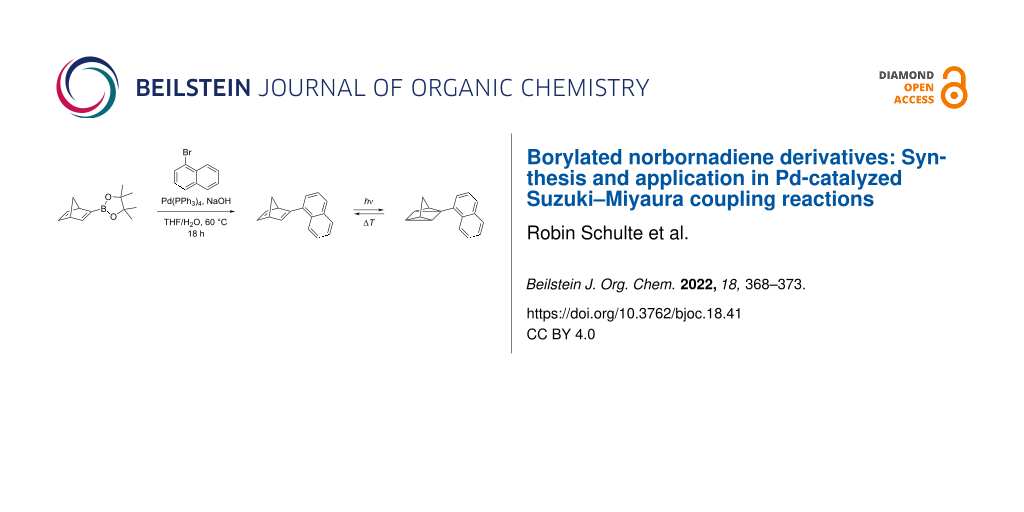
Abstract
The photochromic norbornadiene/quadricyclane system is among the most promising candidates for molecular solar thermal (MOST) energy storage. As in this context there is still the need for new tailor-made derivatives, borylated norbornadienes were synthesized that may be used as versatile building blocks. Thus, the 4,4,5,5-tetramethyl-2-(bicyclo[2.2.1]heptadien-2-yl)-1,3,2-dioxaborolane was prepared and shown to be a suitable substrate for Pd-catalyzed Suzuki–Miyaura coupling reactions with selected haloarenes. It was demonstrated exemplarily that the novel monosubstituted 2-(1-naphthyl)norbornadiene, that is accessible through this route, is transformed to the corresponding quadricyclane upon irradiation, whereas the back reaction can be accomplished by thermal treatment.
Graphical Abstract
Introduction
Norbornadiene (1a, bicyclo[2.2.1]heptadiene) is a photochromic compound that has recently gained considerable attention because of its ability to store light-energy by the photo-induced intramolecular [2 + 2] cycloaddition to the metastable quadricyclane [1-7]. The latter may be transformed back to the starting norbornadiene in an exothermic process upon heating or irradiation [2,8]. Therefore, the photo-switchable norbornadiene–quadricyclane system has been proposed as a promising basis for a molecular solar thermal storage system (MOST) [9-12]. For that purpose, however, the chromophore of the norbornadiene (1a) has to be modified with suitable substituents because the parent system does not operate in the spectrum of the sunlight. Therefore, the efficient and purposeful functionalization of the norbornadiene scaffold is an important and challenging task in this field. Specifically, earlier approaches for the synthesis of norbornadiene derivatives are mainly based on Diels–Alder reactions of cyclopentadiene with alkynes [13-23]. However, since this synthetic route requires strongly electrophilic alkynes, its scope is limited to products that contain at least one electron-acceptor group, such as an ester, a nitrile or amide functionality [13-18,24,25]. At the same time, norbornadiene derivatives are available from metalated substrates. Hence, norbornadiene is deprotonated with the Schlosser base and subsequently trapped by an appropriate electrophile [26,27]. In addition, halogenated norbornadiene derivatives may be metalated in a Li–halogen exchange reaction [27]. In another versatile approach, arylation and alkenylation reactions of the norbornadiene may be accomplished with a Suzuki–Miyaura coupling reaction. In this case, halogenated norbornadienes react with arylboronic acids or their esters to the corresponding aryl-substituted norbornadienes under optimized conditions [28-31]. To the best of our knowledge, however, no borylated norbornadiene derivatives have been employed in Suzuki–Miyaura coupling reactions so far, although this synthetic route appears to be a useful complementary approach to the already established one. Currently, two borylated norbornadienes are already known in literature, namely the dibutyl bicyclo[2.2.1]heptadiene-2-ylboronate, which is not well suited for coupling reactions, and the 2-(3-bromobicyclo[2.2.1]heptadien-2-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane, whose reactivity in Suzuki–Miyaura couplings has not been investigated, so far [32,33]. To explore the suitability of borylated norbornadienes for Suzuki–Miyaura coupling reactions and thus to provide new useful building blocks for the modular construction of norbornadiene derivatives, specifically the so far underrepresented monosubstituted arylnorbornadienes, we examined exemplarily the synthesis of borylated norbornadienes and their Pd-catalyzed Suzuki–Miyaura coupling reactions.
