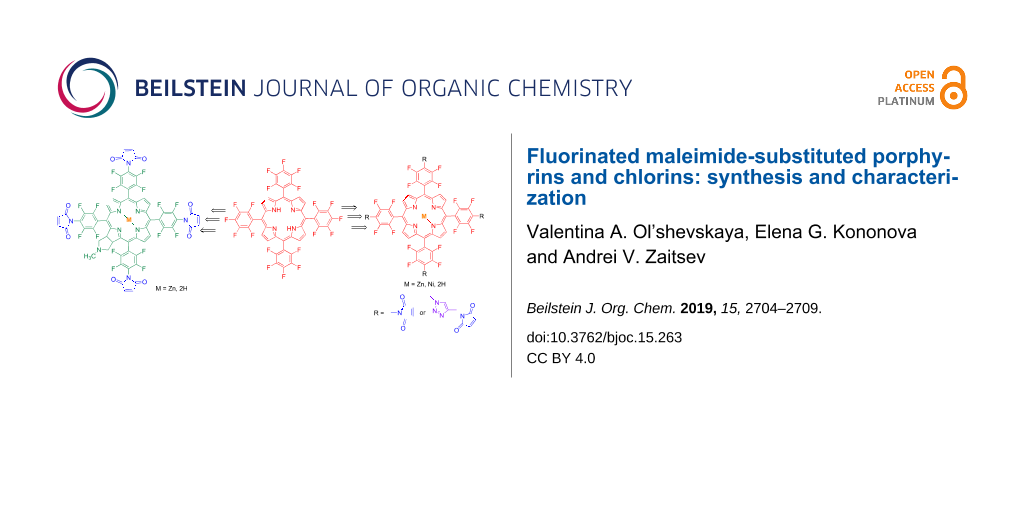
Abstract
Maleimide-containing fluorinated porphyrins and chlorins were prepared based on the reaction of Zn(II) or Ni(II) complexes of 5,10,15,20-tetrakis(4-amino-2,3,5,6-tetrafluorophenyl)porphyrin and chlorin with maleic anhydride. Porphyrin maleimide derivatives were also prepared by the reaction of 5,10,15,20-tetrakis(4-azido-2,3,5,6-tetrafluorophenyl)porphyrinato Zn(II) or Ni(II) with N-propargylmaleimide via the CuAAC click reaction to afford fluorinated porphyrin–triazole–maleimide conjugates. New maleimide derivatives were isolated in reasonable yields and identified by UV–vis, 1H NMR, 19F NMR spectroscopy and mass-spectrometry.
Graphical Abstract
Introduction
Porphyrins belong to a broad class of the natural and synthetic macroheterocyclic compounds with unique properties. They play an important role in photosynthesis [1], catalysis [2,3], nonlinear optics [4,5], polymer synthesis [6] and energy conversions [7]. Porphyrins have been extensively studied as potential photosensitizers in a photodynamic therapy (PDT) [8,9], a promising treatment modality for several cancer and infectious diseases. In PDT, light, O2, and a photosensitizing drug are combined to produce a selective therapeutic effect via the generation of active oxygen forms (1O2, HO−, НО2−˙and O2−˙) upon excitation with monochromatic light which causes the death of the tumor [10-14]. Some other important features, that photosensitizers should have for such applications are their photo and thermal stability, and an ability to selectively accumulate in the target tissue, the absence of toxicity, toxic byproducts and mutagenic effects, and an opportunity for medical administration. An additional advantage of porphyrins is the possibility of functionalization of the macrocycle periphery with various substituents and thus to affect the photophysical, photochemical and tumor-specific properties of the porphyrin system. Such an approach provides a platform to new photosensitizers with optimized characteristics useful for biomedical applications including PDT. Currently a number of investigations directed for the preparation of tumor-targeted photosensitizers have been explored aiming to improve their tumor-specificity [15,16].
