a possible substrate for the conversion into an azide and follow-up click reactions with alkyne-substituted tumor vectors. While heating of amine 28a to the corresponding boroxine 28b lead to formation of a brown solid (presumably due to degradation), the reaction of triflate 21 with the pinacol ester 31 showed no product formation at all (Table 2, entries 8 and 9). Since we were able to investigate the functional group tolerance of the coupling reaction, we shifted our focus towards heterocyclic boronic acids as substrates. Since 4’-pyridinyl- [27,30] and 3’-thienyl- [27,31-33] substituted silicon rhodamines are already known, we investigated the synthesis of these dyes by Suzuki–Miyaura cross coupling. Firstly, pyridinylboronic acid 29a was used as a substrate after heating at 110 °C, but no conversion was observed presumably due to the formation of an internal salt (protonated pyridine ring and deprotonated boronic acid) and ensuing difficult formation of boroxine 29b (Table 2, entry 10). Switching to the neutral heterocyclic boronic acid 30a, the corresponding thienyl-substituted silicon rhodamine 30c could be obtained in 37% (56% brsm) yield with the PdCl2(PPh3)2 catalyst. Remarkably, the yield could be clearly enhanced by catalysis with PdCl2(dppf) and the thienyl-substituted fluorophore 30c could subsequently be synthesized in 91% yield.
Scheme 4: Coupling reactions of silicon xanthone 12 with different boron species (23b–30b, 31).
Scheme 4: Coupling reactions of silicon xanthone 12 with different boron species (23b–30b, 31).
Table 2: Coupling reactions of silicon xanthone 12 with different boron species (23b–30b, 31).
| Entry | Boron source | Catalyst | Yield |
| 1 | 23b | PdCl2(PPh3)2 |
5%a,
46%a,b (23c) |
| 2 | 23b | PdCl2(dppf) |
31%,
56%b (23c) |
| 3 | 24b | PdCl2(PPh3)2 |
traces
(24c) |
| 4 | 25b | PdCl2(PPh3)2 |
43%a,
62%a,b (25c) |
| 5 | 25b | PdCl2(dppf) |
53%,
66%b (25c) |
| 6 | 26b | PdCl2(PPh3)2 | n.r. |
| 7 | 27b | PdCl2(PPh3)2 | n.r. |
| 8 | 28b | PdCl2(PPh3)2 | n.r. |
| 9 | 31 | PdCl2(PPh3)2 | n.r. |
| 10 | 29b | PdCl2(PPh3)2 | n.r. |
| 11 | 30b | PdCl2(PPh3)2 |
37%a,
56%a,b (30c) |
| 12 | 30b | PdCl2(dppf) |
91%
(30c) |
aCorrected yield, contamination with [PPh3Ar]+. bBased on recovered starting material (brsm) 12.
Table 3 compares the reaction outcome of the silicon rhodamine synthesis via Suzuki coupling with other employed methods: synthesis of the phenyl-substituted silicon rhodamine 22 by Suzuki cross coupling affords the product in a similar yield compared to the addition of phenyllithium to xanthone 12 or the attack of the double metallated bis-aniline 4 (R1 = R2 = Me, M = Mg) to the benzoic acid methyl ester [26]. However, the cross coupling of triflate 21 with boroxine 25b led to the ester-substituted rhodamine 25c in a reasonable yield of 53% (66% brsm) while the addition of the lithiated tert-butyl 3-bromobenzoate gave the fluorophore 25c in only 7% yield. Finally, the cross-coupling reaction of 12 and 30b to rhodamine 30c clearly outperforms the addition of lithiated 2-bromothiophene to xanthone 12 since 2-bromothiophene might also undergo lithiation in 5-position in competition to the halogen metal exchange (in general multiple halogenated aryls are problematic nucleophiles for these addition reactions).