Results and Discussion
Synthesis
Norbornadiene (1a) was deprotonated with the Schlosser base, and the reaction of the resulting metalated norbornadiene with bis(pinacolato)diborone gave the 4,4,5,5-tetramethyl-2-(bicyclo[2.2.1]heptadien-2-yl)-1,3,2-dioxaborolane (2a) in 69% yield (Scheme 1). Subsequent treatment of product 2a with aqueous KHF2 solution resulted in the formation of potassium bicyclo[2.2.1]heptadien-2-yltrifluoroborate (3) in 72% yield (Scheme 1), which was obtained in sufficient purity (>97%), as indicated by 1H NMR spectroscopy; however, satisfactory elemental analysis data could not be obtained for this product. The parent bicyclo[2.2.1]heptadien-2-ylboronic acid could not be obtained at all, as neither the reaction of 2a with NaIO4 and hydrochloric acid, nor the reaction of 2a with LiOH and subsequent addition of acid (ammonium chloride, hydrochloric acid) gave the desired boronic acid, but led to decomposition, instead. Obviously, the 2-borylated norbornadiene derivatives are acid-labile, so that the synthesis of the boronic acid was not further investigated. In addition, the 4,4,5,5-tetramethyl-2-(3-methylbicyclo[2.2.1]heptadien-2-yl)-1,3,2-dioxaborolane (2b) was obtained by conversion of the known 2-bromo-3-methylbicyclo[2.2.1]heptadiene (1b) [27] to the metalated intermediate with t-BuLi and the following reaction with bis(pinacolato)diborone in 15% overall yield (Scheme 1) [27]. The low yield of this reaction as compared with the ones of resembling reactions of the substrate 1b [27] may be caused by the unfavorable clash of the sterically demanding borylating reagent and the neighboring methyl group at the reaction center. The novel compounds 2a, 2b, and 3 were identified and characterized by NMR spectroscopy (1H, 13C, COSY, HSQC, HMBC), melting point, and elemental analysis. In addition, the structure of product 2b was supported by high resolution mass spectrometry (HRMS).
Scheme 1: Synthesis of the borylated norbornadienes 2a,b and 3.
Scheme 1: Synthesis of the borylated norbornadienes 2a,b and 3.
To assess the suitability of the boronic esters 2a and 2b to be used as building blocks in Suzuki–Miyaura reactions, the Pd-catalyzed cross-coupling reaction of norbornadiene 2a and bromobenzene (4a) was examined under different conditions (Table 1, Scheme 2). First experiments were conducted with Pd2(dba)3/(t-Bu)3PHBF4 as catalytic system and CsF as additive in THF at room temperature, because these conditions have been shown to be well-suitable for Suzuki–Miyaura reactions of halogen-substituted norbornadiene derivatives with arylboronic acids [28]. However, under these conditions the coupling reaction of 2a with 4a gave the product 5a in only 37% yield (Table 1, entry 6). The use of PdCl2(dppf)·CH2Cl2 as catalyst with different bases resulted in even lower yields (≤15%; Table 1, entries 2–4). In contrast, the best yield was accomplished with Pd(PPh3)4 as catalyst and NaOH as base in THF/water at 60 °C giving 2-phenylbicyclo[2.2.1]heptadiene (5a) in 56% yield. Under these optimized conditions, the Suzuki–Miyaura coupling reaction of 2a was also carried out with other representative haloarenes 5b–j to assess the scope and limits of this synthetic route. Thus, the coupling reaction of 2a with 1-bromonaphthalene (4b), 2-bromotoluene (4c), 3-bromoanisole (4e), 2-bromoanisole (4f) 2-bromonitrobenzene (4h), 3-iodo-4-methoxybenzonitrile (4i), and 2-bromopyridine (4j) gave the corresponding 2-arylnorbornadienes in moderate to good yields (32–67%), whereas the reaction with the 4-cyano- and 4-methoxy-substituted halobenzene derivatives 4d and 4g gave the corresponding products only in low yield or not at all (Scheme 2). It should be noted, however, that the relatively low yields of some coupling reactions are also caused by the difficult purification of the products, because most of them tend to decompose slowly in solution or during chromatographic work-up [31,34]. The novel compounds 5b–j were identified and fully characterized by NMR spectroscopy (1H, 13C, COSY, HSQC, HMBC); however, correct elemental analysis was not obtained in the case of the less-stable products 5d, 5g and 5j.
Table 1: Pd-catalyzed Suzuki–Miyaura coupling reaction of norbornadiene 2a and bromobenzene (4a) at different reaction conditions.
| Entry | Catalyst | Base | Solvent | T / °C | Yield 5a / %a |
|---|---|---|---|---|---|
| 1 | Pd(OAc)2, PPh3 | NaOH | THF/H2O | 60 | 31 |
| 2 | PdCl2(dppf)·CH2Cl2 | K2CO3 | DME | 60 | <5% |
| 3 | PdCl2(dppf)·CH2Cl2 | t-BuNH2 | iPrOH/H2O | 60 | <5% |
| 4 | PdCl2(dppf)·CH2Cl2 | NaOH | THF/H2O | 60 | 15 |
| 5 | Pd(PPh3)4 | NaOH | THF/H2O | 60 | 56 |
| 6 | Pd2(dba)3, (t-Bu)3PHBF4 | CsF | THF | rt | 37 |
aYield of isolated product 5a after column chromatography.
Scheme 2: Suzuki–Miyaura coupling reactions of borono-norbornadienes 2a and 2b with selected haloarenes 4a–k.
Scheme 2: Suzuki–Miyaura coupling reactions of borono-norbornadienes 2a and 2b with selected haloarenes 4a–k.