To continue our ongoing efforts on the preparation of porphyrin-based photosensitizers [17-20], we present herein the synthesis of maleimide-subtituted porphyrins and chlorins based on 5,10,15,20-tetrakis(pentafluorophenyl)porphyrin (1) as the starting compound. The presence of fluorine atoms on the four phenyl rings at the meso-positions of the porphyrin structure can make a strong influence on the hydrophobic interactions and lipophilicity, metabolic stability, thus modulating the biological efficiency of the photosensitizing agents [21,22]. At the same time fluorinated porphyrins are well known for their photostability and efficiency in generating long-lived triplet excited states through intersystem crossing (ISC) with minimal energy loss from excited states, and are widely used to generate singlet oxygen for PDT applications [9,23]. We intend here to combine within one molecule the structural specificity of a meso-fuorinated porphyrin/chlorin macrocycle and maleimide units to develop novel multifunctional compounds with improved properties for various applications. Maleimides are considered as a biologically important scaffold that possess almost all types of biological activities including antibacterial and antifungal activity [24], anticancer activity [25], cox-2 inhibitor and anti-inflammatory, antidiabetic activity [26] and photodynamic activity [27]. Attaching of the maleimide group with its rich biological activity to the tetrapyrrole macrocycles with their unique photophysical properties may result in new conjugates with improved chemical, biological and anticancer characteristics [28]. Moreover, maleimide is a stable functionality that rapidly and covalently conjugates thiol groups of cysteine residues in proteins or peptides by the thio-Michael addition to the double bond of the maleimide to form a corresponding succinimidyl thioether. Conjugation of the cysteine sulfhydryl group with maleimide moieties allows us to prepare the bioconjugates selectively, covalently in high yields with no requirement for prior activation of reactants [29] and thus strengthen the association of a drug molecule with the cell surface. It is important to mention that Kitagishi and co-workers [30] showed that the maleimide-appended porphyrin/cyclodextrine complex was conjugated to a cystein residue of serum albumin via a Michael addition reaction. At the same time, it is well known that 5,10,15,20-tetrakis(pentafluorophenyl)porphyrin and its chlorin derivatives generate singlet oxygen by the light irradiation under atmospheric oxygen [31]. These tetrapyrrole macrocycles and their metal complexes are considered to be efficient precursors for design and selection of new PDT agents, since their reactivity toward various nucleophiles provides a simple, selective and general access to the functionalized derivatives [32-34]. Considering the promise of porphyrins and chlorins for the development of PDT therapeutics and dependence of their biological properties on the structure of peripheral substituents, we developed a simple synthetic approach for new maleimide derivatives of the fluorinated porphyrins and chlorins. We also believe the synthetic potential of maleimide units in these new conjugates allows versatile ways to obtain a series of new photosensitizers for medical applications.
Results and Discussion
Synthesis of maleimide-substituted porphyrins
In this work for the preparation of porphyrins functionalized with maleimde moieties commercially available 5,10,15,20-tetrakis(pentafluorophenyl)porphyrin (1) [35] was used as starting compound. The synthesis includes the metallation of the free base porphyrin 1 with an excess of zinc acetate or nickel acetate resulting in the corresponding porphyrin Zn(II) and Ni(II) complexes 2a [36] and 2b [37], respectively. Complexes 2a and 2b readily reacted with the excess of NaN3 in DMF/DMSO to afford porphyrins 3a and 3b in high yields via the selective substitution of fluorine atoms in the para-position of the pentafluorophenyl substituents with four azide functions. The subsequent reduction of para-tetraazidoporphyrinato Zn(II) 3a with SnCl2 in MeOH [38] at ambient temperature smoothly provided the corresponding tetraamino-substituted porphyrin 4a in 91% yield. (Scheme 1). The free amino groups of porphyrin 4a are useful and reactive functionalities for further modifications. It was shown that the interaction of porphyrin 4a with maleic anhydride by analogy with a well-known standard procedure [39,40] provided the target maleimide-substituted porphyrin 5a in 42% yield, which contains four maleimide moieties in the structure. Treatment of porphyrin 5a with trifluoroacetic acid in CHCl3 resulted in the removal of zinc from the coordination sphere of the porphyrin macrocycle and gave the free base maleimide porphyrin 6 in a quantitative yield. A successful formation of the fluorinated porphyrin azides 3a and 3b allowed their utility as intermediates in the further porphyrin core functionalisation especially with 1,2,3-triazole heterocycles via the copper-catalyzed azide–alkyne cycloaddition reaction (CuAAC) between alkynes and azides, developed independently by Sharpless [41] and Meldal [42]. In addition to the applications of triazoles as pharmacophores in the potential biologically active molecules, these heterocycles have also been used as linkers and for labeling biomolecules in chemical biology [43]. Moreover, this synthetic approach provides high yields, selectivity, mild reaction conditions and simple purification methods. It was demonstrated that the CuAAC reaction of porphyrins 3a and 3b with N-propargylmaleimide [44] was carried out successfully in CH2Cl2 and the fluorinated porphyrin–triazole–maleimide conjugates 7a and 7b were obtained in 54–58% yield. In these conjugates the tetrafluorophenyl units of the porphyrin macrocycle where separated from maleimides with 1,2,3-triazole spacer groups. Removal of zinc in porphyrin 7a under the action of CF3COOH in CHCl3 resulted in porphyrin 8 in a quantitative yield (Scheme 1).