Table 3: Comparison of common methods for silicon rhodamine synthesis.
| Method → |
Addition of lithium organyl
to 12a |
Suzuki–Miyaura
cross coupling |
Attack of 4 (R1 = R2 = Me,
M = Mg) to 5 (R3 = H) |
| Fluorophore ↓ | |||
| phenyl-substituted SiR (22) | 72% |
67%,
73%b |
72% [26] |
| tert-butylbenzoic acid-substituted SiR (25c) | 7% |
53%,
66%b |
– |
| thienyl-substituted SiR (30c) | 77% | 91% | – |
aConditions: 7 equiv aryl bromide, 14 equiv t-BuLi, THF, −78 °C, 30 min, then 1 equiv 12 at −78 °C to rt, overnight, then aq HCl, work-up, purification with DCM/MeOH 99:1 to 9:1. bBased on recovered starting material (brsm) 12.
Conclusion
Since just three literature examples are known to date in which Suzuki–Miyaura cross-coupling reactions gave access to silicon rhodamines in poor to moderate yields (Scheme 2), we wanted to improve these first valuable experimental results. In general, the amount of re-isolated starting material 12 could be significantly reduced when acetonitrile was exchanged with dichloromethane in the triflation reaction to provide triflate 21 neat and more reliable. Screening of different boron species and catalysts showed that, like in the syntheses of O, S, Se, and Te-rhodamines, boroxines were a suitable source, but also potassium trifluoroborates can be taken into consideration for the reaction design, whereas pinacol esters didn’t show any reactivity. While PdCl2(PPh3)2 was a sufficient catalyst for the cross coupling, application of PdCl2(dppf) led to clearly enhanced yields: overall the Suzuki–Miyaura cross-coupling reaction gave access to silicon rhodamines with neutral (hetero)aromatic xanthene substituents (phenyl: 67%, respectively 73% brsm; thienyl: 91%) (even though the term ‘dihydrosilaanthracene’ is correct to name the Si-anthracene moiety, the term ‘Si-xanthene’ is widely used in the literature (see e.g. [30]); also the term Si-xanthone (for derivatives of 12) is established instead of 9-silaanthracen-10(9H)-one). The conditions tolerated also the use of the unprotected acid functionality of the boroxine 23b (23c, 31%, respectively 56% brsm), while application of basic boronic acids failed (28, 29), presumably due to unsuccessful boroxine formation. The main advantage of the cross coupling is the access to acid-functionalized fluorophores like 23c that can be immediately coupled to a molecule of interest (e.g., tumor binding vectors) whereas previously published methodologies need, e.g., an ester, orthoester or oxazoline protecting group for the acid. But also the tert-butyl ester-functionalized boroxine 25 is suitable for the cross coupling. With the current catalytic system, coupling of 2-substituted boroxines (26, 27) remains challenging, but optimizing the catalytic system with ligands suitable for coupling of multisubstituted aryls is under current investigation. In conclusion, several silicon rhodamines could be synthesized under the optimized conditions, without the necessity of HPLC purification, in up to 91% yield whereby the free acids are directly accessible in contrast to the three hitherto described methods.
Supporting Information
| Supporting Information File 1: Experimental procedures and NMR spectra of all synthesized compounds as well as photochromic characterization data (fluorescence spectra, quantum yield) of thienyl-substituted silicon rhodamine 30c. | ||
| Format: PDF | Size: 1.6 MB | Download |
Acknowledgements
We are very grateful to the Wilhelm Sander Stiftung for a grant on bi-modal tumor tracers (2018.024.1). We thank Yvonne Remde for synthetic support. We are thankful to Jessica Matthias (group of Stefan Hell, MPI for Medical Research Heidelberg) for measurement of the UV–vis spectrum of 30c.