The Suzuki–Miyaura coupling reactions of the borononorbornadiene 2a (Scheme 2) show that this substrate can be used as starting material for the synthesis of monosubstituted norbornadiene derivatives and thus represents a useful complementary method to the already known routes to higher substituted derivatives [28-31]. Although the yields are not very high and may have to be optimized in each specific case, e.g. by variation of the catalyst system, this route is so far the only one reported to get access to a series of compounds from this class of norbornadiene derivatives. So far, only few examples of such monosubstituted compounds have been made available [31,34], and in most cases they have been reported as fairly unstable, which is in agreement with our observations during work-up and storage of the products. At the same time, this approach enabled the isolation and identification of novel stable derivatives as well, such as 5b, 5c, and 5i. But unfortunately, there is no clear trend for a substituent effect on the reaction outcome, as both donor- and acceptor-substituted haloarenes resulted in essentially the same range of yields, i.e. from good yields (e.g., 4b, 4h, and 4j) to failed reactions (4d and 4g). In this context, the different reactivity of the methoxy-substituted arene isomers 4d–f and 4i is instructive as the para isomer did not result in product formation, whereas the ortho and meta-isomers gave reasonable yields. Hence, there is no obvious impact of the electron-donating or accepting properties of the substituent in this reaction because the methoxy substituent operates as +M donor in the para and ortho-position and as –I acceptor in the meta-position (as well as in the ortho position in a not conjugated conformation). Also, steric effects do not seem to have a main impact on the reaction outcome as both examples of ortho- and para-substituted arene derivatives give similar yields. These results show that there is obviously a fine balance between stereoelectronic and steric effects that determines the outcome of the reaction, as has been shown for the Suzuki–Miyaura reaction [35]. It should be noted that the inverse coupling, i.e., of 2-bromonorbornadiene with arylboronic acid derivatives is also a reasonable and already tested alternative route, that gives very good yields in the case of higher substituted norbornadiene products [28,29]. However, the approach presented herein avoids the use of the significantly more expensive reagents such as the arylboronic acid derivatives and the (t-Bu)3P ligand. To add to that, the latter is also highly air sensitive and thus requires special handling, but it is essential for a reasonably efficient Pd-mediated coupling of 2-bromonorbornadiene derivatives. In any case, with both complementary methods at hand the synthesis of monoaryl-substituted norbornadiene derivatives is possible in a highly modular approach.
Because the methoxyphenyl-substituted norbornadiene derivative 5c has been reported already [28], we also attempted, for comparison, its complementary synthesis by the Suzuki–Miyaura coupling reaction of norbornadiene 2b with 4-iodoanisole (4k) (Scheme 2). To our surprise, the product 5k was not formed in detectable amounts, neither with Pd(PPh3)4/NaOH, nor with Pd2(dba)3/(t-Bu)3PHBF4/CsF as catalyst and additive. The 1H NMR-spectroscopic analysis of the reaction showed that in both attempts no reaction occurred. It may be concluded that the nucleophilicity of the boronic ester is not sufficient, however, under the employed conditions, it can be hydrolyzed to the more reactive boronic acid. Unfortunately, the latter was not available on preparative scale (see above) so that this aspect could not be clarified with control experiments. At the same time, the coupling reaction may also be sterically hindered by the 3-methyl group of the norbornadiene 2b, which is in agreement with the low yields of the borylation reaction (see above).
Photochromism of naphthylnorbornadiene 6b
The photoisomerization reaction of substrate 5b was monitored by absorption spectroscopy (Figure 1) and by 1H NMR-spectroscopic analysis (see Supporting Information File 1, Figure S51). In MeCN solution, norbornadiene 5b exhibits the lowest-energy maximum at 305 nm, which is comparable to the reported absorption spectra of known naphthyl-substituted norbornadiene derivatives [36,37]. Irradiation of 5b at 315 nm leads to a decrease of the absorption band with simultaneous formation of a blue-shifted maximum at 292 nm, which usually indicates the formation of the corresponding quadricyclane 6b [10-12,29,36]. In addition, the isosbestic points, that developed upon superposition of the spectra obtained during the photoreaction, clearly indicated that only two absorbing species were present in the reaction mixture. The NMR-spectroscopic studies of the latter also confirmed the formation of the quadricyclane product 6b, because the olefinic 1H NMR signals of the norbornadiene at 6.65 ppm, 6.69 ppm, and 6.90 ppm disappeared in favor of signals in the aliphatic region, that are characteristic of the quadricyclane structure [9-12,36]. Moreover, the NMR-spectroscopic analysis of the reaction mixture revealed a photostationary state at λex = 315 nm with a norbornadiene/quadricyclane ratio of 20:80. The quadricyclane 6b could not be transformed back to the norbornadiene 5b by direct irradiation because of the overlap of its absorption with the one of the norbornadiene. However, upon heating to 60 °C in MeCN solution the norbornadiene was regained after 25 h, as shown by photometric monitoring of the reaction (Scheme 3 and Figure 1, inset). These photochemical properties are comparable to the ones of literature-known naphthyl-substituted norbornadienes, which carry, however, additional acceptor substituents [36,37]. Therefore, these results indicate that the latter substituents may not be necessary to accomplish these photochemical properties and may be even omitted for the sake of molecular mass, that should be as low as possible for MOST applications [38,39].
