Abstract
The direct exploitation of ‘electrons’ as reagents in synthetic organic transformations is on the verge of a renaissance by virtue of its greenness, sustainability, atom economy, step economy and inherent safety. Achieving stereocontrol in such organic electrochemical reactions remains a major synthetic challenge and hence demands great expertise. This review provides a comprehensive discussion of the details of stereoselective organic electrochemical reactions along with the synthetic accomplishments achieved with these methods.
Graphical Abstract
Introduction
Electric current-assisted exchange of electrons between an electrode and an organic substrate, resulting in desired redox transformation of the substrate via the intermediacy of a highly reactive electrogenerated reagent (radical, ion or ionic radical), is generally termed ‘organic electrochemical reactions’. Amidst tremendous effort by synthetic organic chemists towards reaching targets selectively from readily available starting materials using low cost, nontoxic reagents and solvents while maintaining high atom and step economy and minimizing waste production with respect to safety standards, electroorganic chemistry (EOC) stands out as a potential greener and sustainable alternative to traditional redox protocols [1-4]. Starting from its inception in 1800, EOC has undergone a series of advances with respect to the design of electrochemical cells; the nature of the electrode materials; the applied current or potential; the available redox mediators, electroauxiliaries, supporting electrolyte, types of catalysts; and many other controlling factors [5-7]. The complex history of organic electrosynthesis has been revisited in detail in a number of articles [8-12]. The two sequential reviews from Baran’s group and Waldvogel’s group focused on the advancements achieved in the development of synthetic methods as well as applications of organic electrosynthesis reported since 2000 [13,14]. Moreover, in his recent article, Moeller have nicely demonstrated that both physical and organic chemistry are indispensable for exploiting electroorganic reactions to their fullest extent [15].
Asymmetric electrochemical synthesis refers to electroorganic reactions resulting in the introduction of one or more new elements of chirality into a target compound. The induction of asymmetry into achiral substrates through electrochemical methods can be achieved by using suitable chiral sources [16]. Several groups of organic chemists have been involved in establishing and improving the effectiveness of chiral inductors for electrosynthesis; as a result, a number of reports have been published in this important field of synthetic organic chemistry. In this review, we aim to focus on methods for achieving stereocontrol in synthetic organic electrochemistry via a systematic description of the reported literature on chiral inductors, followed by their applications in the synthesis of natural products and bioactive compounds including late-stage functionalizations. Although this field has been periodically reviewed over the last few decades, a single article addressing all attempts to date will be useful for organic chemists working in this field and for those who would like to obtain an overview of the current state of the art of asymmetric electrocatalytic reactions.
Review
Classification and systematic description of chiral inductors
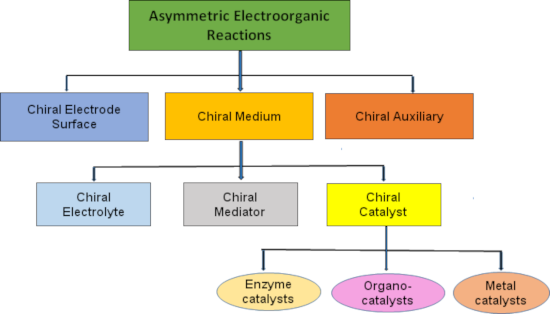
Transformations of achiral organic substrates into chiral products by electrochemical synthetic methods require the active participation of an external source of chirality. Organic chemists working in this area have established a number of asymmetric electrochemical inductors. After a thorough comprehension of the available literature, we propose to classify the chiral electrochemical inductors into three broad categories: chiral electrodes, chiral media and chiral auxiliaries [17]. We have summarized the background and recent advances and arranged this article to provide a systematic description of electrochemical reactions in which chirality has been induced using these three sources (Figure 1).
![[1860-5397-15-264-1]](/bjoc/content/figures/1860-5397-15-264-1.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 1: General classification of asymmetric electroorganic reactions.
Figure 1: General classification of asymmetric electroorganic reactions.
Chemically modified chiral electrodes
Electrochemical asymmetric reductions using chiral electrodes
Electrodes with chiral surfaces have long been prepared through the adsorption of chiral active auxiliaries onto the surface of the metallic electrode. In such cases, the chiral electrode becomes an inherent part of the electrochemical cell and serves as a heterogeneous catalyst. A recent article by Wattanakit described several approaches for the development of chiral metal electrodes for enantioselective recognition and asymmetric synthesis developed over the past decade [18]. Concurrent with pioneering works in the field of asymmetric induction in electrochemical reduction on chiral mercury cathodes in the presence of chiral inductors, including tertiary amines, optically active proteins, and alkaloids [19,20], in 1975, Miller’s group reported the first example of the modification of electrodes via covalent binding [21]. They chemically modified air-oxidized graphite electrodes via treatment with thionyl chloride followed by derivatization with (S)-(−)-phenylalanine methyl ester. The applicability of this modified electrode [(S)-CelPheM] was tested by using it as a cathode for the electrochemical reduction of 4-acetylpyridine (1). GC analysis of the product indicated the formation of the corresponding alcohol 2 with 14.7% optical yield [21]. Control experiments confirmed that the modified electrodes served as chiral inductors (Scheme 1).
Scheme 1: Asymmetric reduction of 4-acetylpyridine using a modified graphite cathode.
Scheme 1: Asymmetric reduction of 4-acetylpyridine using a modified graphite cathode.
In 1982, Fujihira and Osa reported the modification of Raney nickel powder electrodes with optically active tartaric acid and used these electrodes for the asymmetric hydrogenation of ketones [22]. They showed that upon reduction with Raney nickel powder electrodes modified with (R,R)-(+)-tartaric acid, 2-hexanone 3 was converted to (S)-(+)-2-hexanol 4 with an average optical purity of 4%, whereas Raney nickel powder electrodes modified with (S,S)-(−)-tartaric acid produced (R)-(−)-2-hexanol with almost the same optical purity. Similar results were observed when 2-heptanone and 2-octanone were used as substrates (Scheme 2).
Scheme 2: Asymmetric hydrogenation of ketones using Raney nickel powder electrodes modified with optically active tartaric acid.
Scheme 2: Asymmetric hydrogenation of ketones using Raney nickel powder electrodes modified with optically ac...
The above results confirm that chemically modified chiral electrodes are a useful platform for asymmetric induction because they require a small amount of inducing agents. However, poor coverage of the electrode surface with the chiral species was soon noted as one of the major disadvantages of this method, and chemists started developing polymer-coated electrodes as an alternative. In 1983, Nonaka et al. explored the applicability of a poly-ʟ-valine 6 coated graphite cathode for asymmetric electrochemical reductions in two consecutive reports. In the first communication, they carried out the asymmetric reduction of prochiral activated olefins 5 and 8, affording 7 and 9 with optical yields of 25% and 43%, respectively (Scheme 3) [23]. In the second report, the same electrode was applied for the asymmetric reduction of prochiral carbonyl compounds 10a and 12a, oximes 14 and gem-dibromo compounds 16 (Scheme 4). Among all of these the highest optical yield (16.6%) was obtained in the reduction of gem-dibromide substrates 16 to corresponding bromo-cyclopropane 17 [24]. According to the authors, the passage of larger amounts of charge to facilitate product isolation might be responsible for the observed lower asymmetric yields.
Scheme 3: Asymmetric reduction of prochiral activated olefins with a poly-ʟ-valine-coated graphite cathode.
Scheme 3: Asymmetric reduction of prochiral activated olefins with a poly-ʟ-valine-coated graphite cathode.
Scheme 4: Asymmetric reduction of prochiral carbonyl compounds, oximes and gem-dibromides on a poly-ʟ-valine-coated graphite cathode.
Scheme 4: Asymmetric reduction of prochiral carbonyl compounds, oximes and gem-dibromides on a poly-ʟ-valine-...
In 1998, Moutet’s group reported for the first time that transition metal complexes with chiral ligands can be used for the chemical modification of electrodes to achieve asymmetric induction in electrosynthesis [25,26]. They showed that carbon electrodes modified with poly[RuIII(L)2Cl2]+ (generated from the electropolymerization of [RuIII(L)2Cl2]+ complexes 19 on the carbon surface) can effectively catalyze the enantioselective hydrogenation of prochiral ketones 18. The prochiral ketones such as acetophenone, 1-tetralone and 1-indanone were reduced to their corresponding alcohols 20a, 20b and 20c, respectively, with moderate optical yields, with formation of (S) as major enantiomer (Scheme 5).
Scheme 5: Asymmetric hydrogenation of prochiral ketones with poly[RuIII(L)2Cl2]+-modified carbon felt cathodes.
Scheme 5: Asymmetric hydrogenation of prochiral ketones with poly[RuIII(L)2Cl2]+-modified carbon felt cathode...
After a long gap, in 2006, Takano and Seki prepared a PLPy(Pd) electrode coated with a chiral poly-(N-substituted pyrrole) film having ʟ-(+)-lactic acid moieties as the optically active groups and further incorporated with palladium metal [27]. Thus prepared electrodes 21 were used for the electrocatalytic hydrogenation of α-keto esters 12 to afford corresponding hydroxy esters 13b and 13c with appreciable enantioselectivities (Scheme 6).
Scheme 6: Asymmetric hydrogenation of α-keto esters using chiral polypyrrole film-coated cathode incorporated with palladium metal.
Scheme 6: Asymmetric hydrogenation of α-keto esters using chiral polypyrrole film-coated cathode incorporated...
Following the pioneering works by Grimshaw et al. [28] in 1973, Gileadi and co-workers showed that the addition of quinidine or related alkaloids to the reaction mixture during the electroreduction of acetophenone on a mercury cathode induces optical activity in the product (alcohol 24a, Scheme 7) [29]. Additionally, pinacol (23) was also obtained via dimerization along with chiral alcohols. The same method was reinvestigated by Wang and Lu in 2013 using a silver cathode in an undivided cell in the presence of cinchonidine alkaloid as the source of chirality [30]. They modified the electrolysis conditions using a mixture of CH3CN/H2O and tetraethylammonium iodide as the supporting electrolyte and achieved a slight improvement in the enantiomeric excess (Scheme 7, conditions B).
Scheme 7: Quinidine and cinchonidine alkaloid-induced asymmetric electroreduction of acetophenone.
Scheme 7: Quinidine and cinchonidine alkaloid-induced asymmetric electroreduction of acetophenone.
Based on a strychnine alkaloid-induced asymmetric electroreduction, Kopilov, Kariv and Miller showed that 4- and 2-acetylpyridines (1 and 25) could be reduced to the corresponding pyridylethanols (2 and 26, respectively) in presence of a catalytic amount of the alkaloid on a mercury cathode. Notably, the highest optical yields were achieved using strychnine (Scheme 8) [31].
Scheme 8: Asymmetric electroreduction of 4- and 2-acetylpyridines at a mercury cathode in the presence of a catalytic amount of strychnine.
Scheme 8: Asymmetric electroreduction of 4- and 2-acetylpyridines at a mercury cathode in the presence of a c...
Similarly, by applying modified electrolytic conditions, in 1993, Schoo and Schäfer increased the enantioselectivity of the alkaloid-catalyzed enantioselective electroreduction of 4-methylcoumarin (8) relative to that reported previously by Grimshaw’s group in 1970 [28] (the optical yield of 9' was increased from 17% to 47.4% at the expense of a decrease in the chemical yield from 57% to 18%). According to the authors, protonated alkaloid Yoh-H+ initially interacts with 8 to give complex A, which after 2e− reduction forms chiral ion pair B. The intermediate then undergoes enantioselective protonation by Yoh-H+ to generate Yoh-bound 9' (complex C). Subsequent release of the reduced product 9' and reprotonation of yohimbine complete the cycle (Scheme 9) [32,33].
Scheme 9: Enantioselective reduction of 4-methylcoumarin in the presence of catalytic yohimbine.
Scheme 9: Enantioselective reduction of 4-methylcoumarin in the presence of catalytic yohimbine.
In a continuation of their previous work [34], Wang and Lu investigated the enantioselective electrocarboxylation of 4-methylpropiophenone (27) on a stainless steel cathode in which the enantiodiscrimination was controlled by the nucleophilic quinuclidine nitrogen atom and the -OH group of the inductor alkaloid [35]. Mechanistically, cinchonine (CN) abstracts a proton from the cocatalyst (n-butanol), which results in the protonation of the quiniclidine N-atom to give CN–H. At the same time, compound 27 undergoes a one-electron reduction at the cathode to afford radical anion 30, which is protonated by CN–H and generates 31. This results in the installation of an oxygen atom on one face, promoting the addition of CO2 from the other face, resulting in enantioenriched carboxylated product 29 after acidification (Scheme 10). The authors described similar results involving the asymmetric electrocarboxylation of other prochiral aromatic ketones in a following publication [36].
Scheme 10: Cinchonine-induced asymmetric electrocarboxylation of 4-methylpropiophenone.
Scheme 10: Cinchonine-induced asymmetric electrocarboxylation of 4-methylpropiophenone.