References
-
Thompson, A. D.; Omar, M. H.; Rivera-Molina, F.; Xi, Z.; Koleske, A. J.; Toomre, D. K.; Schepartz, A. Angew. Chem., Int. Ed. 2017, 56, 10408–10412. doi:10.1002/anie.201704783
Return to citation in text: [1] -
Butkevich, A. N.; Ta, H.; Ratz, M.; Stoldt, S.; Jakobs, S.; Belov, V. N.; Hell, S. W. ACS Chem. Biol. 2018, 13, 475–480. doi:10.1021/acschembio.7b00616
Return to citation in text: [1] -
Butkevich, A. N.; Mitronova, G. Y.; Sidenstein, S. C.; Klocke, J. L.; Kamin, D.; Meineke, D. N. H.; D'Este, E.; Kraemer, P.-T.; Danzl, J. G.; Belov, V. N.; Hell, S. W. Angew. Chem., Int. Ed. 2016, 55, 3290–3294. doi:10.1002/anie.201511018
Return to citation in text: [1] -
Kozma, E.; Estrada Girona, G.; Paci, G.; Lemke, E. A.; Kele, P. Chem. Commun. 2017, 53, 6696–6699. doi:10.1039/c7cc02212c
Return to citation in text: [1] -
Grimm, J. B.; Klein, T.; Kopek, B. G.; Shtengel, G.; Hess, H. F.; Sauer, M.; Lavis, L. D. Angew. Chem., Int. Ed. 2016, 55, 1723–1727. doi:10.1002/anie.201509649
Return to citation in text: [1] -
Lukinavičius, G.; Reymond, L.; Umezawa, K.; Sallin, O.; D’Este, E.; Göttfert, F.; Ta, H.; Hell, S. W.; Urano, Y.; Johnsson, K. J. Am. Chem. Soc. 2016, 138, 9365–9368. doi:10.1021/jacs.6b04782
Return to citation in text: [1] -
Takakura, H.; Zhang, Y.; Erdmann, R. S.; Thompson, A. D.; Lin, Y.; McNellis, B.; Rivera-Molina, F.; Uno, S.-n.; Kamiya, M.; Urano, Y.; Rothman, J. E.; Bewersdorf, J.; Schepartz, A.; Toomre, D. Nat. Biotechnol. 2017, 35, 773–780. doi:10.1038/nbt.3876
Return to citation in text: [1] -
Lukinavičius, G.; Umezawa, K.; Olivier, N.; Honigmann, A.; Yang, G.; Plass, T.; Mueller, V.; Reymond, L.; Corrêa, I. R., Jr.; Luo, Z.-G.; Schultz, C.; Lemke, E. A.; Heppenstall, P.; Eggeling, C.; Manley, S.; Johnsson, K. Nat. Chem. 2013, 5, 132–139. doi:10.1038/nchem.1546
Return to citation in text: [1] [2] -
Shieh, P.; Siegrist, M. S.; Cullen, A. J.; Bertozzi, C. R. Proc. Natl. Acad. Sci. U. S. A. 2014, 111, 5456–5461. doi:10.1073/pnas.1322727111
Return to citation in text: [1] -
Iwatate, R. J.; Kamiya, M.; Umezawa, K.; Kashima, H.; Nakadate, M.; Kojima, R.; Urano, Y. Bioconjugate Chem. 2018, 29, 241–244. doi:10.1021/acs.bioconjchem.7b00776
Return to citation in text: [1] -
Kim, E.; Yang, K. S.; Kohler, R. H.; Dubach, J. M.; Mikula, H.; Weissleder, R. Bioconjugate Chem. 2015, 26, 1513–1518. doi:10.1021/acs.bioconjchem.5b00152
Return to citation in text: [1] -
Hanaoka, K.; Kagami, Y.; Piao, W.; Myochin, T.; Numasawa, K.; Kuriki, Y.; Ikeno, T.; Ueno, T.; Komatsu, T.; Terai, T.; Nagano, T.; Urano, Y. Chem. Commun. 2018, 54, 6939–6942. doi:10.1039/c8cc02451k
Return to citation in text: [1] -