![[1860-5397-18-41-1]](/bjoc/content/figures/1860-5397-18-41-1.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 1: Photometric monitoring of the photoisomerization of 2-(1-naphthyl)norbornadiene (5b) in MeCN, c = 20 µM, T = 20 °C, λex = 315 nm. The arrows indicate the development of the absorption bands with reaction time. Inset: Thermally induced back conversion of quadricyclane 6b into norbornadiene 5b at 60 °C as monitored by the increase of the norbornadiene absorbance at 301 nm.
Figure 1: Photometric monitoring of the photoisomerization of 2-(1-naphthyl)norbornadiene (5b) in MeCN, c = 2...
Scheme 3: Photo-induced, reversible conversion of the naphthylnorbornadiene 5b to quadricyclane 6b in CH3CN (PSS315: Photostationary state at λ = 315 nm).
Scheme 3: Photo-induced, reversible conversion of the naphthylnorbornadiene 5b to quadricyclane 6b in CH3CN (...
Conclusion
In summary, we presented two new borylated norbornadiene derivatives 2a and 2b and identified suitable reaction conditions of their Suzuki–Miyaura-coupling reaction with halogenated aromatic substrates. Although the sterically more congested derivative 2b has significantly suppressed reactivity, the monosubstituted derivative 2a was shown to be a very useful and complementary building block for the synthesis of monoarylated norbornadiene derivatives that carry no further substituents. It should be noted, however, that the inverse coupling, i.e., of the 2-bromonorbornadiene with arylboronic acid derivatives is also a reasonable and already tested alternative route, but the approach presented herein avoids the use of the usually more expensive arylboronic acid derivatives. In any case, with both complementary methods at hand the synthesis of monoaryl-substituted norbornadiene derivatives is possible in a highly modular approach. Considering the paucity of such derivatives in this field, this synthetic route may pave the way for a new series of promising norbornadiene-based MOST materials.
Supporting Information
| Supporting Information File 1: Experimental protocols and NMR spectra. | ||
| Format: PDF | Size: 3.4 MB | Download |
References
-
Dauben, W. G.; Cargill, R. L. Tetrahedron 1961, 15, 197–201. doi:10.1016/0040-4020(61)80026-4
Return to citation in text: [1] -
Dubonosov, A. D.; Bren, V. A.; Chernoivanov, V. A. Russ. Chem. Rev. 2002, 71, 917–927. doi:10.1070/rc2002v071n11abeh000745
Return to citation in text: [1] [2] -
Hammond, G. S.; Turro, N. J.; Fischer, A. J. Am. Chem. Soc. 1961, 83, 4674–4675. doi:10.1021/ja01483a051
Return to citation in text: [1] -
Herges, R.; Starck, F.; Winkler, T.; Schmittel, M. Chem. – Eur. J. 1999, 5, 2965–2970. doi:10.1002/(sici)1521-3765(19991001)5:10<2965::aid-chem2965>3.0.co;2-6
Return to citation in text: [1] -
Ikezawa, H.; Kutal, C.; Yasufuku, K.; Yamazaki, H. J. Am. Chem. Soc. 1986, 108, 1589–1594. doi:10.1021/ja00267a032
Return to citation in text: [1] -
Lorenz, P.; Hirsch, A. Chem. – Eur. J. 2020, 26, 5220–5230. doi:10.1002/chem.201904679
Return to citation in text: [1] -
Tomioka, H.; Hamano, Y.; Izawa, Y. Bull. Chem. Soc. Jpn. 1987, 60, 821–823. doi:10.1246/bcsj.60.821
Return to citation in text: [1] -
Yoshida, Z.-i. J. Photochem. 1985, 29, 27–40. doi:10.1016/0047-2670(85)87059-3
Return to citation in text: [1] -
Lennartson, A.; Roffey, A.; Moth-Poulsen, K. Tetrahedron Lett. 2015, 56, 1457–1465. doi:10.1016/j.tetlet.2015.01.187
Return to citation in text: [1] [2] -
Orrego-Hernández, J.; Dreos, A.; Moth-Poulsen, K. Acc. Chem. Res. 2020, 53, 1478–1487. doi:10.1021/acs.accounts.0c00235
Return to citation in text: [1] [2] [3] -
