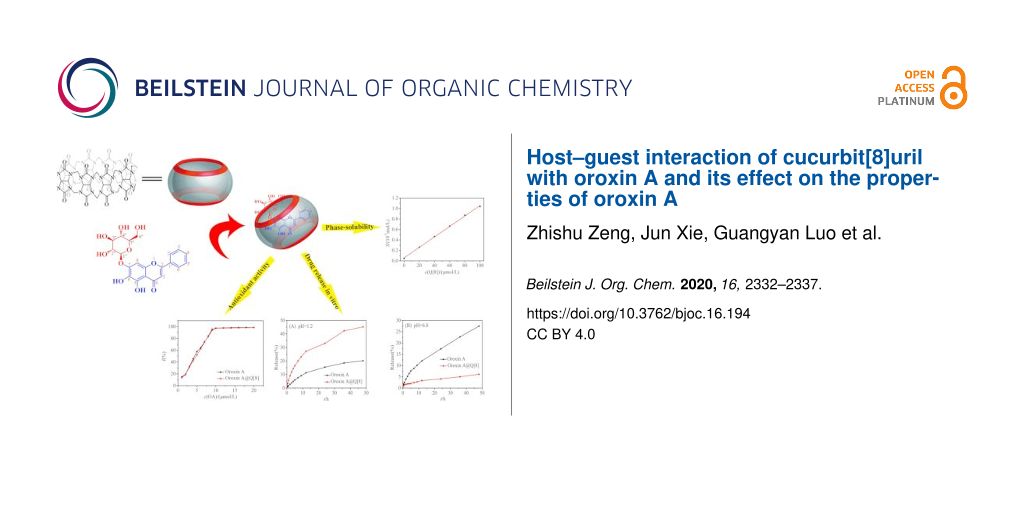
Abstract
In this study, we investigated the host–guest interactions between oroxin A (OA) and cucurbit[8]uril (Q[8]) using 1H NMR, MS, UV–vis and IR spectroscopy. The results showed that OA and Q[8] formed an inclusion compound (OA@Q[8]) with a molar ratio of 1:1 and a binding constant of 1.299 × 107 L·mol−1. In addition, the effect of Q[8] on the properties of OA was investigated through comparative experiments. The solubility of OA in water increased 22.47-fold when the concentration of Q[8] was 1 × 10−4 mol·L−1. Q[8] hardly affected the antioxidant capacity of OA, while the cumulative release of OA in gastric juice increased 2.3-fold after forming the inclusion compound with Q[8].

Graphical Abstract
Introduction
Cucurbit[n]urils (Q[n]s) are a family of macrocyclic cage compounds synthesized by the condensation of glycoluril and formaldehyde in a strong acidic solution [1-3]. As a consequence of the specific structural features of Q[n]s, which have two hydrophilic “portals” decorated with partially negatively charged carbonyl groups and a hydrophobic cavity [4], cucurbit[n]urils are able to form host–guest complexes with a range of drugs [5-7]. These complexes involve three main intermolecular forces: a hydrophobic effect, hydrogen bonding and ion–dipole interactions at the carbonyl portals [7-9]. The high thermal stability [10], ease of synthesis [11], general absence of cytotoxicity or toxicity [12,13] and their good molecular recognition and binding constants [14] have shown that Q[n]s are ideal drug carriers [15,16]. Moreover, Q[n]s can enhance the physical stability [17,18] and increase the solubility [19,20] of drug molecules. After Q[n]s forms a host–guest complex with drug molecules, it can also improve the bioavailability and delivery capacity [21], can help to reduce the side effects and toxicity of the drug [22].
Oroxin A (OA, baicalein-7-O-glucoside, Figure 1A) is one of the active ingredients isolated from the traditional herbal medicine Oroxylum indicum (L.) Kurz of Asian countries [23,24]. Accumulating studies have shown the beneficial biological effects of OA, which include antioxidant, antidiabetic, anticancer, antibacterial, anti-inflammatory and antiviral properties [25-30]. Herein, we selected Q[8] as a host molecule and investigated its host–guest interactions with OA, as well as its effect on the properties of OA. Our results provide an approach and theoretical basis for the development and utilization of oroxin A. Compared with the literature [31,32], it is found that although baicalein, oroxin B and oroxin A have the same aglycone, but the complex inclusion modes with Q[8] are different, It shows that the molecular size of the flavonoids and the length of the sugar chains have a greater impact on the assembly mode of supramolecular systems.
Figure 1: The molecular structure of OA (A) and Q[8] (B).
Figure 1: The molecular structure of OA (A) and Q[8] (B).