Scheme 1: Synthesis of fluorinated maleimide-substituted porphyrins 5a, 6, 7a, 7b, and 8.
Scheme 1: Synthesis of fluorinated maleimide-substituted porphyrins 5a, 6, 7a, 7b, and 8.
Synthesis of maleimide-substituted chlorins
Following the developed procedure, maleimide-substituted chlorins were also prepared. Chlorins reveal a number of applications in the photobiological processes demonstrating suitable properties for PDT [45-47]. In this context the synthesis of new chlorins is one of the most important possibilities for the production of new efficient photosensitizers. The reason for this is that chlorins absorb light intensely at wavelengths where the human tissues are the most transparent [48]. Based upon our previous successful results with porphyrins we studied the possibility to obtain maleimide derivatives of 5,10,15,20-tetrakis(pentafluorophenyl)-17,18-N-methylpyrrolidine chlorin 9 [49] prepared by the reaction of porphyrin 1 with azomethine ylide, generated in situ from sarcosine and paraformaldehyde. The reaction of chlorin 9 with the excess of NaN3 in DMF/DMSO resulted in tetraazide chlorin 10 in 85% yield after the purification. The reduction of azide groups in chlorin 10 with SnCl2 in MeOH gave tetraamino-substituted chlorin 11 which was transformed into the corresponding maleimide 12 in 25% yield on the reaction with maleic anhydride according to the standard procedure. Chlorin 12 was metalated with Zn(OAc)2/CHCl3/MeOH giving chlorin 13 in 92% yield. (Scheme 2).
Scheme 2: Synthesis of fluorinated maleimide-substituted chlorins 12,13.
Scheme 2: Synthesis of fluorinated maleimide-substituted chlorins 12,13.
All prepared compounds have been structurally identified by 1H, 19F NMR, IR and UV–vis absorption spectra (see Supporting Information File 1).
Conclusion
In this work, we developed a facile protocol for the preparation of new porphyrin and chlorin conjugates with maleimide entities which were synthesized in reasonable isolated yields and fully characterized with NMR spectroscopy and mass spectrometry. These novel porphyrins and chlorins are expected to be efficient photosensitizers for cancer treatment. The presence of four maleimide groups in these compounds may provide improved binding with proteins in living systems.
Supporting Information
| Supporting Information File 1: Experimental procedures, characterization data and copies of NMR and mass spectra of the synthesized compounds. | ||
| Format: PDF | Size: 1.5 MB | Download |
References
-
Bottari, G.; Trukhina, O.; Ince, M.; Torres, T. Coord. Chem. Rev. 2012, 256, 2453–2477. doi:10.1016/j.ccr.2012.03.011
Return to citation in text: [1] -
Simonneaux, G.; Le Maux, P.; Ferrand, Y.; Rault-Berthelot, J. Coord. Chem. Rev. 2006, 250, 2212–2221. doi:10.1016/j.ccr.2006.01.014
Return to citation in text: [1] -
Barona-Castaño, J. C.; Carmona-Vargas, C. C.; Brocksom, T. J.; de Oliveira, K. T. Molecules 2016, 21, 310. doi:10.3390/molecules21030310
Return to citation in text: [1] -
Annoni, E.; Pizzotti, M.; Ugo, R.; Quici, S.; Morotti, T.; Bruschi, M.; Mussini, P. Eur. J. Inorg. Chem. 2005, 3857–3874. doi:10.1002/ejic.200500292
Return to citation in text: [1] -
Khadria, A.; Fleischhauer, J.; Boczarow, I.; Wilkinson, J. D.; Kohl, M. M.; Anderson, H. L. iScience 2018, 4, 153–163. doi:10.1016/j.isci.2018.05.015
Return to citation in text: [1] -
Tian, J.; Zhang, W. Prog. Polym. Sci. 2019, 95, 65–117. doi:10.1016/j.progpolymsci.2019.05.002
Return to citation in text: [1] -
Angaridis, P. A.; Lazarides, T.; Coutsolelos, A. C. Polyhedron 2014, 82, 19–32. doi:10.1016/j.poly.2014.04.039
Return to citation in text: [1] -
Pandey, R. K.; Kessel, D.; Dougherty, T. J., Eds. Handbook of Photodynamic Therapy. Updates on Recent Applications of Porphyrin-Based Compounds, 1st ed.; World Scientific Pub Co Inc., 2016. doi:10.1142/9774
Return to citation in text: [1] -
Ormond, A. B.; Freeman, H. S. Materials 2013, 6, 817–840. doi:10.3390/ma6030817
Return to citation in text: [1] [2] -
Dougherty, T. J.; Gomer, C. J.; Henderson, B. W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. J. Natl. Cancer Inst. 1998, 90, 889–905. doi:10.1093/jnci/90.12.889
Return to citation in text: [1] -
Weishaupt, K. R.; Gomer, C. J.; Dougherty, T. J. Cancer Res. 1976, 36, 2326–2329.