Orrego‐Hernández, J.; Hölzel, H.; Quant, M.; Wang, Z.; Moth‐Poulsen, K. Eur. J. Org. Chem. 2021, 5337–5342. doi:10.1002/ejoc.202100795
Return to citation in text: [1] [2] [3] -
Quant, M.; Lennartson, A.; Dreos, A.; Kuisma, M.; Erhart, P.; Börjesson, K.; Moth-Poulsen, K. Chem. – Eur. J. 2016, 22, 13265–13274. doi:10.1002/chem.201602530
Return to citation in text: [1] [2] [3] -
Barlow, M. G.; Suliman, N. N. E.; Tipping, A. E. J. Fluorine Chem. 1995, 70, 109–119. doi:10.1016/0022-1139(94)03103-7
Return to citation in text: [1] [2] -
Cornelius, L. A. M.; Bone, R. G. A.; Hastings, R. H.; Deardorff, M. A.; Scharlach, R. A.; Hauptmann, B. E.; Stankovic, C. S.; Pinnick, H. W. J. Org. Chem. 1993, 58, 3188–3190. doi:10.1021/jo00063a050
Return to citation in text: [1] [2] -
Delaude, L.; Demonceau, A.; Noels, A. F. Macromolecules 1999, 32, 2091–2103. doi:10.1021/ma9812783
Return to citation in text: [1] [2] -
De Lucchi, O.; Licini, G.; Pasquato, L.; Senta, M. Tetrahedron Lett. 1988, 29, 831–834. doi:10.1016/s0040-4039(00)80222-1
Return to citation in text: [1] [2] -
Rainier, J. D.; Xu, Q. Org. Lett. 1999, 1, 27–30. doi:10.1021/ol990532o
Return to citation in text: [1] [2] -
Riera, A.; Martí, M.; Moyano, A.; Pericás, M. A.; Santamaría, J. Tetrahedron Lett. 1990, 31, 2173–2176. doi:10.1016/0040-4039(90)80101-q
Return to citation in text: [1] [2] -
Lowe, A. J.; Dyson, G. A.; Pfeffer, F. M. Eur. J. Org. Chem. 2008, 1559–1567. doi:10.1002/ejoc.200701015
Return to citation in text: [1] -
Schuschke, C.; Hohner, C.; Jevric, M.; Ugleholdt Petersen, A.; Wang, Z.; Schwarz, M.; Kettner, M.; Waidhas, F.; Fromm, L.; Sumby, C. J.; Görling, A.; Brummel, O.; Moth-Poulsen, K.; Libuda, J. Nat. Commun. 2019, 10, 2384. doi:10.1038/s41467-019-10263-4
Return to citation in text: [1] -
Williams, R. V.; Chauhan, K.; Gadgil, V. R. J. Chem. Soc., Chem. Commun. 1994, 1739–1740. doi:10.1039/c39940001739
Return to citation in text: [1] -
Yamashita, Y.; Hanaoka, T.; Takeda, Y.; Mukai, T.; Miyashi, T. Bull. Chem. Soc. Jpn. 1988, 61, 2451–2458. doi:10.1246/bcsj.61.2451
Return to citation in text: [1] -
Zheng, H.; Hall, D. G. Tetrahedron Lett. 2010, 51, 3561–3564. doi:10.1016/j.tetlet.2010.04.132
Return to citation in text: [1] -
Ciganek, E. Org. React. 1984, 32, 1–374. doi:10.1002/0471264180.or032.01
Return to citation in text: [1] -
2.2.2 Cyclopentadienes and Cyclohexadienes. In The Diels–Alder Reaction: Selected Practical Methods; Fringuelli, F.; Taticchi, A., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2002; pp 37–40.
Return to citation in text: [1] -
Gassman, P. G.; Gennick, I. J. Am. Chem. Soc. 1980, 102, 6863–6864. doi:10.1021/ja00542a041
Return to citation in text: [1] -
Tranmer, G. K.; Yip, C.; Handerson, S.; Jordan, R. W.; Tam, W. Can. J. Chem. 2000, 78, 527–535. doi:10.1139/v00-047
Return to citation in text: [1] [2] [3] [4] [5] -
Yoo, W.-J.; Tsui, G. C.; Tam, W. Eur. J. Org. Chem. 2005, 1044–1051. doi:10.1002/ejoc.200400713
Return to citation in text: [1] [2] [3] [4] [5] -
Gray, V.; Lennartson, A.; Ratanalert, P.; Börjesson, K.; Moth-Poulsen, K. Chem. Commun. 2014, 50, 5330–5332. doi:10.1039/c3cc47517d
Return to citation in text: [1] [2] [3] [4] -
Löw, R.; Rusch, T.; Moje, T.; Röhricht, F.; Magnussen, O. M.; Herges, R. Beilstein J. Org. Chem. 2019, 15, 1815–1821. doi:10.3762/bjoc.15.175
Return to citation in text: [1] [2] -
Kunz, A.; Wegner, H. A. ChemSystemsChem 2021, 3, e2000035. doi:10.1002/syst.202000035
Return to citation in text: [1] [2] [3] [4] -
Matteson, D. S.; Peacock, K. J. Am. Chem. Soc. 1960, 82, 5759–5760. doi:10.1021/ja01506a056
Return to citation in text: [1] -
Kim, H.; Yin, Z.; Sakurai, H.; Yoshida, J.-i. React. Chem. Eng. 2018, 3, 635–639. doi:10.1039/c8re00131f