The same group, in 2014, published two concomitant reports on the synthesis of metallo-organic hybrid materials by means of entrapment of alkaloids within silver particles [37,38]. They further used this organically doped metal as cathode for the enantioselective electrohydrogenation of 12b that resulted in improved yield and enantiomeric purity as compared to previously reported methods (Scheme 11). In another report the same reaction was discovered to be catalyzed by applying copper nanoparticle (CuNP) as a cathode for elelctrohydrogenation of 12 [39]. The asymmetry was induced by addition of certain concentration of alkaloid which was found to get adsorbed on CuNP surface (Scheme 12).
Scheme 11: Enantioselective hydrogenation of methyl benzoylformate using an alkaloid entrapped silver cathode.
Scheme 11: Enantioselective hydrogenation of methyl benzoylformate using an alkaloid entrapped silver cathode.
Scheme 12: Alkaloid-induced enantioselective hydrogenation using a Cu nanoparticle cathode.
Scheme 12: Alkaloid-induced enantioselective hydrogenation using a Cu nanoparticle cathode.
In continuation, Wang and Lu, in 2016, developed a ‘copper encapsulated alkaloid composite’ by the entrapment of commercial alkaloids within metallic copper nanoparticles. They compacted this composite as a coin and applied directly as cathode for enantioselective hydrogenation of aromatic ketones. In this case they observed the remarkable stability as well as recyclability of the alkaloid@Cu composite making them reliable for further practical applications [40]. Further, alkaloid-induced asymmetric electrochemical hydrogenation of 2,2,2-trifluoroacetophenone 32 nanoparticles was also developed (Scheme 13). These NPs with low Pt loading were further obtained by reducing platinum precursors on Cu NPs [41].
Scheme 13: Alkaloid-induced enantioselective hydrogenation of aromatic ketones using a bimetallic Pt@Cu cathode.
Scheme 13: Alkaloid-induced enantioselective hydrogenation of aromatic ketones using a bimetallic Pt@Cu cathod...
Yadav’s group, in 2013, described preparative scale enantioselective cathodic reduction of prochiral ketones 34 to their corresponding alcohols 35 at mercury pool in the presence of (−)-N,N'-dimethylquininium tetrafluoroborate (DMQ·2BF4) as chiral inductor using catalytic amounts of (1R,2S)-(−)-N,N'-dimethylephedrinium tetrafluoroborate (Scheme 14) [42,43].
Scheme 14: Enantioselective reduction of ketones at mercury cathode using N,N'-dimethylquininium tetrafluoroborate as chiral inductor.
Scheme 14: Enantioselective reduction of ketones at mercury cathode using N,N'-dimethylquininium tetrafluorobo...
Kawabata and Yoneyama, in 2000, reported for the first time, a crucial modification of a glassy carbon electrode via chemical immobilization of ᴅ-amino acid oxidase (D-AOx) as enzyme and 1-aminopropyl-1'-methyl-4,4'-dipyridinium iodide (ADPy) as electron mediator and thus prepared electrode D-AOx/ADPy/GC. Electrochemical reduction of pyruvic acid (36) using this electrode in a two compartment cell separated by a cation exchange membrane resulted in the production of ᴅ-alanine (37) with almost 100% ee (Scheme 15). As per the proposed mechanism, the imino acid 36A produced from the reaction of acid with NH4OH, gets oxidized to the corresponding amino acid via electrochemical reduction of FAD in AOx by electron mediator ADPy. It should be noted that the authors also reported the limitation of this electrochemical method in terms of substrate selectivity of the enzymes used [44].
Scheme 15: Asymmetric synthesis of an amino acid using an electrode modified with amino acid oxidase and electron mediator.
Scheme 15: Asymmetric synthesis of an amino acid using an electrode modified with amino acid oxidase and elect...
Electrochemical asymmetric oxidation using chirally modified electrodes
Electrochemical oxidation reactions have long served as substantial synthetic tool because of their ability to increase the functionality of organic molecules via reversing the polarity of electron-rich functional groups and thereby generating highly reactive intermediates. Recent advances in anodic cyclization reactions along with their detailed mechanistic rationale have been studied in details in an article published by Moeller’s group [45]. Herein, we present a systematic description of electrooxidation reactions where asymmetry has been induced by means of chiral modification of anodes.
As in case of asymmetric electroreduction, the prime reasonable entry in the area of asymmetric electrooxidation using chemically modified electrode was made by a successive work by Miller’s group in 1976. Using modified graphite electrodes, they successfully reported anodic oxidation of 38 to the corresponding sulfoxide 39 with excellent chemical yield although the value of enantiomeric excess was considerably low (Scheme 16) [46].
Scheme 16: Asymmetric oxidation of p-tolyl methyl sulfide using chemically modified graphite anode.
Scheme 16: Asymmetric oxidation of p-tolyl methyl sulfide using chemically modified graphite anode.
A few years later, Komori and Nonaka, in two consecutive reports, established a modified method for the asymmetric electrochemical oxidation of alkyl aryl sulfides 40 to their corresponding sulfoxides 41 using poly(amino acid)-coated platinum electrodes with moderate yield and good to excellent enantiomeric excess (Scheme 17) [47,48].
Scheme 17: Asymmetric oxidation of unsymmetric sulfides using poly(amino acid)-coated electrodes.
Scheme 17: Asymmetric oxidation of unsymmetric sulfides using poly(amino acid)-coated electrodes.
Osa and co-workers, in 1994, made an important contribution in the area by developing an enantioselective method for the electrocatalytic oxidative coupling of 42 using constant potential electrolysis of the substrates on a graphite felt electrode modified with TEMPO in the presence of (−)-sparteine 43. The electrolysis resulted in (S)-binaphthyl type dimers 44 with excellent yield and enantiomeric excess (Scheme 18) [49].
Scheme 18: Enantioselective, electocatalytic oxidative coupling on TEMPO-modified graphite felt electrode in the presence of (−)-sparteine.
Scheme 18: Enantioselective, electocatalytic oxidative coupling on TEMPO-modified graphite felt electrode in t...
Later, the same group explored another application of TEMPO-modified graphite felt electrodes for enantioselective electrocatalytic oxidation of racemic secondary alcohols 45 and 48 (Scheme 19). (S)-Isomers of alcohol 48 possessing a chiral center at α-position to the hydroxy group were oxidized to the corresponding ketones 49 whereas (R)-50 remained unreacted on a TEMPO-modified graphite felt electrode in the presence of (−)-sparteine. Enantiopurity of remaining alcohols were found to be more than 99% in all cases [50].
Scheme 19: Asymmetric electrocatalytic oxidation of racemic alcohols on a TEMPO-modified graphite felt electrode in the presence of (−)-sparteine.
Scheme 19: Asymmetric electrocatalytic oxidation of racemic alcohols on a TEMPO-modified graphite felt electro...
As a result of their study towards suitable modification of graphite felt electrodes for successful application in asymmetric electroorganic transformations, Kashiwagi’s group came up with two distinct protocols for asymmetric electrochemical lactonization of differently substituted diols. In 1996, they disclosed an asymmetric electrocatalytic method for lactonization of methyl-substituted diols 51 on a TEMPO-modified graphite felt electrode in the presence of the chiral base (−)-sparteine (43) with excellent enantioselectivity (conditions A, Scheme 20) [51]. Later in 2003, they reported another protocol for a graphite felt electrode for asymmetric electrochemical lactonization of diols 51. However, in this method instead of a chiral base, they used 1-azaspiro[5.5]undecane N-oxyl 52 as a radical mediator for modifying the electrodes which resulted in optically active lactones 53 with enantiopurity of up to 99% (conditions B, Scheme 20) [52].
Scheme 20: Asymmetric electrocatalytic lactonization of diols on TEMPO-modified graphite felt electrodes.
Scheme 20: Asymmetric electrocatalytic lactonization of diols on TEMPO-modified graphite felt electrodes.
Chiral medium
In this section, we present a compact overview of different categories of chiral media developed by organic chemists to induce asymmetry in electrochemical transformations. Chiral media can be further classified into subcategories such as chiral solvents, chiral electrolytes, chiral mediators, and chiral catalysts.
Chiral solvents
The only report on asymmetric electrochemical reaction in chiral solvent was published by Seebach and Oei in 1975 [53]. The asymmetric photodimerization of acetone to pinacol was conducted using amino-ether DDB 54 as a chiral solvent. Under the optimized reaction conditions, this electrolysis strategy allowed the electroreduction of acetophenone (22a) to pinacol (23) with 6.4% ee (Scheme 21). As mentioned by the author, the radical intermediate diffuses from the double layer, and dimerization occurs in the chiral solvent sphere in the solution phase.
Scheme 21: Asymmetric electrochemical pinacolization in a chiral solvent.
Scheme 21: Asymmetric electrochemical pinacolization in a chiral solvent.
Chiral supporting electrolyte
Electroorganic transformations using chiral supporting electrolytes have not been extensively explored, and the first example of such a reaction was reported by Horner in 1968 [54]. Interestingly, 4.6% ee was achieved in the electroreduction of acetophenone using ephedrine hydrochloride (55a) as chiral supporting electrolyte. A slight improvement in the enantioselectivity (8.4% ee) was observed using 55b [55]. Kodama explained the reason of chiral induction in terms of ion pair interaction [56]. A further improvement in stereoselectivity was reported by Yadav, who added tetrabutylammonium trifluoroborate as an additional supporting electrolyte along with chiral 55c (Scheme 22) [42,57].
Scheme 22: Asymmetric electroreduction using a chiral supporting electrolyte.
Scheme 22: Asymmetric electroreduction using a chiral supporting electrolyte.
In 2003, Nishiguchi’s group explored the anodic oxidation of enol acetates 57 upon constant current electrolysis in an undivided cell at −78 ºC in a mixture of solvents containing (S)-tetraethylammonium camphorsulfonate 58 as a chiral supporting electrolyte [58]. This resulted in the corresponding α-acetoxy ketones 59, with low to moderate ee values (Scheme 23).
Scheme 23: Asymmetric anodic oxidation of enol acetates using chiral supporting electrolytes.
Scheme 23: Asymmetric anodic oxidation of enol acetates using chiral supporting electrolytes.
Chiral mediators
Chiral mediators are substances that allow or facilitate homogeneous electron transfer from the electrode to the substrate. This could be considered indirect electrolysis as the mediator takes an electron from the solid electrode and performs the organic transformation. This strategy has been shown to be suitable for inducing chirality, particularly in electro-oxidation reactions. Many chiral TEMPO-derived compounds have been explored as chiral mediators in electrochemistry. Notably, the distinction between mediators and catalysts can be difficult, but we have grouped them in terms of their role as per our classification.
In 1999, Kashiwagi and his group explored the applicability of chiral azaspiro-N-oxyl radical SPIROXYL (61) in the kinetic resolution of primary amines 60 via their electrocatalytic oxidation to the corresponding ketones 62, and remaining amines (R)-60 were obtained with very high enantioselectivity. The controlled potential electrolysis was carried out in a divided cell in the presence of a base and a catalytic amount of 61 (Scheme 24) [59]. A similar strategy using N-oxalyl radicals as chiral mediators has also been explored for the enantioselective electrocatalytic oxidation of secondary alcohols [60].
Scheme 24: Kinetic resolution of primary amines using a chiral N-oxyl radical mediator.
Scheme 24: Kinetic resolution of primary amines using a chiral N-oxyl radical mediator.
Tanaka and co-workers subsequently published another article on the kinetic resolution of sec-alcohols 63 via electrochemical oxidation in an undivided cell under constant current [61]. This time, they used catalytic amount of a different chiral N-oxyl radical mediator 64 (Scheme 25). The authors proposed that in the biphasic CH2Cl2/water medium, [Br+] is initially generated from [Br−] by electrooxidation in the aqueous phase. The [Br+] thus generated oxidizes the N-oxyl/N-hydroxy species, leading to the formation of the N-oxoammonium species in CH2Cl2. The nucleophilic addition of racemic 63 to this N-oxoammonium species might further generate 65 and a steric hindered environment would favor the formation of (S)-63 over (R)-63. Hence, (S)-63 would be converted to ketone 62 via hydride transfer, whereas (R)-63 might be predominantly retained.
Scheme 25: Chiral N-oxyl-radical-mediated kinetic resolution of secondary alcohols via electrochemical oxidation in a biphasic system.
Scheme 25: Chiral N-oxyl-radical-mediated kinetic resolution of secondary alcohols via electrochemical oxidati...
Very recently, Wirth’s group demonstrated the use of chiral iodoarene 67 as a redox mediator for the electrochemical lactonization of diketo acid derivatives 66 (Scheme 26). Galvanostatic electrolysis of 66 in an undivided cell using a catalytic amount of 67 resulted in the corresponding lactones 68 in moderate yields and good stereoselectivities. The stereoselectivity was proposed to be controlled through the preferential formation of transition state A over B after reaction of the enolates of 66 with chiral iodoarene 67. Steric factors restrict the formation of transition state B, and the reaction preferentially proceeds via transition state A to stereoselectively form the lactones [62].
Scheme 26: Chiral iodoarene-mediated asymmetric electrochemical lactonization.
Scheme 26: Chiral iodoarene-mediated asymmetric electrochemical lactonization.
Chiral catalysts
Achieving enantiocontrol in electrochemical transformations using various catalysts as chiral sources has been studied in greater detail than the other strategies mentioned in this review. As per our classification, we further categorize this class into metal catalysts, enzyme catalysts and organocatalysts.