Du, M.; Huo, B.; Liu, J.; Li, M.; Fang, L.; Yang, Y. Anal. Chim. Acta 2018, 1030, 172–182. doi:10.1016/j.aca.2018.05.013
Return to citation in text: [1] -
Koide, Y.; Urano, Y.; Hanaoka, K.; Terai, T.; Nagano, T. ACS Chem. Biol. 2011, 6, 600–608. doi:10.1021/cb1002416
Return to citation in text: [1] [2] -
Wang, T.; Zhao, Q.-J.; Hu, H.-G.; Yu, S.-C.; Liu, X.; Liu, L.; Wu, Q.-Y. Chem. Commun. 2012, 48, 8781. doi:10.1039/c2cc34159j
Return to citation in text: [1] [2] -
Wang, B.; Cui, X.; Zhang, Z.; Chai, X.; Ding, H.; Wu, Q.; Guo, Z.; Wang, T. Org. Biomol. Chem. 2016, 14, 6720–6728. doi:10.1039/c6ob00894a
Return to citation in text: [1] -
Egawa, T.; Hanaoka, K.; Koide, Y.; Ujita, S.; Takahashi, N.; Ikegaya, Y.; Matsuki, N.; Terai, T.; Ueno, T.; Komatsu, T.; Nagano, T. J. Am. Chem. Soc. 2011, 133, 14157–14159. doi:10.1021/ja205809h
Return to citation in text: [1] -
Huang, Y.-L.; Walker, A. S.; Miller, E. W. J. Am. Chem. Soc. 2015, 137, 10767–10776. doi:10.1021/jacs.5b06644
Return to citation in text: [1] -
Zhang, H.; Liu, J.; Liu, C.; Yu, P.; Sun, M.; Yan, X.; Guo, J.-P.; Guo, W. Biomaterials 2017, 133, 60–69. doi:10.1016/j.biomaterials.2017.04.023
Return to citation in text: [1] -
Huo, Y.; Miao, J.; Han, L.; Li, Y.; Li, Z.; Shi, Y.; Guo, W. Chem. Sci. 2017, 8, 6857–6864. doi:10.1039/c7sc02608k
Return to citation in text: [1] -
Koide, Y.; Urano, Y.; Hanaoka, K.; Terai, T.; Nagano, T. J. Am. Chem. Soc. 2011, 133, 5680–5682. doi:10.1021/ja111470n
Return to citation in text: [1] -
Umezawa, K.; Yoshida, M.; Kamiya, M.; Yamasoba, T.; Urano, Y. Nat. Chem. 2017, 9, 279–286. doi:10.1038/nchem.2648
Return to citation in text: [1] [2] [3] [4] [5] -
McCann, T. E.; Kosaka, N.; Koide, Y.; Mitsunaga, M.; Choyke, P. L.; Nagano, T.; Urano, Y.; Kobayashi, H. Bioconjugate Chem. 2011, 22, 2531–2538. doi:10.1021/bc2003617
Return to citation in text: [1] [2] -
Koide, Y.; Urano, Y.; Hanaoka, K.; Piao, W.; Kusakabe, M.; Saito, N.; Terai, T.; Okabe, T.; Nagano, T. J. Am. Chem. Soc. 2012, 134, 5029–5031. doi:10.1021/ja210375e
Return to citation in text: [1] [2] [3] -
Wang, B.; Chai, X.; Zhu, W.; Wang, T.; Wu, Q. Chem. Commun. 2014, 50, 14374–14377. doi:10.1039/c4cc06178k
Return to citation in text: [1] -
Fischer, C.; Sparr, C. Angew. Chem., Int. Ed. 2018, 57, 2436–2440. doi:10.1002/anie.201711296
Return to citation in text: [1] [2] [3] -
Grimm, J. B.; Brown, T. A.; Tkachuk, A. N.; Lavis, L. D. ACS Cent. Sci. 2017, 3, 975–985. doi:10.1021/acscentsci.7b00247
Return to citation in text: [1] [2] [3] -
Urano, Y.; Kamiya, M.; Umezawa, K.; Yoshida, M. Glutathione-detecting fluorescent probe. U.S. Pat. Appl. US2017/0045525 A1, Feb 16, 2017.