Return to citation in text: [1] -
Zhang, J.; Jiang, C.; Figueiró Longo, J. P.; Azevedo, R. B.; Zhang, H.; Muehlmann, L. A. Acta Pharm. Sin. B 2018, 8, 137–146. doi:10.1016/j.apsb.2017.09.003
Return to citation in text: [1] -
Kou, J.; Dou Dou, D.; Yang, L. Oncotarget 2017, 8, 81591–81603. doi:10.18632/oncotarget.20189
Return to citation in text: [1] -
Huang, H.; Song, W.; Rieffel, J.; Lovell, J. F. Front. Phys. 2015, 3, No. 23. doi:10.3389/fphy.2015.00023
Return to citation in text: [1] -
Cui, B. C.; Yoon, I.; Li, J. Z.; Lee, W. K.; Shim, Y. K. Int. J. Mol. Sci. 2014, 15, 8091–8105. doi:10.3390/ijms15058091
Return to citation in text: [1] -
Lange, C.; Bednarski, P. Curr. Pharm. Des. 2016, 22, 6956–6974. doi:10.2174/1381612822666161124155344
Return to citation in text: [1] -
Ol'shevskaya, V. A.; Zaytsev, A. V.; Savchenko, A. N.; Shtil, A. A.; Cheong, C.-S.; Kalinin, V. N. Bull. Korean Chem. Soc. 2007, 28, 1910–1916. doi:10.5012/bkcs.2007.28.11.1910
Return to citation in text: [1] -
Ol’shevskaya, V. A.; Zaitsev, A. V.; Kalinin, V. N.; Shtil, A. A. Russ. Chem. Bull. 2014, 63, 2383–2387. doi:10.1007/s11172-014-0751-z
Return to citation in text: [1] -
Omarova, E. O.; Nazarov, P. A.; Firsov, A. M.; Strakhovskaya, M. G.; Arkhipova, A. Y.; Moisenovich, M. M.; Agapov, I. I.; Ol’shevskaya, V. A.; Zaitsev, A. V.; Kalinin, V. N.; Kotova, E. A.; Antonenko, Y. N. PLoS One 2015, 10, e0141990. doi:10.1371/journal.pone.0141990
Return to citation in text: [1] -
Ol’shevskaya, V. A.; Alpatova, V. M.; Konovalova, N. V.; Kononova, E. G.; Rys, E. G.; Bragina, N. A. J. Porphyrins Phthalocyanines 2018, 22, 989–996. doi:10.1142/s1088424618500967
Return to citation in text: [1] -
Gillis, E. P.; Eastman, K. J.; Hill, M. D.; Donnelly, D. J.; Meanwell, N. A. J. Med. Chem. 2015, 58, 8315–8359. doi:10.1021/acs.jmedchem.5b00258
Return to citation in text: [1] -
Costa, J. I. T.; Tomé, A. C.; Neves, M. G. P. M. S.; Cavaleiro, J. A. S. J. Porphyrins Phthalocyanines 2011, 15, 1116–1133. doi:10.1142/s1088424611004294
Return to citation in text: [1] -
Pedrosa, L. F.; de Souza, M. C.; Faustino, M. A. F.; Neves, M. G. P. M. S.; Silva, A. M. S.; Tome, A. C.; Ferreira, V. F.; Cavaleiro, J. A. S. Aust. J. Chem. 2011, 64, 939–944. doi:10.1071/ch11013
Return to citation in text: [1] -
Sortino, M.; Garibotto, F.; Cechinel Filho, V.; Gupta, M.; Enriz, R.; Zacchino, S. Bioorg. Med. Chem. 2011, 19, 2823–2834. doi:10.1016/j.bmc.2011.03.038
Return to citation in text: [1] -
Hanif, M.; Moon, S.; Sullivan, M. P.; Movassaghi, S.; Kubanik, M.; Goldstone, D. C.; Söhnel, T.; Jamieson, S. M. F.; Hartinger, C. G. J. Inorg. Biochem. 2016, 165, 100–107. doi:10.1016/j.jinorgbio.2016.06.025
Return to citation in text: [1] -
Dubernet, M.; Caubert, V.; Guillard, J.; Viaud-Massuard, M.-C. Tetrahedron 2005, 61, 4585–4593. doi:10.1016/j.tet.2005.03.016
Return to citation in text: [1] -