Metal catalysts: In 1992, Amundsen and co-workers developed an electrochemical approach for a Sharpless asymmetric dihydrohydroxylation of alkenes using an osmium complex [63]. In this protocol, the traditional chemical oxidant was replaced by an anode that regenerates [FeCN)6]3− via an Os(VIII) to Os(VI) interconversion. Similarly, Torri and co-workers investigated the asymmetric electrodihydroxylation of olefins 69 in an undivided cell under constant current using potassium osmate as a catalyst in presence of Sharpless ligand and using iodine as the oxidizing mediator (Scheme 27). Two sets of conditions were developed, and they differ in the inorganic bases used; method A involves K2CO3, whereas method B uses a mixture of K3PO4 and K2HPO4. However, olefins 69 were converted to the corresponding dihydroxylated products 70 in excellent yields as well as enantiomeric excesses regardless of the nature of the substituent or reaction conditions [64]. Further improvements of these methods have recently been achieved by Moeller et al. who designed a photovoltaic apparatus for the efficient Sharpless dihydroxylation of styrene in up to 94% enantiomeric excess [65].
Scheme 27: Os-catalyzed electrochemical asymmetric dihydroxylation of olefins using the Sharpless ligand and iodine as the oxidizing mediator.
Scheme 27: Os-catalyzed electrochemical asymmetric dihydroxylation of olefins using the Sharpless ligand and i...
Tanaka and co-workers explored the catalytic applicability of optically active Mn-salen complex 72 towards asymmetric electroepoxidation of olefins 71 in a biphasic CH2Cl2/aqueous NaCl system (Scheme 28). The constant current epoxidation of 71 in an undivided cell resulted in chiral epoxides 73 in good yields and moderate enantioselectivities [66].
Scheme 28: Asymmetric electrochemical epoxidation of olefins catalyzed by a chiral Mn-salen complex.
Scheme 28: Asymmetric electrochemical epoxidation of olefins catalyzed by a chiral Mn-salen complex.
In 2008, Onomura and co-workers described the electrochemical asymmetric oxidation of 1,2-diols 74 and amino alcohols 77 in presence of a Br- mediator using Cu(OTf)2 and (R,R)-Ph-BOX 76 as the catalytic system (Scheme 29). The method enabled an asymmetric synthesis of α-hydroxycycloalkanones 75 and α-amino esters 78 along with the kinetic resolution of racemic 74 and 77, respectively. The racemic substrates were electrolyzed in an undivided cell which resulted in the corresponding oxidized products and their kinetic resolution with good enantioselectivities [67].
Scheme 29: Asymmetric electrooxidation of 1,2-diols, and amino alcohols using a chiral copper catalyst.
Scheme 29: Asymmetric electrooxidation of 1,2-diols, and amino alcohols using a chiral copper catalyst.
In the proposed mechanism, the substrate initially coordinates with the chiral Cu complex 79. The complex 79 consisting of diols and a chiral copper catalyst Cu–L* easily gets deprotonated by cathodically generated MeO− to afford alkoxide anions 80, which reacts with anodically generated Br+ to form O-brominated intermediates 81. Finally, MeO− removed HBr from 81 to afford products 75/78 and regenerate the Cu–L* catalyst (Scheme 30).
Scheme 30: Mechanism of asymmetric electrooxidation of 1,2-diols, and amino alcohols using a chiral copper catalyst.
Scheme 30: Mechanism of asymmetric electrooxidation of 1,2-diols, and amino alcohols using a chiral copper cat...
In 2014, Wang and Lu reported a method for asymmetric fixation of CO2 via electrocarboxylation of 82 catalyzed by an electrogenerated chiral [CoI(salen)]− complex [68]. The potentiostatic electrolysis of 82 in an undivided cell in presence of catalytic chiral Co(II)-(R,R)salen complex 83 in DMF saturated with CO2 resulted in the formation of 84 in a low yield but with good enantioselectivity (Scheme 31).
Scheme 31: Enantioselective electrocarboxylation catalyzed by an electrogenerated chiral [CoI(salen)]− complex.
Scheme 31: Enantioselective electrocarboxylation catalyzed by an electrogenerated chiral [CoI(salen)]− complex....
Very recently, Meggers research group described an application of an asymmetric Lewis acid catalyst using electricity to provide a synthetic route towards chiral 1,4-dicarbonyls bearing tertiary and all-carbon quaternary stereocenters via oxidative cross coupling of 2-acylimidazoles 85 with silyl enol ethers 86 (Scheme 32). Chiral Rh complex 87 was exploited as a Lewis acid catalyst for the purpose of activating the substrate towards anodic oxidation by raising the energy of the HOMO upon enolate formation. Upon constant current electrolysis of a mixture of 85 and 86 in an undivided ElectraSyn 2.0 cell in presence of 2,6-lutidine as an external base and catalyst 87, products with tertiary carbon stereocenters as well as all-carbon quaternary stereocenters 88 were obtained in good yields and enatioselectivities [69].
Scheme 32: Asymmetric oxidative cross coupling of 2-acylimidazoles with silyl enol ethers.
Scheme 32: Asymmetric oxidative cross coupling of 2-acylimidazoles with silyl enol ethers.
In 2002, Dunach’s group explored an electroreductive method for the cleavage of the allyl group of β-keto ester 89 followed by the transfer of the same to the carbonyl group to afford a mixture of 91 (major), 92 and 93 (Scheme 33). Constant current electrolysis of 89 in a single compartment cell using a sacrificial Mg anode was conducted in the presence of a Ni catalyst and chiral ligand 90. After esterification and purification, 91 was isolated in a good yield and with moderate enantioselectivity [70].
Scheme 33: Ni-catalyzed asymmetric electroreductive cleavage of allylic β-keto ester 89.
Scheme 33: Ni-catalyzed asymmetric electroreductive cleavage of allylic β-keto ester 89.
In another recent report, Guo and his group developed an electrochemical strategy for the asymmetric alkylation of 2-acylimidazole derivatives 94 with substituted para-methylphenols 95 using Ni(OAc)2 as a Lewis acid catalyst in presence of chiral diamine ligand 96 (Scheme 34) [71]. Based on their detailed mechanistic studies, the asymmetric induction was proposed to be realized via a combination of chiral Lewis acid-bound radical (generated through a single-electron anodic oxidation) and benzylic radical, generated through the anodic oxidation of 95.
Scheme 34: Asymmetric alkylation using a combination of electrosynthesis and a chiral Ni catalyst.
Scheme 34: Asymmetric alkylation using a combination of electrosynthesis and a chiral Ni catalyst.
As per the proposed catalytic cycle, initial coordination of the Lewis acid catalyst to 2-acylimidazole derivatives 94 generates the Lewis acid/enolate complex 100 upon deprotonation (Scheme 35). This is followed by the formation of intermediate 101 by electrolysis-induced SET oxidation. In a parallel electrochemical cycle, benzylic radical species 95 was delivered by the anodic oxidation. The radical–radical coupling reaction between 101 and 95 is proposed to afford the corresponding intermediate 102. Then release of the Lewis acid catalyst gives the final product 97. The stereocontrol step is related to the in situ generation of radicals with π-systems of chiral enamines and chiral enolates.
Scheme 35: Mechanism of asymmetric alkylation using a combination of electrosynthesis and a chiral Ni catalyst.
Scheme 35: Mechanism of asymmetric alkylation using a combination of electrosynthesis and a chiral Ni catalyst....
Organocatalysts: From the outset of electroorganic chemistry, chemists have devoted substantial effort towards applying organocatalysts in electroorganic synthesis. Recent advances integrating organocatalysis and electroorganic synthesis were elegantly presented by Boydston and Ogawa in their review article [72]. In this section, we will be presenting a concise description of the use of organocatalysts as chiral inductors in electroorganic synthesis.
In 2008, Page, Marken and their group reported a method for electricity-driven asymmetric organocatalytic epoxidation. The percarbonate oxidant required for this process was initially generated through anodic oxidation of aqueous Na2CO3 in an undivided cell under constant current electrolysis. A catalytic amount of chiral iminium salt 103 and olefinic substrate 71' were added to the same reaction mixture, and chiral epoxides 73' were obtained via oxidation by electrogenerated CO42− [73]. Moreover, they showed that electrogenerated persulfate generated in situ via a similar anodic oxidation of H2SO4 could act as an even better oxidant in presence of catalyst 103 to achieve the epoxidation of 71'c with higher ee values (Scheme 36).
Scheme 36: Asymmetric epoxidation by electrogenerated percarbonate and persulfate ions in the presence of chiral iminium salt.
Scheme 36: Asymmetric epoxidation by electrogenerated percarbonate and persulfate ions in the presence of chir...
In 2009, Jang’s group disclosed the first example of the anodic oxidation of enamines (SOMO protocol) in terms of asymmetric α-oxyamination of aldehydes [74]. Galvanostatic electrolysis of aldehydes 105 in a single compartment cell using TEMPO (106) in presence of chiral secondary amine catalyst 107 resulted in the corresponding coupling products 108 in moderate yields and good enantioselectivities. After detailed electrochemical analysis, the authors proposed that the reaction proceeds through the intermediacy of radical cation 111, generated via anodic oxidation of enamine 110 (Scheme 37).
Scheme 37: α-Oxyamination of aldehydes via anodic oxidation catalyzed by chiral secondary amines.
Scheme 37: α-Oxyamination of aldehydes via anodic oxidation catalyzed by chiral secondary amines.
The same group further extended the application of the SOMO protocol to the enantioselective α-alkylation of aldehydes [75]. Using modified chiral secondary amine 114, the authors showed that constant current electrolysis of aldehydes 105' with xanthenes 113 in an undivided cell resulted in α-alkylated aldehydes 115 with moderate enantioselectivity (Scheme 38).
Scheme 38: The α-alkylation of aldehydes via anodic oxidation catalyzed by chiral secondary amines.
Scheme 38: The α-alkylation of aldehydes via anodic oxidation catalyzed by chiral secondary amines.
As shown in Scheme 39, the mechanism involved initial formation of radical cation 117 via anodic oxidation of enamine 116 (obtained from the condensation of 114 and 105'), which then coupled with xanthene radical 119 (Scheme 39). Finally, hydrolysis of intermediate 118 takes place to obtained the final compound with regeneration of chiral catalyst 114.
![[1860-5397-15-264-i39]](/bjoc/content/inline/1860-5397-15-264-i39.png?scale=2.0&max-width=1024&background=FFFFFF)
Scheme 39: Mechanism of α-alkylation of aldehydes via anodic oxidation catalyzed by chiral secondary amines.
Scheme 39: Mechanism of α-alkylation of aldehydes via anodic oxidation catalyzed by chiral secondary amines.
At almost the same time, Jorgensen et al. demonstrated that the intermolecular α-arylation of aldehydes via anodic oxidation could be performed using chiral secondary amine catalyst [76]. Constant current electrolysis of a mixture of aldehydes 105' and N-protected aminophenol 120 in an undivided cell in the presence of catalyst 107 resulted in diastereomerically pure 122, which were further reduced to the corresponding alcohol 123 and were isolated in good yields and excellent enantioselectivities (Scheme 40).
Scheme 40: Electrochemical chiral secondary amine-catalyzed intermolecular α-arylation of aldehydes.
Scheme 40: Electrochemical chiral secondary amine-catalyzed intermolecular α-arylation of aldehydes.
As per the proposed mechanism, products 122 were obtained via nucleophilic addition of enamine 124' to electrophilic intermediate 127 (generated from the anodic oxidation of 120) followed by sequential hydrolysis, proton transfer and immediate intramolecular condensation (Scheme 41).
Scheme 41: Mechanism of electrochemical chiral secondary amine-catalyzed intermolecular α-arylation of aldehydes.
Scheme 41: Mechanism of electrochemical chiral secondary amine-catalyzed intermolecular α-arylation of aldehyd...
Very recently, Luo’s group disclosed another potential application of integrating electrochemical oxidation with chiral amine catalysis [77]. Using chiral primary amine 130 as a catalyst, the authors reported an electricity-driven cross-dehydrogenative coupling of ketones 129 with tertiary amines 128. The potentiostatic electrolysis of a mixture of ketones 129 with tertiary amines 128 in an undivided cell under standard conditions furnished C1-alkylated tetrahydroisoquinolines 131 in high yields with excellent enantioselectivity (Scheme 42).
Scheme 42: Asymmetric cross-dehydrogenative coupling of tertiary amines with simple ketones via an electrochemical oxidation catalyzed by chiral primary amines.
Scheme 42: Asymmetric cross-dehydrogenative coupling of tertiary amines with simple ketones via an electrochem...
Enzyme catalysts: In 1981, Tischer’s group reported the electroenzymatic asymmetric reduction of 135 using enolate reductase that afforded the corresponding chiral acid 136 in 95% yield and 95% ee. It has been shown that the cofactor NADH is oxidized during this process and can be regenerated using methyl viologen 137 as a reductive mediator (Scheme 43) [78].
Scheme 43: Electroenzymatic asymmetric reduction using enoate reductase.
Scheme 43: Electroenzymatic asymmetric reduction using enoate reductase.
In 1997, Yoneyama published a similar report on the asymmetric reduction of ketones 138 using alcohol dehydrogenase (ADH) as a catalyst [79]. In most cases, they achieved excellent stereocontrol; however, the method was ineffective in the case of phenyl ketoacid resulting in the formation of racemic alcohol 139d (Scheme 44).