Return to citation in text: [1] [2] -
Calitree, B. D.; Detty, M. R. Synlett 2010, 89–92. doi:10.1055/s-0029-1218535
Return to citation in text: [1] [2] [3] [4] [5] -
Zhang, H.; Li, K.; Li, L.-L.; Yu, K.-K.; Liu, X.-Y.; Li, M.-Y.; Wang, N.; Liu, Y.-H.; Yu, X.-Q. Chin. Chem. Lett. 2019, 30, 1063–1066. doi:10.1016/j.cclet.2019.03.017
Return to citation in text: [1] [2] -
Groves, K.; Bruff, R. Substituted silaxanthenium red to near-infrared fluorochromes for in vitro and in vivo imaging and detection. WO Pat. Appl. WO2014/144793 A1, Sept 18, 2014.
Return to citation in text: [1] -
Gamsey, S.; Bernat, V.; Kutyavin, A.; Clary, J. W.; Pradhan, S. Near-IR Glucose Sensors. U. S..Pat. Appl. US20180179233 A1, June 28, 2018.
Return to citation in text: [1] -
Urano, Y.; Kamiya, M.; Tachibana, R. Preparation of spiro compounds as red fluorescent probes for use in detection of peptdase activity. WO Pat. Appl. WO 2018151260 A1, Aug 23, 2018.
Return to citation in text: [1]
| 26. | Fischer, C.; Sparr, C. Angew. Chem., Int. Ed. 2018, 57, 2436–2440. doi:10.1002/anie.201711296 |
| 26. | Fischer, C.; Sparr, C. Angew. Chem., Int. Ed. 2018, 57, 2436–2440. doi:10.1002/anie.201711296 |
| 30. | Zhang, H.; Li, K.; Li, L.-L.; Yu, K.-K.; Liu, X.-Y.; Li, M.-Y.; Wang, N.; Liu, Y.-H.; Yu, X.-Q. Chin. Chem. Lett. 2019, 30, 1063–1066. doi:10.1016/j.cclet.2019.03.017 |
| 1. | Thompson, A. D.; Omar, M. H.; Rivera-Molina, F.; Xi, Z.; Koleske, A. J.; Toomre, D. K.; Schepartz, A. Angew. Chem., Int. Ed. 2017, 56, 10408–10412. doi:10.1002/anie.201704783 |
| 2. | Butkevich, A. N.; Ta, H.; Ratz, M.; Stoldt, S.; Jakobs, S.; Belov, V. N.; Hell, S. W. ACS Chem. Biol. 2018, 13, 475–480. doi:10.1021/acschembio.7b00616 |
| 3. | Butkevich, A. N.; Mitronova, G. Y.; Sidenstein, S. C.; Klocke, J. L.; Kamin, D.; Meineke, D. N. H.; D'Este, E.; Kraemer, P.-T.; Danzl, J. G.; Belov, V. N.; Hell, S. W. Angew. Chem., Int. Ed. 2016, 55, 3290–3294. doi:10.1002/anie.201511018 |
| 4. | Kozma, E.; Estrada Girona, G.; Paci, G.; Lemke, E. A.; Kele, P. Chem. Commun. 2017, 53, 6696–6699. doi:10.1039/c7cc02212c |
| 5. | Grimm, J. B.; Klein, T.; Kopek, B. G.; Shtengel, G.; Hess, H. F.; Sauer, M.; Lavis, L. D. Angew. Chem., Int. Ed. 2016, 55, 1723–1727. doi:10.1002/anie.201509649 |