Return to citation in text: [1] -
Baumgärtel, O.; Szeimies, G. Chem. Ber. 1983, 116, 2180–2204. doi:10.1002/cber.19831160612
Return to citation in text: [1] [2] -
Sawatlon, B.; Wodrich, M. D.; Corminboeuf, C. Org. Lett. 2020, 22, 7936–7941. doi:10.1021/acs.orglett.0c02862
Return to citation in text: [1] -
Jevric, M.; Petersen, A. U.; Mansø, M.; Kumar Singh, S.; Wang, Z.; Dreos, A.; Sumby, C.; Nielsen, M. B.; Börjesson, K.; Erhart, P.; Moth-Poulsen, K. Chem. – Eur. J. 2018, 24, 12767–12772. doi:10.1002/chem.201802932
Return to citation in text: [1] [2] [3] [4] -
Mansø, M.; Fernandez, L.; Wang, Z.; Moth-Poulsen, K.; Nielsen, M. B. Molecules 2020, 25, 322. doi:10.3390/molecules25020322
Return to citation in text: [1] [2] -
Kucharski, T. J.; Tian, Y.; Akbulatov, S.; Boulatov, R. Energy Environ. Sci. 2011, 4, 4449–4472. doi:10.1039/c1ee01861b
Return to citation in text: [1] -
Sun, C.-L.; Wang, C.; Boulatov, R. ChemPhotoChem 2019, 3, 268–283. doi:10.1002/cptc.201900030
Return to citation in text: [1]
| 28. | Yoo, W.-J.; Tsui, G. C.; Tam, W. Eur. J. Org. Chem. 2005, 1044–1051. doi:10.1002/ejoc.200400713 |
| 35. | Sawatlon, B.; Wodrich, M. D.; Corminboeuf, C. Org. Lett. 2020, 22, 7936–7941. doi:10.1021/acs.orglett.0c02862 |
| 28. | Yoo, W.-J.; Tsui, G. C.; Tam, W. Eur. J. Org. Chem. 2005, 1044–1051. doi:10.1002/ejoc.200400713 |
| 29. | Gray, V.; Lennartson, A.; Ratanalert, P.; Börjesson, K.; Moth-Poulsen, K. Chem. Commun. 2014, 50, 5330–5332. doi:10.1039/c3cc47517d |
| 1. | Dauben, W. G.; Cargill, R. L. Tetrahedron 1961, 15, 197–201. doi:10.1016/0040-4020(61)80026-4 |
| 2. | Dubonosov, A. D.; Bren, V. A.; Chernoivanov, V. A. Russ. Chem. Rev. 2002, 71, 917–927. doi:10.1070/rc2002v071n11abeh000745 |
| 3. | Hammond, G. S.; Turro, N. J.; Fischer, A. J. Am. Chem. Soc. 1961, 83, 4674–4675. doi:10.1021/ja01483a051 |
| 4. | Herges, R.; Starck, F.; Winkler, T.; Schmittel, M. Chem. – Eur. J. 1999, 5, 2965–2970. doi:10.1002/(sici)1521-3765(19991001)5:10<2965::aid-chem2965>3.0.co;2-6 |
| 5. | Ikezawa, H.; Kutal, C.; Yasufuku, K.; Yamazaki, H. J. Am. Chem. Soc. 1986, 108, 1589–1594. doi:10.1021/ja00267a032 |
| 6. | Lorenz, P.; Hirsch, A. Chem. – Eur. J. 2020, 26, 5220–5230. doi:10.1002/chem.201904679 |
| 7. | Tomioka, H.; Hamano, Y.; Izawa, Y. Bull. Chem. Soc. Jpn. 1987, 60, 821–823. doi:10.1246/bcsj.60.821 |
| 13. | Barlow, M. G.; Suliman, N. N. E.; Tipping, A. E. J. Fluorine Chem. 1995, 70, 109–119. doi:10.1016/0022-1139(94)03103-7 |
| 14. | Cornelius, L. A. M.; Bone, R. G. A.; Hastings, R. H.; Deardorff, M. A.; Scharlach, R. A.; Hauptmann, B. E.; Stankovic, C. S.; Pinnick, H. W. J. Org. Chem. 1993, 58, 3188–3190. doi:10.1021/jo00063a050 |
| 15. | Delaude, L.; Demonceau, A.; Noels, A. F. Macromolecules 1999, 32, 2091–2103. doi:10.1021/ma9812783 |
| 16. | De Lucchi, O.; Licini, G.; Pasquato, L.; Senta, M. Tetrahedron Lett. 1988, 29, 831–834. doi:10.1016/s0040-4039(00)80222-1 |
| 17. | Rainier, J. D.; Xu, Q. Org. Lett. 1999, 1, 27–30. doi:10.1021/ol990532o |
| 18. | Riera, A.; Martí, M.; Moyano, A.; Pericás, M. A.; Santamaría, J. Tetrahedron Lett. 1990, 31, 2173–2176. doi:10.1016/0040-4039(90)80101-q |
| 24. | Ciganek, E. Org. React. 1984, 32, 1–374. doi:10.1002/0471264180.or032.01 |
| 25. | 2.2.2 Cyclopentadienes and Cyclohexadienes. In The Diels–Alder Reaction: Selected Practical Methods; Fringuelli, F.; Taticchi, A., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2002; pp 37–40. |
| 28. | Yoo, W.-J.; Tsui, G. C.; Tam, W. Eur. J. Org. Chem. 2005, 1044–1051. doi:10.1002/ejoc.200400713 |