Scheme 44: Assymetric reduction using alcohol dehydrogenase as the electrocatalyst.
Scheme 44: Assymetric reduction using alcohol dehydrogenase as the electrocatalyst.
In 2007, Schmid explored the application of Rh complex 141 as a mediator for NADPH recycling [80]. This report showed that asymmetric electroreduction of cyclohexanone 140 in organic/aqueous media can be efficiently catalyzed by thermophilic NAD-dependent alcohol dehydrogenase (TADH) and result in the corresponding chiral alcohol 142 with a diastereomeric excess of 96% (Scheme 45).
Scheme 45: Asymmetric electroreduction catalyzed by thermophilic NAD-dependent alcohol dehydrogenase.
Scheme 45: Asymmetric electroreduction catalyzed by thermophilic NAD-dependent alcohol dehydrogenase.
The same group explored the practicality of direct electrochemical regeneration of a flavin-dependent monooxygenase as a catalyst for the asymmetric electrochemical epoxidation of styrenes [81]. They showed that upon potentiostatic electrolysis, styrene derivatives 143 could be transformed into their asymmetric epoxides 144 via direct electrochemical regeneration of FADH2. The authors claimed that this method is superior with respect to substitution of the complex native regeneration cycle, which consists of three enzymes (StyA, StyB, and an NADH-regenerating enzyme) and two cofactors (NADH and FAD), with only an oxygenase component and its flavin prosthetic group (Scheme 46).
Scheme 46: Asymmetric epoxidation of styrene by electrochemical regeneration of flavin-dependent monooxygenase.
Scheme 46: Asymmetric epoxidation of styrene by electrochemical regeneration of flavin-dependent monooxygenase....
In 2004, Liese reported the asymmetric sulfoxidation of thioanisole 145 with high productivity and excellent enantioselectivity using a chloroperoxidase catalyst. H2O2 generated in situ from the cathodic reduction of oxygen was proposed to be responsible for the enzyme-mediated thiol ether oxidation (Scheme 47) [82].
Scheme 47: Asymmetric electroreduction using a chloroperoxidase catalyst.
Scheme 47: Asymmetric electroreduction using a chloroperoxidase catalyst.
Vitamin B12-dependent enzymes are an exciting representative in the family of chiral inductors for electroorganic chemistry. These enzymes involve cobalt in the catalytically active center. In his article, Prof. Hisaeda reviewed vitamin B12-mediated electrochemical reactions in organic solvents in detail [83]. A number of pioneering results have been published in this area by leading electroorganic chemists [84-86].
In 1994 and 1995, Murakami and his group published two sequential reports on the controlled potential electrolysis of racemic 147 catalyzed by a number of hydrophobic vitamin B12 enzymes 148 to afford hydrogen-substituted product 149 with good enantioselectivity (Scheme 48) [87,88].
Scheme 48: Asymmetric electrochemical transformation mediated by hydrophobic vitamin B12.
Scheme 48: Asymmetric electrochemical transformation mediated by hydrophobic vitamin B12.
Chiral auxiliary approach
In 1994, Zielinski and Schäfer made a vital contribution in the field of asymmetric electrosynthesis in terms of the diastereoselective cathodic reduction of the carbonyl group of chiral phenylglyoxamides 150 and 152 [89,90]. After initial conversion to its corresponding amides using chiral auxiliaries phenylglyoxalic acid was further converted to mandelic acid derivatives 151 and 153, respectively, upon reduction at a mercury pool cathode. The electrolysis afforded an excellent chemical yield along with moderate to good diastereomeric excesses. Moreover, the recovery of chiral auxiliaries upon hydrolysis of the major diastereomers of 151a and 153a, respectively, allowed access to optically active mandelic acid esters (Scheme 49).
Scheme 49: Diastereoselective cathodic reduction of phenylglyoxalic acids substituted with amines as chiral auxiliaries.
Scheme 49: Diastereoselective cathodic reduction of phenylglyoxalic acids substituted with amines as chiral au...
In 1997, Durandetti’s group published a Ni-catalyzed method for the electroreductive coupling between aryl halides and α-chloropropionic acid derivatives 153 in which the asymmetry was induced by chiral auxiliaries attached to the propionic acid derivatives [91]. Cross-coupling products 155 were obtained in satisfactory yields with good diastereomeric ratios. Moreover, the enantiomeric excess of products 155 were measured after their conversion to the corresponding esters or imides and no racemization was observed during the reaction (Scheme 50).
Scheme 50: Ni-catalyzed asymmetric electroreductive cross coupling of aryl halides with α-chloropropanoic acid derivatives bearing chiral auxiliaries.
Scheme 50: Ni-catalyzed asymmetric electroreductive cross coupling of aryl halides with α-chloropropanoic acid...
In 2000, Pilli and co-workers published a vinylogous Mannich addition of silyloxyfuran to chiral N-acyliminium ions generated in situ from 157 which had been obtained from the anodic oxidation of 156 bearing a cyclohexyl-based chiral auxiliary ([92]. The authors established that the Mannich addition occurred exclusively on the Si-face of the N-acyliminium ions, resulting in the threo-isomer as the major isomer (with moderate yields and good diastereomeric ratios). Upon catalytic hydrogenation followed by methanolysis, threo-158a, and 159a were further converted to the corresponding lactams 160a and 160b enabling efficient recovery of chiral auxiliaries (Scheme 51). Furthermore, the same group reported an identical method for the TiCl4-promoted addition of allyltrimethylsilane to N-acyliminium ions containing the same cyclohexyl-based chiral auxiliaries [93].
Scheme 51: Electrochemical Mannich addition of silyloxyfuran to in situ-generated N-acyliminium ions.
Scheme 51: Electrochemical Mannich addition of silyloxyfuran to in situ-generated N-acyliminium ions.
While studying the effect of chiral auxiliaries on the enantioselective electroreduction of cinnamic acid derivatives, which resulted in the corresponding hydrodimers, Kise and his group investigated a number of chiral auxiliaries and found that chiral auxiliary derived from (+)-camphor was the most effective one for asymmetric electroreductive hydrocoupling of cinnamates 161 [94,95]. The constant current electrolysis of 161 in an undivided cell resulted in acyclic homodimer 162 (with good yield and excellent diastereoselectivity) along with a certain percentage of 163. The chiral auxiliary was released almost quantitatively after LAH reduction of 162. The authors proposed a hydrocoupling mechanism involving the one-electron reduction of 161 to generate radical anion 164 followed by the coupling of another radical anion from the Si-face (Scheme 52).
Scheme 52: Stereoselective electroreductive homodimerization of cinnamates attached to a camphor-derived chiral auxiliary.
Scheme 52: Stereoselective electroreductive homodimerization of cinnamates attached to a camphor-derived chira...
In two sequential reports, Feroci and Inesi discussed the electrochemical carboxylation of chiral α-bromocarboxylic acid derivatives 165 substituted with Evans-type chiral auxiliaries [96,97]. The cathodic reduction of the C–Br bond in presence of CO2 followed by treatment with diazomethane resulted in the corresponding malonic ester derivatives 166, and the diastereomeric ratio was greatly affected by various electrochemical conditions, and higher yields (88%) were obtained at the expense of diastereoselectivity (dr 61:39) [166a + 166'a]. The authors next modified the chiral auxiliary and obtained the best results with α-bromocarboxylic acid derivatives 167 substituted with Oppolzer’s camphor sultam, which provided carboxylated products 168 in good yield (80%) and excellent diastereomeric ratio (98:2) (Scheme 53).
Scheme 53: Diastereoselective electrochemical carboxylation of chiral α-bromocarboxylic acid derivatives.
Scheme 53: Diastereoselective electrochemical carboxylation of chiral α-bromocarboxylic acid derivatives.
The same group reported an electrocatalytic Michael addition of chiral acetoacetic derivatives 169 substituted with chiral auxiliaries to methyl vinyl ketone for stereoselective construction of the quaternary carbon centers in 170 (Scheme 54). While screening a number of chiral auxiliaries, the authors found that upon electrolysis under galvanostatic conditions at low temperature, the acetoacetates of Oppolzer’s sultam and Evans’ oxazolidin-2-ones afforded the best yields and diastereomeric ratios of addition products 170 relative to what was achieved with other chiral auxiliaries [98].
Scheme 54: Electrocatalytic stereoselective conjugate addition of chiral β-dicarbonyl compounds to methyl vinyl ketone.
Scheme 54: Electrocatalytic stereoselective conjugate addition of chiral β-dicarbonyl compounds to methyl viny...
As a part of studies on the effects of chiral auxiliaries on the stereoselectivity in electrochemical transformations, Feroci and Inesi developed a stereoselective carboxylation of cinnamic acid derivatives 171 substituted with chiral auxiliaries [99]. The substrate 171 was subjected to galvanostatic reduction under CO2 atmosphere in an undivided cell. Carboxylation followed by treatment with diazomethane resulted in the corresponding 2-phenylsuccinate esters 172 and 172' in satisfactory yields. The best diastereoselectivities were obtained using Oppolzer’s camphor sultam (172f + 172f') and 4R-(diphenylmethyl)oxazolidin-2-one (172c + 172'c) as chiral auxiliaries (Scheme 55).
Scheme 55: Stereoselective electrochemical carboxylation of chiral cinnamic acid derivatives under a CO2 atmosphere.
Scheme 55: Stereoselective electrochemical carboxylation of chiral cinnamic acid derivatives under a CO2 atmos...
Martens and his group synthesized chiral compounds based on phosphorus esters and investigated their effects as chiral auxiliaries in the α-alkylation of secondary amines via anodic oxidation [100]. The constant current methoxylation of N-protected chiral pyrrolidines 173 in an undivided cell resulted in 174 in excellent yield. Further nucleophilic substitution of 174 with allyltrimethylsilane in the presence of a Lewis acid afforded α-alkylated products 175. The highest de was obtained in the case of 175d. The deprotection of 175 with LAH enabled access to α-alkylated pyrrolidines 176 with the release of 177 (Scheme 56).
Scheme 56: Electrochemical diastereoselective α-alkylation of pyrrolidines attached with phosphorus-derived chiral auxiliaries.
Scheme 56: Electrochemical diastereoselective α-alkylation of pyrrolidines attached with phosphorus-derived ch...
In 2007, Feroci disclosed an electrochemical strategy for the cis-stereoselective synthesis of chiral β-lactams 180 via a 4-exo-tet cyclization of bromo amides 178 with an acidic methylene group and bearing a chiral auxiliary [101]. The cyclization occurred via deprotonation of the acidic methylene group by a base (cyanomethyl anion) obtained from the galvanostatic reduction of acetonitrile/tetraethylammonium hexafluorophosphate (Scheme 57).
Scheme 57: Electrogenerated cyanomethyl anion-induced synthesis of chiral cis-β-lactams from amides bearing chiral auxiliaries.
Scheme 57: Electrogenerated cyanomethyl anion-induced synthesis of chiral cis-β-lactams from amides bearing ch...
Two years later, Lee and co-workers explored the efficacy of isoborneol-based chiral auxiliaries for asymmetric induction in intramolecular anodic oxidations of ω-hydroxyl amides [102]. Upon constant current electrolysis, substrates 181 were oxidized to 182, which underwent in situ cyclization by intramolecular nucleophilic hydroxy group addition to afford a diastereomeric mixture of 183. The study also revealed that when cyclic amines were used as substrates (181a, 181b), the products were formed with 100% diastereoselectivity (183a and 183b), whereas acyclic amines resulted in lower diastereoselectivity (183c and 183d) even though the intramolecular attack of the hydroxy group to the iminium bond in 182 preferentially occurred from the Si-face over the Re-face in both cases (Scheme 58).
Scheme 58: Diastereoselective anodic oxidation followed by intramolecular cyclization of ω-hydroxyl amides bearing isoborneol-based chiral auxiliaries.
Scheme 58: Diastereoselective anodic oxidation followed by intramolecular cyclization of ω-hydroxyl amides bea...
Magdesieva and his group developed an electrochemical method for the deprotonation of a Ni(II) glycinate complex containing (S)-o-[N-(N-benzylprolyl)amino]benzophenone [(S)-BPB] 188 as an chiral auxiliary moiety and explored its applicability in diastereoselective Michael addition reactions [103,104]. The glycine–nickel complex 184 was deprotonated using a radical anion generated from the electrochemical reduction of azobenzene. The anionic Ni complex 185 acted as a good C-nucleophile towards Michael acceptors 186 resulting in diastereoisomeric mixtures of 187 and 187'. Moreover, the modified Ni-complexes obtained after Michael addition could be easily decomposed using HCl in MeOH, releasing functionalized amino acid 189 along with the quantitative recovery of the chiral auxiliary (S)-BPB 188 (Scheme 59).
Scheme 59: Electrochemical deprotonation of Ni(II) glycinate containing (S)-BPB as a chiral auxiliary: diastereoselective nucleophilic addition to Michael acceptors.
Scheme 59: Electrochemical deprotonation of Ni(II) glycinate containing (S)-BPB as a chiral auxiliary: diaster...