| 6. | Lukinavičius, G.; Reymond, L.; Umezawa, K.; Sallin, O.; D’Este, E.; Göttfert, F.; Ta, H.; Hell, S. W.; Urano, Y.; Johnsson, K. J. Am. Chem. Soc. 2016, 138, 9365–9368. doi:10.1021/jacs.6b04782 |
| 7. | Takakura, H.; Zhang, Y.; Erdmann, R. S.; Thompson, A. D.; Lin, Y.; McNellis, B.; Rivera-Molina, F.; Uno, S.-n.; Kamiya, M.; Urano, Y.; Rothman, J. E.; Bewersdorf, J.; Schepartz, A.; Toomre, D. Nat. Biotechnol. 2017, 35, 773–780. doi:10.1038/nbt.3876 |
| 8. | Lukinavičius, G.; Umezawa, K.; Olivier, N.; Honigmann, A.; Yang, G.; Plass, T.; Mueller, V.; Reymond, L.; Corrêa, I. R., Jr.; Luo, Z.-G.; Schultz, C.; Lemke, E. A.; Heppenstall, P.; Eggeling, C.; Manley, S.; Johnsson, K. Nat. Chem. 2013, 5, 132–139. doi:10.1038/nchem.1546 |
| 18. | Huang, Y.-L.; Walker, A. S.; Miller, E. W. J. Am. Chem. Soc. 2015, 137, 10767–10776. doi:10.1021/jacs.5b06644 |
| 8. | Lukinavičius, G.; Umezawa, K.; Olivier, N.; Honigmann, A.; Yang, G.; Plass, T.; Mueller, V.; Reymond, L.; Corrêa, I. R., Jr.; Luo, Z.-G.; Schultz, C.; Lemke, E. A.; Heppenstall, P.; Eggeling, C.; Manley, S.; Johnsson, K. Nat. Chem. 2013, 5, 132–139. doi:10.1038/nchem.1546 |
| 15. | Wang, T.; Zhao, Q.-J.; Hu, H.-G.; Yu, S.-C.; Liu, X.; Liu, L.; Wu, Q.-Y. Chem. Commun. 2012, 48, 8781. doi:10.1039/c2cc34159j |
| 14. | Koide, Y.; Urano, Y.; Hanaoka, K.; Terai, T.; Nagano, T. ACS Chem. Biol. 2011, 6, 600–608. doi:10.1021/cb1002416 |
| 13. | Du, M.; Huo, B.; Liu, J.; Li, M.; Fang, L.; Yang, Y. Anal. Chim. Acta 2018, 1030, 172–182. doi:10.1016/j.aca.2018.05.013 |
| 14. | Koide, Y.; Urano, Y.; Hanaoka, K.; Terai, T.; Nagano, T. ACS Chem. Biol. 2011, 6, 600–608. doi:10.1021/cb1002416 |
| 15. | Wang, T.; Zhao, Q.-J.; Hu, H.-G.; Yu, S.-C.; Liu, X.; Liu, L.; Wu, Q.-Y. Chem. Commun. 2012, 48, 8781. doi:10.1039/c2cc34159j |
| 16. | Wang, B.; Cui, X.; Zhang, Z.; Chai, X.; Ding, H.; Wu, Q.; Guo, Z.; Wang, T. Org. Biomol. Chem. 2016, 14, 6720–6728. doi:10.1039/c6ob00894a |
| 17. | Egawa, T.; Hanaoka, K.; Koide, Y.; Ujita, S.; Takahashi, N.; Ikegaya, Y.; Matsuki, N.; Terai, T.; Ueno, T.; Komatsu, T.; Nagano, T. J. Am. Chem. Soc. 2011, 133, 14157–14159. doi:10.1021/ja205809h |
| 26. | Fischer, C.; Sparr, C. Angew. Chem., Int. Ed. 2018, 57, 2436–2440. doi:10.1002/anie.201711296 |
| 9. | Shieh, P.; Siegrist, M. S.; Cullen, A. J.; Bertozzi, C. R. Proc. Natl. Acad. Sci. U. S. A. 2014, 111, 5456–5461. doi:10.1073/pnas.1322727111 |