Sandland, J.; Boyle, R. W. Bioconjugate Chem. 2019, 30, 975–993. doi:10.1021/acs.bioconjchem.9b00055
Return to citation in text: [1] -
Guo, X.; Wang, L.; Wang, S.; Li, Y.; Zhang, F.; Song, B.; Zhao, W. Bioorg. Med. Chem. Lett. 2015, 25, 4078–4081. doi:10.1016/j.bmcl.2015.08.036
Return to citation in text: [1] -
Giuntini, F.; Alonso, C. M. A.; Boyle, R. W. Photochem. Photobiol. Sci. 2011, 10, 759–791. doi:10.1039/c0pp00366b
Return to citation in text: [1] -
Kitagishi, H.; Kawasaki, H.; Kano, K. Chem. – Asian J. 2015, 10, 1768–1775. doi:10.1002/asia.201500451
Return to citation in text: [1] -
Obata, M.; Hirohara, S.; Tanaka, R.; Kinoshita, I.; Ohkubo, K.; Fukuzumi, S.; Tanihara, M.; Yano, S. J. Med. Chem. 2009, 52, 2747–2753. doi:10.1021/jm8015427
Return to citation in text: [1] -
Samaroo, D.; Vinodu, M.; Chen, X.; Drain, C. M. J. Comb. Chem. 2007, 9, 998–1011. doi:10.1021/cc070067j
Return to citation in text: [1] -
Hirohara, S.; Oka, C.; Totani, M.; Obata, M.; Yuasa, J.; Ito, H.; Tamura, M.; Matsui, H.; Kakiuchi, K.; Kawai, T.; Kawaichi, M.; Tanihara, M. J. Med. Chem. 2015, 58, 8658–8670. doi:10.1021/acs.jmedchem.5b01262
Return to citation in text: [1] -
Hirohara, S.; Kawasaki, Y.; Funasako, R.; Yasui, N.; Totani, M.; Alitomo, H.; Yuasa, J.; Kawai, T.; Oka, C.; Kawaichi, M.; Obata, M.; Tanihara, M. Bioconjugate Chem. 2012, 23, 1881–1890. doi:10.1021/bc300223j
Return to citation in text: [1] -
Banfi, S.; Caruso, E.; Caprioli, S.; Mazzagatti, L.; Canti, G.; Ravizza, R.; Gariboldi, M.; Monti, E. Bioorg. Med. Chem. 2004, 12, 4853–4860. doi:10.1016/j.bmc.2004.07.011
Return to citation in text: [1] -
Das, S. K.; Song, B.; Mahler, A.; Nesterov, V. N.; Wilson, A. K.; Ito, O.; D’Souza, F. J. Phys. Chem. C 2014, 118, 3994–4006. doi:10.1021/jp4118166
Return to citation in text: [1] -
Peters, M. K.; Herges, R. Inorg. Chem. 2018, 57, 3177–3182. doi:10.1021/acs.inorgchem.7b03164
Return to citation in text: [1] -
Maiti, S. N.; Singh, M. P.; Micetich, R. G. Tetrahedron Lett. 1986, 27, 1423–1424. doi:10.1016/s0040-4039(00)84275-6
Return to citation in text: [1] -
Song, H. Y.; Ngai, M. H.; Song, Z. Y.; MacAry, P. A.; Hobley, J.; Lear, M. J. Org. Biomol. Chem. 2009, 7, 3400–3406. doi:10.1039/b904060a
Return to citation in text: [1] -
Chen, Y.; Parr, T.; Holmes, A. E.; Nakanishi, K. Bioconjugate Chem. 2008, 19, 5–9. doi:10.1021/bc700267f
Return to citation in text: [1] -
Rostovtsev, V. V.; Green, L. G.; Fokin, V. V.; Sharpless, K. B. Angew. Chem., Int. Ed. 2002, 41, 2596–2599. doi:10.1002/1521-3773(20020715)41:14<2596::aid-anie2596>3.0.co;2-4
Return to citation in text: [1] -
Tornøe, C. W.; Christensen, C.; Meldal, M. J. Org. Chem. 2002, 67, 3057–3064. doi:10.1021/jo011148j
Return to citation in text: [1] -
Sletten, E. M.; Bertozzi, C. R. Acc. Chem. Res. 2011, 44, 666–676. doi:10.1021/ar200148z
Return to citation in text: [1] -
Yan, J.; Wang, R.; Pan, D.; Yang, R.; Xu, Y.; Wang, L.; Yang, M. Polym. Chem. 2016, 7, 6241–6249. doi:10.1039/c6py01344a