Recently, Kise’s group reported an efficient method for the enantioselective synthesis of 4,5,5-trisubstituted γ-butyrolactones 193 using an electroreductive coupling of diaryl ketones 191 with α,β-unsaturated carbonyl compounds 190 bearing chiral auxiliaries derived from imidazolidin-2-one and oxazolidine-2-ones (Scheme 60). Compound 191 underwent 2e− reduction under electrochemical conditions followed by O-silylation and afforded 192 with high diastereoselectivity. Further treatment of 192 with TBAF resulted in highly substituted optically active 193 with concomitant release of chiral auxiliary 194 (Table 1) [105].
Scheme 60: Enantioselective electroreductive coupling of diaryl ketones with α,β-unsaturated carbonyl compounds containing chiral auxiliaries.
Scheme 60: Enantioselective electroreductive coupling of diaryl ketones with α,β-unsaturated carbonyl compound...
Table 1: Enantioselective electroreductive coupling of diaryl ketones with α,β-unsaturated carbonyl compounds containing chiral auxiliaries.
| 191 | 190 | 193 | Yield (%) | ee (%) 193 |
|
191a |
190a |
193a |
63 | 99 |
|
190b |
193b |
60 | 93 | |
|
190c |
193’a |
68 | 95 | |
|
190d |
193’b |
47 | 99 | |
|
191b |
190a |
193c Ar = p-OMeC6H4 |
56 | 99 |
|
191c |
190b |
193d |
67 | 93 |
Application in the synthesis of natural products and bio-relevant compounds
Application of organo-electrosynthesis in complex molecule synthesis has recently been reviewed by Lundberg and Kärkäs [106]. In the following section of this review, we describe potential practical applications of asymmetric electroorganic reactions in the synthesis of different complex and biologically relevant compounds as well as natural products.
In 2008, Pilli and Santos developed an innovative method for the enantioselective total synthesis of the local anesthetic drug ropivacaine (197c) and its analogues levobupivacaine (197b) and mepivacaine (197a) [107]. The key steps in the synthesis involved the initial anodic oxidation of cyclic N-carbamate 194 bearing an 8-phenylmenthyl group as a chiral auxiliary which generates in situ N-acyliminium ion 195 and this 195 upon reaction with nucleophilic CN− in presence of catalytic TMSOTf and β-cyclodextrin as the cocatalyst provide 196 in a good yield and excellent stereoselectivity (Scheme 61).
Scheme 61: Asymmetric total synthesis of ropivacaine and its analogues using a electroorganic reaction as a key step.
Scheme 61: Asymmetric total synthesis of ropivacaine and its analogues using a electroorganic reaction as a ke...
In 2011, Hurvois and his group reported a stereoselective electrochemical total synthesis of the tetrahydroisoquinoline alkaloid (−)-crispine A (200) and its natural enantiomer [108]. The initial steps involved the synthesis of precursor (+)-198 from phenylacetic acid and (−)-α-PEA via sequential amidation, reduction and Pictet–Spengler cyclization reactions. The key anodic oxidation in the presence of NaCN converted (+)-198 to α-amino nitrile (+)-199 with excellent diastereoselectivity. Upon further alkylation, reduction and catalytic hydrogenolysis, (+)-199 was converted to desired product (−)-200 with 80% ee (Scheme 62).
Scheme 62: Asymmetric total synthesis of (−)-crispine A and its natural enantiomer via anodic cyanation of tetrahydroisoquinoline.
Scheme 62: Asymmetric total synthesis of (−)-crispine A and its natural enantiomer via anodic cyanation of tet...
An electrochemical method for the asymmetric oxidative dimerization of cinnamic acid derivatives was developed by Watanabe in 2016 [109]. The substrates for the electrochemical oxidation 201 were prepared from the corresponding cinnamic acids via condensation with ʟ-proline tert-butyl ester followed by ester hydrolysis. Upon oxidative dimerization under Ronlan’s electrochemical conditions, substrates 201 were converted to bislactones 202 with good enantioselectivity. The authors extended their method towards the asymmetric syntheses of furofuran lignans 203 from chiral bislactones 202. Furofuran lignans 203 were synthesized in good to excellent enantioselectivity (Scheme 63).
Scheme 63: Asymmetric oxidative electrodimerization of cinnamic acid derivatives as key step for the synthesis of furofuran lignans.
Scheme 63: Asymmetric oxidative electrodimerization of cinnamic acid derivatives as key step for the synthesis...
Conclusion and Future Directions
Extensive studies of electroorganic reactions have opened alternative pathways for synthetic transformations that are otherwise quite challenging. Not only the environmental advantages with respect to substituting toxic chemical reagents with mass-free electrons and minimizing the reagent waste generated, but also in terms of shortening of tedious multistep reaction sequences, these strategies enable advancements in reaction scalability as well as tunability.
Having described the research that has been conducted to achieve suitable ‘electrical’ replacements of conventional ‘chemical’ organic transformations, it is clear that the establishment of stereoselective variants of these synthetic operations remains challenging, making the use of electrochemistry to achieve stereoselective transformations an often unsolved problem. Modern synthetic laboratories have, however, made advances in this regard due to arduous studies resulting in a number of landmark publications with successful stereoselective electroorganic transformations. This review sought to compile significant accomplishments in this area and will hopefully serve as a handbook for practicing organic chemists and inspire them to focus on this relatively underdeveloped area of synthetic organic chemistry. Several challenges need to be addressed in order to see widespread application. While recent advancements in electrochemistry have been promising, readily available parallel screening technologies similar to those in homogeneous, heterogeneous, photo- as well as biocatalysis need to be developed. Parallel electrochemical reaction set-ups will, without doubt, accelerate further developments in the field of asymmetric electrochemistry and electrocatalysis. So far, most of the electrochemical reaction set-ups in this area rely on the use of less favorable electrodes, including Pt, Hg or Pb electrodes. Thus, more economical and ecological alternatives need to be examined, in particular if larger scale production is envisaged. Regarding stereochemical induction, the design of chiral media, similar to chiral solvents in asymmetric catalysis, is still limited. While the chemical modification of electrodes for the generation of chiral surfaces has shown some success, it seems that the most promising area in the further development of asymmetric electrochemistry and electrocatalysis will rely on the use of chiral mediators and chiral catalysts. Given the almost unlimited variations in combining electrochemistry with photo-, organo-, bio- and metal catalysts, many new and unexpected asymmetric reactions will be developed resulting in the further development of catalysis and synthesis in general.
References
-
Frontana-Uribe, B. A.; Little, R. D.; Ibanez, J. G.; Palma, A.; Vasquez-Medrano, R. Green Chem. 2010, 12, 2099–2119. doi:10.1039/c0gc00382d
Return to citation in text: [1] -
Schäfer, H. J. C. R. Chim. 2011, 14, 745–765. doi:10.1016/j.crci.2011.01.002
Return to citation in text: [1] -
Horn, E. J.; Rosen, B. R.; Baran, P. S. ACS Cent. Sci. 2016, 2, 302–308. doi:10.1021/acscentsci.6b00091
Return to citation in text: [1] -
Tang, S.; Liu, Y.; Lei, A. Chem 2018, 4, 27–45. doi:10.1016/j.chempr.2017.10.001
Return to citation in text: [1] -
Meyer, T. H.; Finger, L. H.; Gandeepan, P.; Ackermann, L. Trends Chem. 2019, 1, 63–76. doi:10.1016/j.trechm.2019.01.011
Return to citation in text: [1] -
Sauermann, N.; Meyer, T. H.; Qiu, Y.; Ackermann, L. ACS Catal. 2018, 8, 7086–7103. doi:10.1021/acscatal.8b01682
Return to citation in text: [1] -
Qiu, Y.; Struwe, J.; Ackermann, L. Synlett 2019, 30, 1164–1173. doi:10.1055/s-0037-1611568
Return to citation in text: [1] -
Shono, T. Tetrahedron 1984, 40, 811–850. doi:10.1016/s0040-4020(01)91472-3
Return to citation in text: [1] -
Utley, J. Chem. Soc. Rev. 1997, 26, 157–167. doi:10.1039/cs9972600157
Return to citation in text: [1] -
Lund, H. J. Electrochem. Soc. 2002, 149, S21–S33. doi:10.1149/1.1462037
Return to citation in text: [1] -
Tomilov, A. P.; Turygin, V. V.; Kaabak, L. V. Russ. J. Electrochem. 2007, 43, 1106–1122. doi:10.1134/s1023193507100023
Return to citation in text: [1] -
Yoshida, J.-i.; Kataoka, K.; Horcajada, R.; Nagaki, A. Chem. Rev. 2008, 108, 2265–2299. doi:10.1021/cr0680843
Return to citation in text: [1] -
Yan, M.; Kawamata, Y.; Baran, P. S. Chem. Rev. 2017, 117, 13230–13319. doi:10.1021/acs.chemrev.7b00397
Return to citation in text: [1] -
Wiebe, A.; Gieshoff, T.; Möhle, S.; Rodrigo, E.; Zirbes, M.; Waldvogel, S. R. Angew. Chem., Int. Ed. 2018, 57, 5594–5619. doi:10.1002/anie.201711060
Return to citation in text: [1] -
Moeller, K. D. Chem. Rev. 2018, 118, 4817–4833. doi:10.1021/acs.chemrev.7b00656
Return to citation in text: [1] -
Onomura, O. Electrochemical Asymmetric Synthesis. In Encyclopedia of Applied Electrochemistry; Kreysa, G.; Ota, K.; Savinell, R. F., Eds.; Springer: New York, NY, 2014.