| 29. | Gray, V.; Lennartson, A.; Ratanalert, P.; Börjesson, K.; Moth-Poulsen, K. Chem. Commun. 2014, 50, 5330–5332. doi:10.1039/c3cc47517d |
| 30. | Löw, R.; Rusch, T.; Moje, T.; Röhricht, F.; Magnussen, O. M.; Herges, R. Beilstein J. Org. Chem. 2019, 15, 1815–1821. doi:10.3762/bjoc.15.175 |
| 31. | Kunz, A.; Wegner, H. A. ChemSystemsChem 2021, 3, e2000035. doi:10.1002/syst.202000035 |
| 13. | Barlow, M. G.; Suliman, N. N. E.; Tipping, A. E. J. Fluorine Chem. 1995, 70, 109–119. doi:10.1016/0022-1139(94)03103-7 |
| 14. | Cornelius, L. A. M.; Bone, R. G. A.; Hastings, R. H.; Deardorff, M. A.; Scharlach, R. A.; Hauptmann, B. E.; Stankovic, C. S.; Pinnick, H. W. J. Org. Chem. 1993, 58, 3188–3190. doi:10.1021/jo00063a050 |
| 15. | Delaude, L.; Demonceau, A.; Noels, A. F. Macromolecules 1999, 32, 2091–2103. doi:10.1021/ma9812783 |
| 16. | De Lucchi, O.; Licini, G.; Pasquato, L.; Senta, M. Tetrahedron Lett. 1988, 29, 831–834. doi:10.1016/s0040-4039(00)80222-1 |
| 17. | Rainier, J. D.; Xu, Q. Org. Lett. 1999, 1, 27–30. doi:10.1021/ol990532o |
| 18. | Riera, A.; Martí, M.; Moyano, A.; Pericás, M. A.; Santamaría, J. Tetrahedron Lett. 1990, 31, 2173–2176. doi:10.1016/0040-4039(90)80101-q |
| 19. | Lowe, A. J.; Dyson, G. A.; Pfeffer, F. M. Eur. J. Org. Chem. 2008, 1559–1567. doi:10.1002/ejoc.200701015 |
| 20. | Schuschke, C.; Hohner, C.; Jevric, M.; Ugleholdt Petersen, A.; Wang, Z.; Schwarz, M.; Kettner, M.; Waidhas, F.; Fromm, L.; Sumby, C. J.; Görling, A.; Brummel, O.; Moth-Poulsen, K.; Libuda, J. Nat. Commun. 2019, 10, 2384. doi:10.1038/s41467-019-10263-4 |
| 21. | Williams, R. V.; Chauhan, K.; Gadgil, V. R. J. Chem. Soc., Chem. Commun. 1994, 1739–1740. doi:10.1039/c39940001739 |
| 22. | Yamashita, Y.; Hanaoka, T.; Takeda, Y.; Mukai, T.; Miyashi, T. Bull. Chem. Soc. Jpn. 1988, 61, 2451–2458. doi:10.1246/bcsj.61.2451 |
| 23. | Zheng, H.; Hall, D. G. Tetrahedron Lett. 2010, 51, 3561–3564. doi:10.1016/j.tetlet.2010.04.132 |
| 31. | Kunz, A.; Wegner, H. A. ChemSystemsChem 2021, 3, e2000035. doi:10.1002/syst.202000035 |
| 34. | Baumgärtel, O.; Szeimies, G. Chem. Ber. 1983, 116, 2180–2204. doi:10.1002/cber.19831160612 |
| 9. | Lennartson, A.; Roffey, A.; Moth-Poulsen, K. Tetrahedron Lett. 2015, 56, 1457–1465. doi:10.1016/j.tetlet.2015.01.187 |
| 10. | Orrego-Hernández, J.; Dreos, A.; Moth-Poulsen, K. Acc. Chem. Res. 2020, 53, 1478–1487. doi:10.1021/acs.accounts.0c00235 |
| 11. | Orrego‐Hernández, J.; Hölzel, H.; Quant, M.; Wang, Z.; Moth‐Poulsen, K. Eur. J. Org. Chem. 2021, 5337–5342. doi:10.1002/ejoc.202100795 |
| 12. | Quant, M.; Lennartson, A.; Dreos, A.; Kuisma, M.; Erhart, P.; Börjesson, K.; Moth-Poulsen, K. Chem. – Eur. J. 2016, 22, 13265–13274. doi:10.1002/chem.201602530 |
| 28. | Yoo, W.-J.; Tsui, G. C.; Tam, W. Eur. J. Org. Chem. 2005, 1044–1051. doi:10.1002/ejoc.200400713 |
| 38. | Kucharski, T. J.; Tian, Y.; Akbulatov, S.; Boulatov, R. Energy Environ. Sci. 2011, 4, 4449–4472. doi:10.1039/c1ee01861b |
| 39. | Sun, C.-L.; Wang, C.; Boulatov, R. ChemPhotoChem 2019, 3, 268–283. doi:10.1002/cptc.201900030 |
| 2. | Dubonosov, A. D.; Bren, V. A.; Chernoivanov, V. A. Russ. Chem. Rev. 2002, 71, 917–927. doi:10.1070/rc2002v071n11abeh000745 |
| 8. | Yoshida, Z.-i. J. Photochem. 1985, 29, 27–40. doi:10.1016/0047-2670(85)87059-3 |
| 31. | Kunz, A.; Wegner, H. A. ChemSystemsChem 2021, 3, e2000035. doi:10.1002/syst.202000035 |
| 34. | Baumgärtel, O.; Szeimies, G. Chem. Ber. 1983, 116, 2180–2204. doi:10.1002/cber.19831160612 |
| 32. | Matteson, D. S.; Peacock, K. J. Am. Chem. Soc. 1960, 82, 5759–5760. doi:10.1021/ja01506a056 |
| 33. | Kim, H.; Yin, Z.; Sakurai, H.; Yoshida, J.-i. React. Chem. Eng. 2018, 3, 635–639. doi:10.1039/c8re00131f |