Return to citation in text: [1] -
Dror, S. B.; Bronshtein, I.; Garini, Y.; O'Neal, W. G.; Jacobi, P. A.; Ehrenberg, B. Photochem. Photobiol. Sci. 2009, 8, 354–361. doi:10.1039/b814970d
Return to citation in text: [1] -
Monteiro, C. J. P.; Pina, J.; Pereira, M. M.; Arnaut, L. G. Photochem. Photobiol. Sci. 2012, 11, 1233–1238. doi:10.1039/c2pp25021g
Return to citation in text: [1] -
Allison, R. R.; Downie, G. H.; Cuenca, R.; Hu, X.-H.; Childs, C. J.; Sibata, C. H. Photodiagn. Photodyn. Ther. 2004, 1, 27–42. doi:10.1016/s1572-1000(04)00007-9
Return to citation in text: [1] -
Spikes, J. D. J. Photochem. Photobiol., B 1990, 6, 259–274. doi:10.1016/1011-1344(90)85096-f
Return to citation in text: [1] -
Jiménez-Osés, G.; García, J. I.; Silva, A. M. G.; Santos, A. R. N.; Tomé, A. C.; Neves, M. G. P. M. S.; Cavaleiro, J. A. S. Tetrahedron 2008, 64, 7937–7943. doi:10.1016/j.tet.2008.06.018
Return to citation in text: [1]
| 43. | Sletten, E. M.; Bertozzi, C. R. Acc. Chem. Res. 2011, 44, 666–676. doi:10.1021/ar200148z |
| 44. | Yan, J.; Wang, R.; Pan, D.; Yang, R.; Xu, Y.; Wang, L.; Yang, M. Polym. Chem. 2016, 7, 6241–6249. doi:10.1039/c6py01344a |
| 45. | Dror, S. B.; Bronshtein, I.; Garini, Y.; O'Neal, W. G.; Jacobi, P. A.; Ehrenberg, B. Photochem. Photobiol. Sci. 2009, 8, 354–361. doi:10.1039/b814970d |
| 46. | Monteiro, C. J. P.; Pina, J.; Pereira, M. M.; Arnaut, L. G. Photochem. Photobiol. Sci. 2012, 11, 1233–1238. doi:10.1039/c2pp25021g |
| 47. | Allison, R. R.; Downie, G. H.; Cuenca, R.; Hu, X.-H.; Childs, C. J.; Sibata, C. H. Photodiagn. Photodyn. Ther. 2004, 1, 27–42. doi:10.1016/s1572-1000(04)00007-9 |
| 1. | Bottari, G.; Trukhina, O.; Ince, M.; Torres, T. Coord. Chem. Rev. 2012, 256, 2453–2477. doi:10.1016/j.ccr.2012.03.011 |
| 7. | Angaridis, P. A.; Lazarides, T.; Coutsolelos, A. C. Polyhedron 2014, 82, 19–32. doi:10.1016/j.poly.2014.04.039 |
| 27. | Sandland, J.; Boyle, R. W. Bioconjugate Chem. 2019, 30, 975–993. doi:10.1021/acs.bioconjchem.9b00055 |
| 6. | Tian, J.; Zhang, W. Prog. Polym. Sci. 2019, 95, 65–117. doi:10.1016/j.progpolymsci.2019.05.002 |
| 28. | Guo, X.; Wang, L.; Wang, S.; Li, Y.; Zhang, F.; Song, B.; Zhao, W. Bioorg. Med. Chem. Lett. 2015, 25, 4078–4081. doi:10.1016/j.bmcl.2015.08.036 |
| 4. | Annoni, E.; Pizzotti, M.; Ugo, R.; Quici, S.; Morotti, T.; Bruschi, M.; Mussini, P. Eur. J. Inorg. Chem. 2005, 3857–3874. doi:10.1002/ejic.200500292 |
| 5. | Khadria, A.; Fleischhauer, J.; Boczarow, I.; Wilkinson, J. D.; Kohl, M. M.; Anderson, H. L. iScience 2018, 4, 153–163. doi:10.1016/j.isci.2018.05.015 |
| 25. | Hanif, M.; Moon, S.; Sullivan, M. P.; Movassaghi, S.; Kubanik, M.; Goldstone, D. C.; Söhnel, T.; Jamieson, S. M. F.; Hartinger, C. G. J. Inorg. Biochem. 2016, 165, 100–107. doi:10.1016/j.jinorgbio.2016.06.025 |
| 2. | Simonneaux, G.; Le Maux, P.; Ferrand, Y.; Rault-Berthelot, J. Coord. Chem. Rev. 2006, 250, 2212–2221. doi:10.1016/j.ccr.2006.01.014 |