Return to citation in text: [1] -
Lin, Q.; Li, L.; Luo, S. Chem. – Eur. J. 2019, 25, 10033–10044. doi:10.1002/chem.201901284
Return to citation in text: [1] -
Wattanakit, C. Curr. Opin. Electrochem. 2018, 7, 54–60. doi:10.1016/j.coelec.2017.09.027
Return to citation in text: [1] -
Horner, L.; Skaletz, D. H. Tetrahedron Lett. 1970, 11, 3679–3681. doi:10.1016/s0040-4039(01)98560-0
Return to citation in text: [1] -
Kopilov, J.; Shatzmiller, S.; Kariv, E. Electrochim. Acta 1976, 21, 535–536. doi:10.1016/0013-4686(76)85144-4
Return to citation in text: [1] -
Watkins, B. F.; Behling, J. R.; Kariv, E.; Miller, L. L. J. Am. Chem. Soc. 1975, 97, 3549–3550. doi:10.1021/ja00845a061
Return to citation in text: [1] [2] -
Fujihira, M.; Yokozawa, A.; Kinoshita, H.; Osa, T. Chem. Lett. 1982, 11, 1089–1092. doi:10.1246/cl.1982.1089
Return to citation in text: [1] -
Abe, S.; Nonaka, T.; Fuchigami, T. J. Am. Chem. Soc. 1983, 105, 3630–3632. doi:10.1021/ja00349a046
Return to citation in text: [1] -
Abe, S.; Fuchigami, T.; Nonaka, T. Chem. Lett. 1983, 12, 1033–1036. doi:10.1246/cl.1983.1033
Return to citation in text: [1] -
Moutet, J.-C.; Duboc-Toia, C.; Ménage, S.; Tingry, S. Adv. Mater. (Weinheim, Ger.) 1998, 10, 665–667. doi:10.1002/(sici)1521-4095(199806)10:9<665::aid-adma665>3.0.co;2-5
Return to citation in text: [1] -
Moutet, J.-C.; Cho, L. Y.; Duboc-Toia, C.; Ménage, S.; Riesgo, E. C.; Thummel, R. P. New J. Chem. 1999, 23, 939–944. doi:10.1039/a904477i
Return to citation in text: [1] -
Takano, N.; Seki, C. Electrochemistry 2006, 74, 596–598. doi:10.5796/electrochemistry.74.596
Return to citation in text: [1] -
Gourley, R. N.; Grimshaw, J.; Millar, P. G. J. Chem. Soc. C 1970, 2318–2323. doi:10.1039/j39700002318
Return to citation in text: [1] [2] -
Kariv, E.; Terni, H. A.; Gileadi, E. Electrochim. Acta 1973, 18, 433–441. doi:10.1016/0013-4686(73)80048-9
Return to citation in text: [1] -
Chen, B.-L.; Xiao, Y.; Xu, X.-M.; Yang, H.-P.; Wang, H.; Lu, J.-X. Electrochim. Acta 2013, 107, 320–326. doi:10.1016/j.electacta.2013.06.082
Return to citation in text: [1] -
Kopilov, J.; Kariv, E.; Miller, L. L. J. Am. Chem. Soc. 1977, 99, 3450–3454. doi:10.1021/ja00452a043
Return to citation in text: [1] -
Schoo, N.; Schäfer, H.-J. Liebigs Ann. Chem. 1993, 601–607. doi:10.1002/jlac.199319930198
Return to citation in text: [1] -
Nielsen, M. F.; Batanero, B.; Rolvering, S.; Schäfer, H. J. Enantioselective Cathodic Hydrogenation of Prostereogenic Activated Double Bonds. Novel Trends in Electroorganic Synthesis; Springer-Verlag: Tokyo, 1998; pp 229–232. doi:10.1007/978-4-431-65924-2_69
Return to citation in text: [1] -
Zhang, K.; Wang, H.; Zhao, S.-F.; Niu, D.-F.; Lu, J.-X. J. Electroanal. Chem. 2009, 630, 35–41. doi:10.1016/j.jelechem.2009.02.013
Return to citation in text: [1] -
Zhao, S.-F.; Zhu, M.-X.; Zhang, K.; Wang, H.; Lu, J.-X. Tetrahedron Lett. 2011, 52, 2702–2705. doi:10.1016/j.tetlet.2011.03.076
Return to citation in text: [1] -
Chen, B.-L.; Tu, Z.-Y.; Zhu, H.-W.; Sun, W.-W.; Wang, H.; Lu, J.-X. Electrochim. Acta 2014, 116, 475–483. doi:10.1016/j.electacta.2013.11.001
Return to citation in text: [1] -
Yang, H.; Chi, D.; Sun, Q.; Sun, W.; Wang, H.; Lu, J. Chem. Commun. 2014, 50, 8868–8870. doi:10.1039/c4cc02823f
Return to citation in text: [1] -
Yang, H.; Lv, T.; Sun, W.; Du, Y.; Wang, H.; Lu, J. RSC Adv. 2014, 4, 30584–30586. doi:10.1039/c4ra03368j
Return to citation in text: [1] -
Yang, H.-P.; Wang, H.; Lu, J.-X. Electrochem. Commun. 2015, 55, 18–21. doi:10.1016/j.elecom.2015.03.006
Return to citation in text: [1] -
Yang, H.-P.; Fen, Q.; Wang, H.; Lu, J.-X. Electrochem. Commun. 2016, 71, 38–42. doi:10.1016/j.elecom.2016.08.004
Return to citation in text: [1] -
Yue, Y.-N.; Wu, D.; Zeng, S.; Yang, M.-P.; Wang, H.; Lu, J.-X. New J. Chem. 2017, 41, 7853–7856. doi:10.1039/c7nj00844a
Return to citation in text: [1] -
Yadav, A. K.; Singh, A. Bull. Chem. Soc. Jpn. 2002, 75, 587–588. doi:10.1246/bcsj.75.587
Return to citation in text: [1] [2] -
Yadav, A. K.; Manju, M.; Chhinpa, P. R. Tetrahedron: Asymmetry 2003, 14, 1079–1081. doi:10.1016/s0957-4166(03)00169-1
Return to citation in text: [1] -
Kawabata, S.; Iwata, N.; Yoneyama, H. Chem. Lett. 2000, 29, 110–111. doi:10.1246/cl.2000.110
Return to citation in text: [1] -
Feng, R.; Smith, J. A.; Moeller, K. D. Acc. Chem. Res. 2017, 50, 2346–2352. doi:10.1021/acs.accounts.7b00287
Return to citation in text: [1] -
Firth, B. E.; Miller, L. L.; Mitani, M.; Rogers, T.; Lennox, J.; Murray, R. W. J. Am. Chem. Soc. 1976, 98, 8271–8272. doi:10.1021/ja00441a069
Return to citation in text: [1] -
Komori, T.; Nonaka, T. J. Am. Chem. Soc. 1983, 105, 5690–5691. doi:10.1021/ja00355a029
Return to citation in text: [1] -
Komori, T.; Nonaka, T. J. Am. Chem. Soc. 1984, 106, 2656–2659. doi:10.1021/ja00321a028
Return to citation in text: [1] -
Osa, T.; Kashiwagi, Y.; Yanagisawa, Y.; Bobbitt, J. M. J. Chem. Soc., Chem. Commun. 1994, 2535–2537. doi:10.1039/c39940002535
Return to citation in text: [1] -
Kashiwagi, Y.; Yanagisawa, Y.; Kurashima, F.; Anzai, J.-i.; Osa, T.; Bobbitt, J. M. Chem. Commun. 1996, 2745–2746. doi:10.1039/cc9960002745
Return to citation in text: [1] -
Yanagisawa, Y.; Kashiwagi, Y.; Kurashima, F.; Anzai, J.-i.; Osa, T.; Bobbitt, J. M. Chem. Lett. 1996, 25, 1043–1044. doi:10.1246/cl.1996.1043
Return to citation in text: [1] -
Kashiwagi, Y.; Kurashima, F.; Chiba, S.; Anzai, J.-i.; Osa, T.; Bobbitt, J. M. Chem. Commun. 2003, 114–115. doi:10.1039/b209871g
Return to citation in text: [1] -
Seebach, D.; Oei, H. A. Angew. Chem., Int. Ed. Engl. 1975, 14, 634–636. doi:10.1002/anie.197506342
Return to citation in text: [1] -
Horner, L.; Degner, D. Tetrahedron Lett. 1968, 9, 5889–5892. doi:10.1016/s0040-4039(00)75431-1
Return to citation in text: [1] -
Horner, L.; Degner, D. Electrochim. Acta 1974, 19, 611–627. doi:10.1016/0013-4686(74)85018-8
Return to citation in text: [1] -
Kodama, Y.; Fujiwara, A.; Kawamoto, H.; Ohta, N.; Kitani, A.; Ito, S. Chem. Lett. 2001, 30, 240–241. doi:10.1246/cl.2001.240
Return to citation in text: [1] -
Yadav, A. K.; Manju, M. Indian J. Chem. 2006, 45B, 2770–2772.
Return to citation in text: [1] -
Maekawa, H.; Itoh, K.; Goda, S.; Nishiguchi, I. Chirality 2003, 15, 95–100. doi:10.1002/chir.10165
Return to citation in text: [1] -
Kashiwagi, Y.; Kurashima, F.; Kikuchi, C.; Anzai, J.-i.; Osa, T.; Bobbitt, J. M. Chem. Commun. 1999, 1983–1984. doi:10.1039/a905894j
Return to citation in text: [1] -
Kashiwagi, Y.; Kurashima, F.; Kikuchi, C.; Anzai, J.-i.; Osa, T.; Bobbitt, J. M. Tetrahedron Lett. 1999, 40, 6469–6472. doi:10.1016/s0040-4039(99)01325-8
Return to citation in text: [1] -
Kuroboshi, M.; Yoshihisa, H.; Cortona, M. N.; Kawakami, Y.; Gao, Z.; Tanaka, H. Tetrahedron Lett. 2000, 41, 8131–8135. doi:10.1016/s0040-4039(00)01419-2
Return to citation in text: [1] -
Gao, W.-C.; Xiong, Z.-Y.; Pirhaghani, S.; Wirth, T. Synthesis 2019, 51, 276–284. doi:10.1055/s-0037-1610373
Return to citation in text: [1] -
Amundsen, A. R.; Balko, E. N. J. Appl. Electrochem. 1992, 22, 810–816. doi:10.1007/bf01023722
Return to citation in text: [1] -
Torii, S.; Liu, P.; Bhuvaneswari, N.; Amatore, C.; Jutand, A. J. Org. Chem. 1996, 61, 3055–3060. doi:10.1021/jo952137r
Return to citation in text: [1] -
Nguyen, B. H.; Perkins, R. J.; Smith, J. A.; Moeller, K. D. Beilstein J. Org. Chem. 2015, 11, 280–287. doi:10.3762/bjoc.11.32
Return to citation in text: [1] -
Tanaka, H.; Kuroboshi, M.; Takeda, H.; Kanda, H.; Torii, S. J. Electroanal. Chem. 2001, 507, 75–81. doi:10.1016/s0022-0728(01)00387-4
Return to citation in text: [1] -
Minato, D.; Arimoto, H.; Nagasue, Y.; Demizu, Y.; Onomura, O. Tetrahedron 2008, 64, 6675–6683. doi:10.1016/j.tet.2008.05.015
Return to citation in text: [1] -
Chen, B.-L.; Zhu, H.-W.; Xiao, Y.; Sun, Q.-L.; Wang, H.; Lu, J.-X. Electrochem. Commun. 2014, 42, 55–59. doi:10.1016/j.elecom.2014.02.009
Return to citation in text: [1] -
Huang, X.; Zhang, Q.; Lin, J.; Harms, K.; Meggers, E. Nat. Catal. 2019, 2, 34–40. doi:10.1038/s41929-018-0198-y
Return to citation in text: [1] -
Franco, D.; Riahi, A.; Hénin, F.; Muzart, J.; Duñach, E. Eur. J. Org. Chem. 2002, 2257–2259. doi:10.1002/1099-0690(200207)2002:14<2257::aid-ejoc2257>3.0.co;2-s
Return to citation in text: [1] -
Zhang, Q.; Chang, X.; Peng, L.; Guo, C. Angew. Chem., Int. Ed. 2019, 58, 6999–7003. doi:10.1002/anie.201901801
Return to citation in text: [1] -
Ogawa, K. A.; Boydston, A. J. Chem. Lett. 2015, 44, 10–16. doi:10.1246/cl.140915
Return to citation in text: [1] -
Page, P. C. B.; Marken, F.; Williamson, C.; Chan, Y.; Buckley, B. R.; Bethell, D. Adv. Synth. Catal. 2008, 350, 1149–1154. doi:10.1002/adsc.200800064
Return to citation in text: [1] -
Bui, N.-N.; Ho, X.-H.; Mho, S.-i.; Jang, H.-Y. Eur. J. Org. Chem. 2009, 5309–5312. doi:10.1002/ejoc.200900871
Return to citation in text: [1] -
Ho, X.-H.; Mho, S.-i.; Kang, H.; Jang, H.-Y. Eur. J. Org. Chem. 2010, 4436–4441. doi:10.1002/ejoc.201000453
Return to citation in text: [1] -
Jensen, K. L.; Franke, P. T.; Nielsen, L. T.; Daasbjerg, K.; Jorgensen, K. A. Angew. Chem., Int. Ed. 2010, 49, 129–133. doi:10.1002/anie.200904754
Return to citation in text: [1] -
Fu, N.; Li, L.; Yang, Q.; Luo, S. Org. Lett. 2017, 19, 2122–2125. doi:10.1021/acs.orglett.7b00746
Return to citation in text: [1] -
Simon, H.; Günther, H.; Bader, J.; Tischer, W. Angew. Chem., Int. Ed. Engl. 1981, 20, 861–863. doi:10.1002/anie.198108611
Return to citation in text: [1] -