| 10. | Iwatate, R. J.; Kamiya, M.; Umezawa, K.; Kashima, H.; Nakadate, M.; Kojima, R.; Urano, Y. Bioconjugate Chem. 2018, 29, 241–244. doi:10.1021/acs.bioconjchem.7b00776 |
| 11. | Kim, E.; Yang, K. S.; Kohler, R. H.; Dubach, J. M.; Mikula, H.; Weissleder, R. Bioconjugate Chem. 2015, 26, 1513–1518. doi:10.1021/acs.bioconjchem.5b00152 |
| 12. | Hanaoka, K.; Kagami, Y.; Piao, W.; Myochin, T.; Numasawa, K.; Kuriki, Y.; Ikeno, T.; Ueno, T.; Komatsu, T.; Terai, T.; Nagano, T.; Urano, Y. Chem. Commun. 2018, 54, 6939–6942. doi:10.1039/c8cc02451k |
| 27. | Grimm, J. B.; Brown, T. A.; Tkachuk, A. N.; Lavis, L. D. ACS Cent. Sci. 2017, 3, 975–985. doi:10.1021/acscentsci.7b00247 |
| 24. | Koide, Y.; Urano, Y.; Hanaoka, K.; Piao, W.; Kusakabe, M.; Saito, N.; Terai, T.; Okabe, T.; Nagano, T. J. Am. Chem. Soc. 2012, 134, 5029–5031. doi:10.1021/ja210375e |
| 24. | Koide, Y.; Urano, Y.; Hanaoka, K.; Piao, W.; Kusakabe, M.; Saito, N.; Terai, T.; Okabe, T.; Nagano, T. J. Am. Chem. Soc. 2012, 134, 5029–5031. doi:10.1021/ja210375e |
| 23. | McCann, T. E.; Kosaka, N.; Koide, Y.; Mitsunaga, M.; Choyke, P. L.; Nagano, T.; Urano, Y.; Kobayashi, H. Bioconjugate Chem. 2011, 22, 2531–2538. doi:10.1021/bc2003617 |
| 25. | Wang, B.; Chai, X.; Zhu, W.; Wang, T.; Wu, Q. Chem. Commun. 2014, 50, 14374–14377. doi:10.1039/c4cc06178k |
| 23. | McCann, T. E.; Kosaka, N.; Koide, Y.; Mitsunaga, M.; Choyke, P. L.; Nagano, T.; Urano, Y.; Kobayashi, H. Bioconjugate Chem. 2011, 22, 2531–2538. doi:10.1021/bc2003617 |
| 19. | Zhang, H.; Liu, J.; Liu, C.; Yu, P.; Sun, M.; Yan, X.; Guo, J.-P.; Guo, W. Biomaterials 2017, 133, 60–69. doi:10.1016/j.biomaterials.2017.04.023 |
| 20. | Huo, Y.; Miao, J.; Han, L.; Li, Y.; Li, Z.; Shi, Y.; Guo, W. Chem. Sci. 2017, 8, 6857–6864. doi:10.1039/c7sc02608k |
| 21. | Koide, Y.; Urano, Y.; Hanaoka, K.; Terai, T.; Nagano, T. J. Am. Chem. Soc. 2011, 133, 5680–5682. doi:10.1021/ja111470n |
| 22. | Umezawa, K.; Yoshida, M.; Kamiya, M.; Yamasoba, T.; Urano, Y. Nat. Chem. 2017, 9, 279–286. doi:10.1038/nchem.2648 |
| 24. | Koide, Y.; Urano, Y.; Hanaoka, K.; Piao, W.; Kusakabe, M.; Saito, N.; Terai, T.; Okabe, T.; Nagano, T. J. Am. Chem. Soc. 2012, 134, 5029–5031. doi:10.1021/ja210375e |
| 22. | Umezawa, K.; Yoshida, M.; Kamiya, M.; Yamasoba, T.; Urano, Y. Nat. Chem. 2017, 9, 279–286. doi:10.1038/nchem.2648 |