| 27. | Tranmer, G. K.; Yip, C.; Handerson, S.; Jordan, R. W.; Tam, W. Can. J. Chem. 2000, 78, 527–535. doi:10.1139/v00-047 |
| 9. | Lennartson, A.; Roffey, A.; Moth-Poulsen, K. Tetrahedron Lett. 2015, 56, 1457–1465. doi:10.1016/j.tetlet.2015.01.187 |
| 10. | Orrego-Hernández, J.; Dreos, A.; Moth-Poulsen, K. Acc. Chem. Res. 2020, 53, 1478–1487. doi:10.1021/acs.accounts.0c00235 |
| 11. | Orrego‐Hernández, J.; Hölzel, H.; Quant, M.; Wang, Z.; Moth‐Poulsen, K. Eur. J. Org. Chem. 2021, 5337–5342. doi:10.1002/ejoc.202100795 |
| 12. | Quant, M.; Lennartson, A.; Dreos, A.; Kuisma, M.; Erhart, P.; Börjesson, K.; Moth-Poulsen, K. Chem. – Eur. J. 2016, 22, 13265–13274. doi:10.1002/chem.201602530 |
| 36. | Jevric, M.; Petersen, A. U.; Mansø, M.; Kumar Singh, S.; Wang, Z.; Dreos, A.; Sumby, C.; Nielsen, M. B.; Börjesson, K.; Erhart, P.; Moth-Poulsen, K. Chem. – Eur. J. 2018, 24, 12767–12772. doi:10.1002/chem.201802932 |
| 28. | Yoo, W.-J.; Tsui, G. C.; Tam, W. Eur. J. Org. Chem. 2005, 1044–1051. doi:10.1002/ejoc.200400713 |
| 29. | Gray, V.; Lennartson, A.; Ratanalert, P.; Börjesson, K.; Moth-Poulsen, K. Chem. Commun. 2014, 50, 5330–5332. doi:10.1039/c3cc47517d |
| 30. | Löw, R.; Rusch, T.; Moje, T.; Röhricht, F.; Magnussen, O. M.; Herges, R. Beilstein J. Org. Chem. 2019, 15, 1815–1821. doi:10.3762/bjoc.15.175 |
| 31. | Kunz, A.; Wegner, H. A. ChemSystemsChem 2021, 3, e2000035. doi:10.1002/syst.202000035 |
| 27. | Tranmer, G. K.; Yip, C.; Handerson, S.; Jordan, R. W.; Tam, W. Can. J. Chem. 2000, 78, 527–535. doi:10.1139/v00-047 |
| 36. | Jevric, M.; Petersen, A. U.; Mansø, M.; Kumar Singh, S.; Wang, Z.; Dreos, A.; Sumby, C.; Nielsen, M. B.; Börjesson, K.; Erhart, P.; Moth-Poulsen, K. Chem. – Eur. J. 2018, 24, 12767–12772. doi:10.1002/chem.201802932 |
| 37. | Mansø, M.; Fernandez, L.; Wang, Z.; Moth-Poulsen, K.; Nielsen, M. B. Molecules 2020, 25, 322. doi:10.3390/molecules25020322 |
| 27. | Tranmer, G. K.; Yip, C.; Handerson, S.; Jordan, R. W.; Tam, W. Can. J. Chem. 2000, 78, 527–535. doi:10.1139/v00-047 |
| 36. | Jevric, M.; Petersen, A. U.; Mansø, M.; Kumar Singh, S.; Wang, Z.; Dreos, A.; Sumby, C.; Nielsen, M. B.; Börjesson, K.; Erhart, P.; Moth-Poulsen, K. Chem. – Eur. J. 2018, 24, 12767–12772. doi:10.1002/chem.201802932 |
| 37. | Mansø, M.; Fernandez, L.; Wang, Z.; Moth-Poulsen, K.; Nielsen, M. B. Molecules 2020, 25, 322. doi:10.3390/molecules25020322 |
| 26. | Gassman, P. G.; Gennick, I. J. Am. Chem. Soc. 1980, 102, 6863–6864. doi:10.1021/ja00542a041 |
| 27. | Tranmer, G. K.; Yip, C.; Handerson, S.; Jordan, R. W.; Tam, W. Can. J. Chem. 2000, 78, 527–535. doi:10.1139/v00-047 |
| 27. | Tranmer, G. K.; Yip, C.; Handerson, S.; Jordan, R. W.; Tam, W. Can. J. Chem. 2000, 78, 527–535. doi:10.1139/v00-047 |
| 10. | Orrego-Hernández, J.; Dreos, A.; Moth-Poulsen, K. Acc. Chem. Res. 2020, 53, 1478–1487. doi:10.1021/acs.accounts.0c00235 |
| 11. | Orrego‐Hernández, J.; Hölzel, H.; Quant, M.; Wang, Z.; Moth‐Poulsen, K. Eur. J. Org. Chem. 2021, 5337–5342. doi:10.1002/ejoc.202100795 |
| 12. | Quant, M.; Lennartson, A.; Dreos, A.; Kuisma, M.; Erhart, P.; Börjesson, K.; Moth-Poulsen, K. Chem. – Eur. J. 2016, 22, 13265–13274. doi:10.1002/chem.201602530 |
| 29. | Gray, V.; Lennartson, A.; Ratanalert, P.; Börjesson, K.; Moth-Poulsen, K. Chem. Commun. 2014, 50, 5330–5332. doi:10.1039/c3cc47517d |
| 36. | Jevric, M.; Petersen, A. U.; Mansø, M.; Kumar Singh, S.; Wang, Z.; Dreos, A.; Sumby, C.; Nielsen, M. B.; Börjesson, K.; Erhart, P.; Moth-Poulsen, K. Chem. – Eur. J. 2018, 24, 12767–12772. doi:10.1002/chem.201802932 |
© 2022 Schulte and Ihmels; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.