Yuan, R.; Watanabe, S.; Kuwabata, S.; Yoneyama, H. J. Org. Chem. 1997, 62, 2494–2499. doi:10.1021/jo9620573
Return to citation in text: [1] -
Höllrigl, V.; Otto, K.; Schmid, A. Adv. Synth. Catal. 2007, 349, 1337–1340. doi:10.1002/adsc.200700059
Return to citation in text: [1] -
Hollmann, F.; Hofstetter, K.; Habicher, T.; Hauer, B.; Schmid, A. J. Am. Chem. Soc. 2005, 127, 6540–6541. doi:10.1021/ja050997b
Return to citation in text: [1] -
Lütz, S.; Steckhan, E.; Liese, A. Electrochem. Commun. 2004, 6, 583–587. doi:10.1016/j.elecom.2004.04.009
Return to citation in text: [1] -
Hisaeda, Y.; Nishioka, T.; Inoue, Y.; Asada, K.; Hayashi, T. Coord. Chem. Rev. 2000, 198, 21–37. doi:10.1016/s0010-8545(99)00222-2
Return to citation in text: [1] -
Anderson, R. J.; Dixon, R. M.; Golding, B. T. J. Organomet. Chem. 1992, 437, 227–237. doi:10.1016/0022-328x(92)83446-o
Return to citation in text: [1] -
Bonhôte, P.; Scheffold, R. Helv. Chim. Acta 1991, 74, 1425–1444. doi:10.1002/hlca.19910740706
Return to citation in text: [1] -
Murakami, Y.; Hisaeda, Y.; Kohno, H.; Ohno, T. Chem. Lett. 1992, 21, 909–912. doi:10.1246/cl.1992.909
Return to citation in text: [1] -
Ohno, T.; Nishioka, T.; Hisaeda, Y.; Murakami, Y. J. Mol. Struct.: THEOCHEM 1994, 308, 207–218. doi:10.1016/0166-1280(94)80103-7
Return to citation in text: [1] -
Murakami, Y.; Hisaeda, Y.; Ohno, T.; Kohno, H.; Nishioka, T. J. Chem. Soc., Perkin Trans. 2 1995, 1175–1183. doi:10.1039/p29950001175
Return to citation in text: [1] -
Zielinski, C.; Schäfer, H. J. Tetrahedron Lett. 1994, 35, 5621–5624. doi:10.1016/s0040-4039(00)77263-7
Return to citation in text: [1] -
Reufer, C.; Zielinski, C.; Schäfer, H. J.; Fröhlich, R. Acta Chem. Scand. 1999, 53, 1023–1037. doi:10.3891/acta.chem.scand.53-1023
Return to citation in text: [1] -
Durandetti, M.; Périchon, J.; Nédélec, J.-Y. J. Org. Chem. 1997, 62, 7914–7915. doi:10.1021/jo971279d
Return to citation in text: [1] -
D'Oca, M. G. M.; Pilli, R. A.; Vencato, I. Tetrahedron Lett. 2000, 41, 9709–9712. doi:10.1016/s0040-4039(00)01749-4
Return to citation in text: [1] -
D'Oca, M. G. M.; Pilli, R. A.; Pardini, V. L.; Curi, D.; Comninos, F. C. M. J. Braz. Chem. Soc. 2001, 12, 507–513. doi:10.1590/s0103-50532001000400011
Return to citation in text: [1] -
Kise, N.; Iwasaki, K.; Tokieda, N.; Ueda, N. Org. Lett. 2001, 3, 3241–3244. doi:10.1021/ol016566p
Return to citation in text: [1] -
Kise, N.; Iitaka, S.; Iwasaki, K.; Ueda, N. J. Org. Chem. 2002, 67, 8305–8315. doi:10.1021/jo026183k
Return to citation in text: [1] -
Feroci, M.; Inesi, A.; Orsini, M.; Palombi, L. Org. Lett. 2002, 4, 2617–2620. doi:10.1021/ol025939z
Return to citation in text: [1] -
Feroci, M.; Orsini, M.; Palombi, L.; Sotgiu, G.; Colapietro, M.; Inesi, A. J. Org. Chem. 2004, 69, 487–494. doi:10.1021/jo0343836
Return to citation in text: [1] -
Palombi, L.; Feroci, M.; Orsini, M.; Inesi, A. Tetrahedron: Asymmetry 2002, 13, 2311–2316. doi:10.1016/s0957-4166(02)00636-5
Return to citation in text: [1] -
Orsini, M.; Feroci, M.; Sotgiu, G.; Inesi, A. Org. Biomol. Chem. 2005, 3, 1202–1208. doi:10.1039/b500570a
Return to citation in text: [1] -
Sierecki, E.; Turcaud, S.; Martens, T.; Royer, J. Synthesis 2006, 3199–3208. doi:10.1055/s-2006-950226
Return to citation in text: [1] -
Feroci, M. Adv. Synth. Catal. 2007, 349, 2177–2181. doi:10.1002/adsc.200700156
Return to citation in text: [1] -
Lee, D.-S. Tetrahedron: Asymmetry 2009, 20, 2014–2020. doi:10.1016/j.tetasy.2009.08.017
Return to citation in text: [1] -
Magdesieva, T. V.; Levitskiy, O. A.; Grishin, Y. K.; Ambartsumyan, A. A.; Kiskin, M. A.; Churakov, A. V.; Babievsky, K. K.; Kochetkov, K. A. Organometallics 2014, 33, 4629–4638. doi:10.1021/om500070n
Return to citation in text: [1] -
Levitskiy, O. A.; Grishin, Y. K.; Paseshnichenko, K. A.; Kochetkov, K. A.; Magdesieva, T. V. Tetrahedron Lett. 2018, 59, 2831–2834. doi:10.1016/j.tetlet.2018.06.025
Return to citation in text: [1] -
Kise, N.; Hamada, Y.; Sakurai, T. Org. Lett. 2014, 16, 3348–3351. doi:10.1021/ol5013789
Return to citation in text: [1] -
Shatskiy, A.; Lundberg, H.; Kärkäs, M. D. ChemElectroChem 2019, 6, 4067–4092. doi:10.1002/celc.201900435
Return to citation in text: [1] -
Shankaraiah, N.; Pilli, R. A.; Santos, L. S. Tetrahedron Lett. 2008, 49, 5098–5100. doi:10.1016/j.tetlet.2008.06.028
Return to citation in text: [1] -
Louafi, F.; Moreau, J.; Shahane, S.; Golhen, S.; Roisnel, T.; Sinbandhit, S.; Hurvois, J.-P. J. Org. Chem. 2011, 76, 9720–9732. doi:10.1021/jo2017982
Return to citation in text: [1] -
Mori, N.; Furuta, A.; Watanabe, H. Tetrahedron 2016, 72, 8393–8399. doi:10.1016/j.tet.2016.10.058
Return to citation in text: [1]
| 40. | Yang, H.-P.; Fen, Q.; Wang, H.; Lu, J.-X. Electrochem. Commun. 2016, 71, 38–42. doi:10.1016/j.elecom.2016.08.004 |
| 41. | Yue, Y.-N.; Wu, D.; Zeng, S.; Yang, M.-P.; Wang, H.; Lu, J.-X. New J. Chem. 2017, 41, 7853–7856. doi:10.1039/c7nj00844a |
| 91. | Durandetti, M.; Périchon, J.; Nédélec, J.-Y. J. Org. Chem. 1997, 62, 7914–7915. doi:10.1021/jo971279d |
| 42. | Yadav, A. K.; Singh, A. Bull. Chem. Soc. Jpn. 2002, 75, 587–588. doi:10.1246/bcsj.75.587 |
| 43. | Yadav, A. K.; Manju, M.; Chhinpa, P. R. Tetrahedron: Asymmetry 2003, 14, 1079–1081. doi:10.1016/s0957-4166(03)00169-1 |
| 92. | D'Oca, M. G. M.; Pilli, R. A.; Vencato, I. Tetrahedron Lett. 2000, 41, 9709–9712. doi:10.1016/s0040-4039(00)01749-4 |
| 87. | Ohno, T.; Nishioka, T.; Hisaeda, Y.; Murakami, Y. J. Mol. Struct.: THEOCHEM 1994, 308, 207–218. doi:10.1016/0166-1280(94)80103-7 |
| 88. | Murakami, Y.; Hisaeda, Y.; Ohno, T.; Kohno, H.; Nishioka, T. J. Chem. Soc., Perkin Trans. 2 1995, 1175–1183. doi:10.1039/p29950001175 |
| 89. | Zielinski, C.; Schäfer, H. J. Tetrahedron Lett. 1994, 35, 5621–5624. doi:10.1016/s0040-4039(00)77263-7 |
| 90. | Reufer, C.; Zielinski, C.; Schäfer, H. J.; Fröhlich, R. Acta Chem. Scand. 1999, 53, 1023–1037. doi:10.3891/acta.chem.scand.53-1023 |
| 84. | Anderson, R. J.; Dixon, R. M.; Golding, B. T. J. Organomet. Chem. 1992, 437, 227–237. doi:10.1016/0022-328x(92)83446-o |
| 85. | Bonhôte, P.; Scheffold, R. Helv. Chim. Acta 1991, 74, 1425–1444. doi:10.1002/hlca.19910740706 |
| 86. | Murakami, Y.; Hisaeda, Y.; Kohno, H.; Ohno, T. Chem. Lett. 1992, 21, 909–912. doi:10.1246/cl.1992.909 |
| 51. | Yanagisawa, Y.; Kashiwagi, Y.; Kurashima, F.; Anzai, J.-i.; Osa, T.; Bobbitt, J. M. Chem. Lett. 1996, 25, 1043–1044. doi:10.1246/cl.1996.1043 |
| 52. | Kashiwagi, Y.; Kurashima, F.; Chiba, S.; Anzai, J.-i.; Osa, T.; Bobbitt, J. M. Chem. Commun. 2003, 114–115. doi:10.1039/b209871g |
| 49. | Osa, T.; Kashiwagi, Y.; Yanagisawa, Y.; Bobbitt, J. M. J. Chem. Soc., Chem. Commun. 1994, 2535–2537. doi:10.1039/c39940002535 |
| 99. | Orsini, M.; Feroci, M.; Sotgiu, G.; Inesi, A. Org. Biomol. Chem. 2005, 3, 1202–1208. doi:10.1039/b500570a |
| 50. | Kashiwagi, Y.; Yanagisawa, Y.; Kurashima, F.; Anzai, J.-i.; Osa, T.; Bobbitt, J. M. Chem. Commun. 1996, 2745–2746. doi:10.1039/cc9960002745 |
| 46. | Firth, B. E.; Miller, L. L.; Mitani, M.; Rogers, T.; Lennox, J.; Murray, R. W. J. Am. Chem. Soc. 1976, 98, 8271–8272. doi:10.1021/ja00441a069 |
| 96. | Feroci, M.; Inesi, A.; Orsini, M.; Palombi, L. Org. Lett. 2002, 4, 2617–2620. doi:10.1021/ol025939z |
| 97. | Feroci, M.; Orsini, M.; Palombi, L.; Sotgiu, G.; Colapietro, M.; Inesi, A. J. Org. Chem. 2004, 69, 487–494. doi:10.1021/jo0343836 |
| 47. | Komori, T.; Nonaka, T. J. Am. Chem. Soc. 1983, 105, 5690–5691. doi:10.1021/ja00355a029 |
| 48. | Komori, T.; Nonaka, T. J. Am. Chem. Soc. 1984, 106, 2656–2659. doi:10.1021/ja00321a028 |
| 98. | Palombi, L.; Feroci, M.; Orsini, M.; Inesi, A. Tetrahedron: Asymmetry 2002, 13, 2311–2316. doi:10.1016/s0957-4166(02)00636-5 |
| 44. | Kawabata, S.; Iwata, N.; Yoneyama, H. Chem. Lett. 2000, 29, 110–111. doi:10.1246/cl.2000.110 |
| 93. | D'Oca, M. G. M.; Pilli, R. A.; Pardini, V. L.; Curi, D.; Comninos, F. C. M. J. Braz. Chem. Soc. 2001, 12, 507–513. doi:10.1590/s0103-50532001000400011 |
| 45. | Feng, R.; Smith, J. A.; Moeller, K. D. Acc. Chem. Res. 2017, 50, 2346–2352. doi:10.1021/acs.accounts.7b00287 |
| 94. | Kise, N.; Iwasaki, K.; Tokieda, N.; Ueda, N. Org. Lett. 2001, 3, 3241–3244. doi:10.1021/ol016566p |
| 95. | Kise, N.; Iitaka, S.; Iwasaki, K.; Ueda, N. J. Org. Chem. 2002, 67, 8305–8315. doi:10.1021/jo026183k |
| 53. | Seebach, D.; Oei, H. A. Angew. Chem., Int. Ed. Engl. 1975, 14, 634–636. doi:10.1002/anie.197506342 |
| 54. | Horner, L.; Degner, D. Tetrahedron Lett. 1968, 9, 5889–5892. doi:10.1016/s0040-4039(00)75431-1 |
| 105. | Kise, N.; Hamada, Y.; Sakurai, T. Org. Lett. 2014, 16, 3348–3351. doi:10.1021/ol5013789 |
| 55. | Horner, L.; Degner, D. Electrochim. Acta 1974, 19, 611–627. doi:10.1016/0013-4686(74)85018-8 |
| 106. | Shatskiy, A.; Lundberg, H.; Kärkäs, M. D. ChemElectroChem 2019, 6, 4067–4092. doi:10.1002/celc.201900435 |
| 102. | Lee, D.-S. Tetrahedron: Asymmetry 2009, 20, 2014–2020. doi:10.1016/j.tetasy.2009.08.017 |
| 103. | Magdesieva, T. V.; Levitskiy, O. A.; Grishin, Y. K.; Ambartsumyan, A. A.; Kiskin, M. A.; Churakov, A. V.; Babievsky, K. K.; Kochetkov, K. A. Organometallics 2014, 33, 4629–4638. doi:10.1021/om500070n |
| 104. | Levitskiy, O. A.; Grishin, Y. K.; Paseshnichenko, K. A.; Kochetkov, K. A.; Magdesieva, T. V. Tetrahedron Lett. 2018, 59, 2831–2834. doi:10.1016/j.tetlet.2018.06.025 |
| 100. | Sierecki, E.; Turcaud, S.; Martens, T.; Royer, J. Synthesis 2006, 3199–3208. doi:10.1055/s-2006-950226 |
| 101. | Feroci, M. Adv. Synth. Catal. 2007, 349, 2177–2181. doi:10.1002/adsc.200700156 |