| 22. | Umezawa, K.; Yoshida, M.; Kamiya, M.; Yamasoba, T.; Urano, Y. Nat. Chem. 2017, 9, 279–286. doi:10.1038/nchem.2648 |
| 28. | Urano, Y.; Kamiya, M.; Umezawa, K.; Yoshida, M. Glutathione-detecting fluorescent probe. U.S. Pat. Appl. US2017/0045525 A1, Feb 16, 2017. |
| 29. | Calitree, B. D.; Detty, M. R. Synlett 2010, 89–92. doi:10.1055/s-0029-1218535 |
| 27. | Grimm, J. B.; Brown, T. A.; Tkachuk, A. N.; Lavis, L. D. ACS Cent. Sci. 2017, 3, 975–985. doi:10.1021/acscentsci.7b00247 |
| 30. | Zhang, H.; Li, K.; Li, L.-L.; Yu, K.-K.; Liu, X.-Y.; Li, M.-Y.; Wang, N.; Liu, Y.-H.; Yu, X.-Q. Chin. Chem. Lett. 2019, 30, 1063–1066. doi:10.1016/j.cclet.2019.03.017 |
| 27. | Grimm, J. B.; Brown, T. A.; Tkachuk, A. N.; Lavis, L. D. ACS Cent. Sci. 2017, 3, 975–985. doi:10.1021/acscentsci.7b00247 |
| 31. | Groves, K.; Bruff, R. Substituted silaxanthenium red to near-infrared fluorochromes for in vitro and in vivo imaging and detection. WO Pat. Appl. WO2014/144793 A1, Sept 18, 2014. |
| 32. | Gamsey, S.; Bernat, V.; Kutyavin, A.; Clary, J. W.; Pradhan, S. Near-IR Glucose Sensors. U. S..Pat. Appl. US20180179233 A1, June 28, 2018. |
| 33. | Urano, Y.; Kamiya, M.; Tachibana, R. Preparation of spiro compounds as red fluorescent probes for use in detection of peptdase activity. WO Pat. Appl. WO 2018151260 A1, Aug 23, 2018. |
| 22. | Umezawa, K.; Yoshida, M.; Kamiya, M.; Yamasoba, T.; Urano, Y. Nat. Chem. 2017, 9, 279–286. doi:10.1038/nchem.2648 |
| 29. | Calitree, B. D.; Detty, M. R. Synlett 2010, 89–92. doi:10.1055/s-0029-1218535 |
| 29. | Calitree, B. D.; Detty, M. R. Synlett 2010, 89–92. doi:10.1055/s-0029-1218535 |
| 29. | Calitree, B. D.; Detty, M. R. Synlett 2010, 89–92. doi:10.1055/s-0029-1218535 |
| 29. | Calitree, B. D.; Detty, M. R. Synlett 2010, 89–92. doi:10.1055/s-0029-1218535 |
| 22. | Umezawa, K.; Yoshida, M.; Kamiya, M.; Yamasoba, T.; Urano, Y. Nat. Chem. 2017, 9, 279–286. doi:10.1038/nchem.2648 |
| 28. | Urano, Y.; Kamiya, M.; Umezawa, K.; Yoshida, M. Glutathione-detecting fluorescent probe. U.S. Pat. Appl. US2017/0045525 A1, Feb 16, 2017. |
© 2019 Kanagasundaram et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0). Please note that the reuse, redistribution and reproduction in particular requires that the authors and source are credited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (https://www.beilstein-journals.org/bjoc)