| 3. | Barona-Castaño, J. C.; Carmona-Vargas, C. C.; Brocksom, T. J.; de Oliveira, K. T. Molecules 2016, 21, 310. doi:10.3390/molecules21030310 |
| 26. | Dubernet, M.; Caubert, V.; Guillard, J.; Viaud-Massuard, M.-C. Tetrahedron 2005, 61, 4585–4593. doi:10.1016/j.tet.2005.03.016 |
| 17. | Ol'shevskaya, V. A.; Zaytsev, A. V.; Savchenko, A. N.; Shtil, A. A.; Cheong, C.-S.; Kalinin, V. N. Bull. Korean Chem. Soc. 2007, 28, 1910–1916. doi:10.5012/bkcs.2007.28.11.1910 |
| 18. | Ol’shevskaya, V. A.; Zaitsev, A. V.; Kalinin, V. N.; Shtil, A. A. Russ. Chem. Bull. 2014, 63, 2383–2387. doi:10.1007/s11172-014-0751-z |
| 19. | Omarova, E. O.; Nazarov, P. A.; Firsov, A. M.; Strakhovskaya, M. G.; Arkhipova, A. Y.; Moisenovich, M. M.; Agapov, I. I.; Ol’shevskaya, V. A.; Zaitsev, A. V.; Kalinin, V. N.; Kotova, E. A.; Antonenko, Y. N. PLoS One 2015, 10, e0141990. doi:10.1371/journal.pone.0141990 |
| 20. | Ol’shevskaya, V. A.; Alpatova, V. M.; Konovalova, N. V.; Kononova, E. G.; Rys, E. G.; Bragina, N. A. J. Porphyrins Phthalocyanines 2018, 22, 989–996. doi:10.1142/s1088424618500967 |
| 9. | Ormond, A. B.; Freeman, H. S. Materials 2013, 6, 817–840. doi:10.3390/ma6030817 |
| 23. | Pedrosa, L. F.; de Souza, M. C.; Faustino, M. A. F.; Neves, M. G. P. M. S.; Silva, A. M. S.; Tome, A. C.; Ferreira, V. F.; Cavaleiro, J. A. S. Aust. J. Chem. 2011, 64, 939–944. doi:10.1071/ch11013 |
| 15. | Cui, B. C.; Yoon, I.; Li, J. Z.; Lee, W. K.; Shim, Y. K. Int. J. Mol. Sci. 2014, 15, 8091–8105. doi:10.3390/ijms15058091 |
| 16. | Lange, C.; Bednarski, P. Curr. Pharm. Des. 2016, 22, 6956–6974. doi:10.2174/1381612822666161124155344 |
| 24. | Sortino, M.; Garibotto, F.; Cechinel Filho, V.; Gupta, M.; Enriz, R.; Zacchino, S. Bioorg. Med. Chem. 2011, 19, 2823–2834. doi:10.1016/j.bmc.2011.03.038 |
| 10. | Dougherty, T. J.; Gomer, C. J.; Henderson, B. W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. J. Natl. Cancer Inst. 1998, 90, 889–905. doi:10.1093/jnci/90.12.889 |
| 11. | Weishaupt, K. R.; Gomer, C. J.; Dougherty, T. J. Cancer Res. 1976, 36, 2326–2329. |
| 12. | Zhang, J.; Jiang, C.; Figueiró Longo, J. P.; Azevedo, R. B.; Zhang, H.; Muehlmann, L. A. Acta Pharm. Sin. B 2018, 8, 137–146. doi:10.1016/j.apsb.2017.09.003 |
| 13. | Kou, J.; Dou Dou, D.; Yang, L. Oncotarget 2017, 8, 81591–81603. doi:10.18632/oncotarget.20189 |
| 14. | Huang, H.; Song, W.; Rieffel, J.; Lovell, J. F. Front. Phys. 2015, 3, No. 23. doi:10.3389/fphy.2015.00023 |
| 48. | Spikes, J. D. J. Photochem. Photobiol., B 1990, 6, 259–274. doi:10.1016/1011-1344(90)85096-f |
| 8. | Pandey, R. K.; Kessel, D.; Dougherty, T. J., Eds. Handbook of Photodynamic Therapy. Updates on Recent Applications of Porphyrin-Based Compounds, 1st ed.; World Scientific Pub Co Inc., 2016. doi:10.1142/9774 |
| 9. | Ormond, A. B.; Freeman, H. S. Materials 2013, 6, 817–840. doi:10.3390/ma6030817 |