| 62. | Gao, W.-C.; Xiong, Z.-Y.; Pirhaghani, S.; Wirth, T. Synthesis 2019, 51, 276–284. doi:10.1055/s-0037-1610373 |
| 63. | Amundsen, A. R.; Balko, E. N. J. Appl. Electrochem. 1992, 22, 810–816. doi:10.1007/bf01023722 |
| 60. | Kashiwagi, Y.; Kurashima, F.; Kikuchi, C.; Anzai, J.-i.; Osa, T.; Bobbitt, J. M. Tetrahedron Lett. 1999, 40, 6469–6472. doi:10.1016/s0040-4039(99)01325-8 |
| 61. | Kuroboshi, M.; Yoshihisa, H.; Cortona, M. N.; Kawakami, Y.; Gao, Z.; Tanaka, H. Tetrahedron Lett. 2000, 41, 8131–8135. doi:10.1016/s0040-4039(00)01419-2 |
| 58. | Maekawa, H.; Itoh, K.; Goda, S.; Nishiguchi, I. Chirality 2003, 15, 95–100. doi:10.1002/chir.10165 |
| 109. | Mori, N.; Furuta, A.; Watanabe, H. Tetrahedron 2016, 72, 8393–8399. doi:10.1016/j.tet.2016.10.058 |
| 59. | Kashiwagi, Y.; Kurashima, F.; Kikuchi, C.; Anzai, J.-i.; Osa, T.; Bobbitt, J. M. Chem. Commun. 1999, 1983–1984. doi:10.1039/a905894j |
| 56. | Kodama, Y.; Fujiwara, A.; Kawamoto, H.; Ohta, N.; Kitani, A.; Ito, S. Chem. Lett. 2001, 30, 240–241. doi:10.1246/cl.2001.240 |
| 107. | Shankaraiah, N.; Pilli, R. A.; Santos, L. S. Tetrahedron Lett. 2008, 49, 5098–5100. doi:10.1016/j.tetlet.2008.06.028 |
| 42. | Yadav, A. K.; Singh, A. Bull. Chem. Soc. Jpn. 2002, 75, 587–588. doi:10.1246/bcsj.75.587 |
| 57. | Yadav, A. K.; Manju, M. Indian J. Chem. 2006, 45B, 2770–2772. |
| 108. | Louafi, F.; Moreau, J.; Shahane, S.; Golhen, S.; Roisnel, T.; Sinbandhit, S.; Hurvois, J.-P. J. Org. Chem. 2011, 76, 9720–9732. doi:10.1021/jo2017982 |
| 65. | Nguyen, B. H.; Perkins, R. J.; Smith, J. A.; Moeller, K. D. Beilstein J. Org. Chem. 2015, 11, 280–287. doi:10.3762/bjoc.11.32 |
| 66. | Tanaka, H.; Kuroboshi, M.; Takeda, H.; Kanda, H.; Torii, S. J. Electroanal. Chem. 2001, 507, 75–81. doi:10.1016/s0022-0728(01)00387-4 |
| 64. | Torii, S.; Liu, P.; Bhuvaneswari, N.; Amatore, C.; Jutand, A. J. Org. Chem. 1996, 61, 3055–3060. doi:10.1021/jo952137r |
| 1. | Frontana-Uribe, B. A.; Little, R. D.; Ibanez, J. G.; Palma, A.; Vasquez-Medrano, R. Green Chem. 2010, 12, 2099–2119. doi:10.1039/c0gc00382d |
| 2. | Schäfer, H. J. C. R. Chim. 2011, 14, 745–765. doi:10.1016/j.crci.2011.01.002 |
| 3. | Horn, E. J.; Rosen, B. R.; Baran, P. S. ACS Cent. Sci. 2016, 2, 302–308. doi:10.1021/acscentsci.6b00091 |
| 4. | Tang, S.; Liu, Y.; Lei, A. Chem 2018, 4, 27–45. doi:10.1016/j.chempr.2017.10.001 |
| 15. | Moeller, K. D. Chem. Rev. 2018, 118, 4817–4833. doi:10.1021/acs.chemrev.7b00656 |
| 25. | Moutet, J.-C.; Duboc-Toia, C.; Ménage, S.; Tingry, S. Adv. Mater. (Weinheim, Ger.) 1998, 10, 665–667. doi:10.1002/(sici)1521-4095(199806)10:9<665::aid-adma665>3.0.co;2-5 |
| 26. | Moutet, J.-C.; Cho, L. Y.; Duboc-Toia, C.; Ménage, S.; Riesgo, E. C.; Thummel, R. P. New J. Chem. 1999, 23, 939–944. doi:10.1039/a904477i |
| 73. | Page, P. C. B.; Marken, F.; Williamson, C.; Chan, Y.; Buckley, B. R.; Bethell, D. Adv. Synth. Catal. 2008, 350, 1149–1154. doi:10.1002/adsc.200800064 |
| 13. | Yan, M.; Kawamata, Y.; Baran, P. S. Chem. Rev. 2017, 117, 13230–13319. doi:10.1021/acs.chemrev.7b00397 |
| 14. | Wiebe, A.; Gieshoff, T.; Möhle, S.; Rodrigo, E.; Zirbes, M.; Waldvogel, S. R. Angew. Chem., Int. Ed. 2018, 57, 5594–5619. doi:10.1002/anie.201711060 |
| 27. | Takano, N.; Seki, C. Electrochemistry 2006, 74, 596–598. doi:10.5796/electrochemistry.74.596 |
| 8. | Shono, T. Tetrahedron 1984, 40, 811–850. doi:10.1016/s0040-4020(01)91472-3 |
| 9. | Utley, J. Chem. Soc. Rev. 1997, 26, 157–167. doi:10.1039/cs9972600157 |
| 10. | Lund, H. J. Electrochem. Soc. 2002, 149, S21–S33. doi:10.1149/1.1462037 |
| 11. | Tomilov, A. P.; Turygin, V. V.; Kaabak, L. V. Russ. J. Electrochem. 2007, 43, 1106–1122. doi:10.1134/s1023193507100023 |
| 12. | Yoshida, J.-i.; Kataoka, K.; Horcajada, R.; Nagaki, A. Chem. Rev. 2008, 108, 2265–2299. doi:10.1021/cr0680843 |
| 23. | Abe, S.; Nonaka, T.; Fuchigami, T. J. Am. Chem. Soc. 1983, 105, 3630–3632. doi:10.1021/ja00349a046 |
| 71. | Zhang, Q.; Chang, X.; Peng, L.; Guo, C. Angew. Chem., Int. Ed. 2019, 58, 6999–7003. doi:10.1002/anie.201901801 |
| 5. | Meyer, T. H.; Finger, L. H.; Gandeepan, P.; Ackermann, L. Trends Chem. 2019, 1, 63–76. doi:10.1016/j.trechm.2019.01.011 |
| 6. | Sauermann, N.; Meyer, T. H.; Qiu, Y.; Ackermann, L. ACS Catal. 2018, 8, 7086–7103. doi:10.1021/acscatal.8b01682 |
| 7. | Qiu, Y.; Struwe, J.; Ackermann, L. Synlett 2019, 30, 1164–1173. doi:10.1055/s-0037-1611568 |
| 24. | Abe, S.; Fuchigami, T.; Nonaka, T. Chem. Lett. 1983, 12, 1033–1036. doi:10.1246/cl.1983.1033 |
| 72. | Ogawa, K. A.; Boydston, A. J. Chem. Lett. 2015, 44, 10–16. doi:10.1246/cl.140915 |
| 19. | Horner, L.; Skaletz, D. H. Tetrahedron Lett. 1970, 11, 3679–3681. doi:10.1016/s0040-4039(01)98560-0 |
| 20. | Kopilov, J.; Shatzmiller, S.; Kariv, E. Electrochim. Acta 1976, 21, 535–536. doi:10.1016/0013-4686(76)85144-4 |
| 21. | Watkins, B. F.; Behling, J. R.; Kariv, E.; Miller, L. L. J. Am. Chem. Soc. 1975, 97, 3549–3550. doi:10.1021/ja00845a061 |
| 69. | Huang, X.; Zhang, Q.; Lin, J.; Harms, K.; Meggers, E. Nat. Catal. 2019, 2, 34–40. doi:10.1038/s41929-018-0198-y |
| 18. | Wattanakit, C. Curr. Opin. Electrochem. 2018, 7, 54–60. doi:10.1016/j.coelec.2017.09.027 |
| 22. | Fujihira, M.; Yokozawa, A.; Kinoshita, H.; Osa, T. Chem. Lett. 1982, 11, 1089–1092. doi:10.1246/cl.1982.1089 |
| 70. | Franco, D.; Riahi, A.; Hénin, F.; Muzart, J.; Duñach, E. Eur. J. Org. Chem. 2002, 2257–2259. doi:10.1002/1099-0690(200207)2002:14<2257::aid-ejoc2257>3.0.co;2-s |
| 17. | Lin, Q.; Li, L.; Luo, S. Chem. – Eur. J. 2019, 25, 10033–10044. doi:10.1002/chem.201901284 |
| 67. | Minato, D.; Arimoto, H.; Nagasue, Y.; Demizu, Y.; Onomura, O. Tetrahedron 2008, 64, 6675–6683. doi:10.1016/j.tet.2008.05.015 |
| 16. | Onomura, O. Electrochemical Asymmetric Synthesis. In Encyclopedia of Applied Electrochemistry; Kreysa, G.; Ota, K.; Savinell, R. F., Eds.; Springer: New York, NY, 2014. |
| 21. | Watkins, B. F.; Behling, J. R.; Kariv, E.; Miller, L. L. J. Am. Chem. Soc. 1975, 97, 3549–3550. doi:10.1021/ja00845a061 |
| 68. | Chen, B.-L.; Zhu, H.-W.; Xiao, Y.; Sun, Q.-L.; Wang, H.; Lu, J.-X. Electrochem. Commun. 2014, 42, 55–59. doi:10.1016/j.elecom.2014.02.009 |
| 30. | Chen, B.-L.; Xiao, Y.; Xu, X.-M.; Yang, H.-P.; Wang, H.; Lu, J.-X. Electrochim. Acta 2013, 107, 320–326. doi:10.1016/j.electacta.2013.06.082 |
| 28. | Gourley, R. N.; Grimshaw, J.; Millar, P. G. J. Chem. Soc. C 1970, 2318–2323. doi:10.1039/j39700002318 |
| 29. | Kariv, E.; Terni, H. A.; Gileadi, E. Electrochim. Acta 1973, 18, 433–441. doi:10.1016/0013-4686(73)80048-9 |
| 76. | Jensen, K. L.; Franke, P. T.; Nielsen, L. T.; Daasbjerg, K.; Jorgensen, K. A. Angew. Chem., Int. Ed. 2010, 49, 129–133. doi:10.1002/anie.200904754 |
| 77. | Fu, N.; Li, L.; Yang, Q.; Luo, S. Org. Lett. 2017, 19, 2122–2125. doi:10.1021/acs.orglett.7b00746 |
| 74. | Bui, N.-N.; Ho, X.-H.; Mho, S.-i.; Jang, H.-Y. Eur. J. Org. Chem. 2009, 5309–5312. doi:10.1002/ejoc.200900871 |
| 75. | Ho, X.-H.; Mho, S.-i.; Kang, H.; Jang, H.-Y. Eur. J. Org. Chem. 2010, 4436–4441. doi:10.1002/ejoc.201000453 |
| 37. | Yang, H.; Chi, D.; Sun, Q.; Sun, W.; Wang, H.; Lu, J. Chem. Commun. 2014, 50, 8868–8870. doi:10.1039/c4cc02823f |
| 38. | Yang, H.; Lv, T.; Sun, W.; Du, Y.; Wang, H.; Lu, J. RSC Adv. 2014, 4, 30584–30586. doi:10.1039/c4ra03368j |
| 39. | Yang, H.-P.; Wang, H.; Lu, J.-X. Electrochem. Commun. 2015, 55, 18–21. doi:10.1016/j.elecom.2015.03.006 |
| 35. | Zhao, S.-F.; Zhu, M.-X.; Zhang, K.; Wang, H.; Lu, J.-X. Tetrahedron Lett. 2011, 52, 2702–2705. doi:10.1016/j.tetlet.2011.03.076 |
| 82. | Lütz, S.; Steckhan, E.; Liese, A. Electrochem. Commun. 2004, 6, 583–587. doi:10.1016/j.elecom.2004.04.009 |
| 36. | Chen, B.-L.; Tu, Z.-Y.; Zhu, H.-W.; Sun, W.-W.; Wang, H.; Lu, J.-X. Electrochim. Acta 2014, 116, 475–483. doi:10.1016/j.electacta.2013.11.001 |
| 83. | Hisaeda, Y.; Nishioka, T.; Inoue, Y.; Asada, K.; Hayashi, T. Coord. Chem. Rev. 2000, 198, 21–37. doi:10.1016/s0010-8545(99)00222-2 |
| 32. | Schoo, N.; Schäfer, H.-J. Liebigs Ann. Chem. 1993, 601–607. doi:10.1002/jlac.199319930198 |
| 33. | Nielsen, M. F.; Batanero, B.; Rolvering, S.; Schäfer, H. J. Enantioselective Cathodic Hydrogenation of Prostereogenic Activated Double Bonds. Novel Trends in Electroorganic Synthesis; Springer-Verlag: Tokyo, 1998; pp 229–232. doi:10.1007/978-4-431-65924-2_69 |
| 80. | Höllrigl, V.; Otto, K.; Schmid, A. Adv. Synth. Catal. 2007, 349, 1337–1340. doi:10.1002/adsc.200700059 |
| 34. | Zhang, K.; Wang, H.; Zhao, S.-F.; Niu, D.-F.; Lu, J.-X. J. Electroanal. Chem. 2009, 630, 35–41. doi:10.1016/j.jelechem.2009.02.013 |
| 81. | Hollmann, F.; Hofstetter, K.; Habicher, T.; Hauer, B.; Schmid, A. J. Am. Chem. Soc. 2005, 127, 6540–6541. doi:10.1021/ja050997b |
| 31. | Kopilov, J.; Kariv, E.; Miller, L. L. J. Am. Chem. Soc. 1977, 99, 3450–3454. doi:10.1021/ja00452a043 |
| 78. | Simon, H.; Günther, H.; Bader, J.; Tischer, W. Angew. Chem., Int. Ed. Engl. 1981, 20, 861–863. doi:10.1002/anie.198108611 |
| 28. | Gourley, R. N.; Grimshaw, J.; Millar, P. G. J. Chem. Soc. C 1970, 2318–2323. doi:10.1039/j39700002318 |
| 79. | Yuan, R.; Watanabe, S.; Kuwabata, S.; Yoneyama, H. J. Org. Chem. 1997, 62, 2494–2499. doi:10.1021/jo9620573 |
© 2019 Ghosh et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0). Please note that the reuse, redistribution and reproduction in particular requires that the authors and source are credited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (https://www.beilstein-journals.org/bjoc)