| 21. | Gillis, E. P.; Eastman, K. J.; Hill, M. D.; Donnelly, D. J.; Meanwell, N. A. J. Med. Chem. 2015, 58, 8315–8359. doi:10.1021/acs.jmedchem.5b00258 |
| 22. | Costa, J. I. T.; Tomé, A. C.; Neves, M. G. P. M. S.; Cavaleiro, J. A. S. J. Porphyrins Phthalocyanines 2011, 15, 1116–1133. doi:10.1142/s1088424611004294 |
| 49. | Jiménez-Osés, G.; García, J. I.; Silva, A. M. G.; Santos, A. R. N.; Tomé, A. C.; Neves, M. G. P. M. S.; Cavaleiro, J. A. S. Tetrahedron 2008, 64, 7937–7943. doi:10.1016/j.tet.2008.06.018 |
| 31. | Obata, M.; Hirohara, S.; Tanaka, R.; Kinoshita, I.; Ohkubo, K.; Fukuzumi, S.; Tanihara, M.; Yano, S. J. Med. Chem. 2009, 52, 2747–2753. doi:10.1021/jm8015427 |
| 29. | Giuntini, F.; Alonso, C. M. A.; Boyle, R. W. Photochem. Photobiol. Sci. 2011, 10, 759–791. doi:10.1039/c0pp00366b |
| 30. | Kitagishi, H.; Kawasaki, H.; Kano, K. Chem. – Asian J. 2015, 10, 1768–1775. doi:10.1002/asia.201500451 |
| 41. | Rostovtsev, V. V.; Green, L. G.; Fokin, V. V.; Sharpless, K. B. Angew. Chem., Int. Ed. 2002, 41, 2596–2599. doi:10.1002/1521-3773(20020715)41:14<2596::aid-anie2596>3.0.co;2-4 |
| 42. | Tornøe, C. W.; Christensen, C.; Meldal, M. J. Org. Chem. 2002, 67, 3057–3064. doi:10.1021/jo011148j |
| 38. | Maiti, S. N.; Singh, M. P.; Micetich, R. G. Tetrahedron Lett. 1986, 27, 1423–1424. doi:10.1016/s0040-4039(00)84275-6 |
| 39. | Song, H. Y.; Ngai, M. H.; Song, Z. Y.; MacAry, P. A.; Hobley, J.; Lear, M. J. Org. Biomol. Chem. 2009, 7, 3400–3406. doi:10.1039/b904060a |
| 40. | Chen, Y.; Parr, T.; Holmes, A. E.; Nakanishi, K. Bioconjugate Chem. 2008, 19, 5–9. doi:10.1021/bc700267f |
| 36. | Das, S. K.; Song, B.; Mahler, A.; Nesterov, V. N.; Wilson, A. K.; Ito, O.; D’Souza, F. J. Phys. Chem. C 2014, 118, 3994–4006. doi:10.1021/jp4118166 |
| 37. | Peters, M. K.; Herges, R. Inorg. Chem. 2018, 57, 3177–3182. doi:10.1021/acs.inorgchem.7b03164 |
| 32. | Samaroo, D.; Vinodu, M.; Chen, X.; Drain, C. M. J. Comb. Chem. 2007, 9, 998–1011. doi:10.1021/cc070067j |
| 33. | Hirohara, S.; Oka, C.; Totani, M.; Obata, M.; Yuasa, J.; Ito, H.; Tamura, M.; Matsui, H.; Kakiuchi, K.; Kawai, T.; Kawaichi, M.; Tanihara, M. J. Med. Chem. 2015, 58, 8658–8670. doi:10.1021/acs.jmedchem.5b01262 |
| 34. | Hirohara, S.; Kawasaki, Y.; Funasako, R.; Yasui, N.; Totani, M.; Alitomo, H.; Yuasa, J.; Kawai, T.; Oka, C.; Kawaichi, M.; Obata, M.; Tanihara, M. Bioconjugate Chem. 2012, 23, 1881–1890. doi:10.1021/bc300223j |
| 35. | Banfi, S.; Caruso, E.; Caprioli, S.; Mazzagatti, L.; Canti, G.; Ravizza, R.; Gariboldi, M.; Monti, E. Bioorg. Med. Chem. 2004, 12, 4853–4860. doi:10.1016/j.bmc.2004.07.011 |
© 2019 Ol’shevskaya et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0). Please note that the reuse, redistribution and reproduction in particular requires that the authors and source are credited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (https://www.beilstein-journals.org/bjoc)