Results and Discussion
Host–guest interactions
The host–guest interaction can be effectively observed using 1H NMR spectroscopy, and the mode of action of the cucurbit[n]uril-guest can be inferred from the chemical shift changes of the guest proton resonance peaks. 1H NMR titration experiments were performed in D2O containing 10% DMSO by volume at room temperature. As shown in Figure 2 and Table 1, upon the addition of Q[8], some of the peaks of the protons of the OA aglycone shifted upfield, while the peaks due to the glycosidic proton shifted downfield. At the same time, the proton peaks of Q[8] were split, indicating that OA interacted with Q[8]. When the host–guest molar ratio was 1:1, all of the OA aglycone proton peaks moved upfield, indicating the entry into the cavity of Q[8]. The proton peaks of the glycosidic H and glucose were shifted downfield, indicating that they were located at the portal of Q[8]. However, when OA is present in excess, some of the proton peaks of the OA aglycon moved upfield, and some move downfield, indicating that in the case of an excess of OA, the port interactions and inclusion interactions of OA andQ[8] can exist simultaneously.
![[1860-5397-16-194-2]](/bjoc/content/figures/1860-5397-16-194-2.jpg?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: 1H NMR titration of OA with Q[8] were performed in D2O containing 10% DMSO by volume, OA (500 μmol·L−1) upon the addition of different molar equivalents of Q[8]: (a) 0, ( b ) 0.35, (c) 0.44, (d) 1.03, (e) 1.60 and (f) neat Q[8].
Figure 2: 1H NMR titration of OA with Q[8] were performed in D2O containing 10% DMSO by volume, OA (500 μmol·L...
To further determine the host–guest ratio of the inclusion complex formed by Q[8] and OA, their interaction was investigated using UV–visible absorption spectroscopy via a molar ratio method and Job's method. Figure 3A shows the UV–visible absorption spectra of the interaction between Q[8] and OA. It can be seen that the UV absorption of OA at 275 nm and 316 nm decreased significantly as the concentration of Q[8] was increased. When n(Q[8])/n(OA) = 1, there was a clear transition of the absorbance of the system. Upon further addition of Q[8], the absorption value of the system tended to be constant, indicating the formation of a 1:1 complex with a binding constant K = 1.299 × 107 L·mol−1. The result of the Job’s plot also confirmed the combination of Q[8] and OA in a 1:1 mode (Figure 3B).
![[1860-5397-16-194-3]](/bjoc/content/figures/1860-5397-16-194-3.jpg?scale=2.0&max-width=1024&background=FFFFFF)
Figure 3: (A) The UV–vis absorption spectra recorded for OA in the presence of Q[8] (c(Q[8]), labeled a–k: 0, 0.2, 0.4, 0.6, ..., 2.0 × 10–5 mol·L−1) and (B) Job’s plot obtained for OA in the presence of Q[8].
Figure 3: (A) The UV–vis absorption spectra recorded for OA in the presence of Q[8] (c(Q[8]), labeled a–k: 0,...
Figure 4 shows the IR spectra recorded for Q[8] (a), OA (b), a physical mixture of Q[8] and OA (n(Q[8]):n(OA) = 1:1) (c) and the OA@Q[8] inclusion complex (d). Curve (c) contains characteristic peaks of curves (a) and (b) without interaction in the physical mixture. Comparing spectra (c) and (d), the peaks at 1617.41, 1482.23 and 1451.06 cm−1 due to stretching vibrations of the two benzene rings disappeared, and the peak at 1079.42 cm−1 due to C–O stretching vibrations was obviously weakened in the inclusion complex, which were caused by Q[8].
![[1860-5397-16-194-4]](/bjoc/content/figures/1860-5397-16-194-4.jpg?scale=2.0&max-width=1024&background=FFFFFF)
Figure 4: IR spectra recorded for (a) Q[8], (b) OA, (c) a physical mixture of Q[8] and OA, and (d) the OA@Q[8] inclusion complex.
Figure 4: IR spectra recorded for (a) Q[8], (b) OA, (c) a physical mixture of Q[8] and OA, and (d) the OA@Q[8...
The mass spectrum of the OA@Q[8] inclusion complex featured the parent ion peak at m/z 1783.5716 [M + Na]+ (calcd. 1783.4983 [M + Na]+) (Supporting Information File 1, Figure S1), further supporting the formation of a 1:1 inclusion complex between OA and Q[8]. The possible host–guest mode is shown in Figure 5.
![[1860-5397-16-194-5]](/bjoc/content/figures/1860-5397-16-194-5.jpg?scale=2.0&max-width=1024&background=FFFFFF)
Figure 5: The possible interaction mode for OA@Q[8].
Figure 5: The possible interaction mode for OA@Q[8].
The effect of OA on the properties of cucurbit[8]uril
Phase-solubility
Phase-solubility studies were conducted to investigate the solubility of OA in the presence of Q[8]. As can be seen from Figure 6, the solubility of OA in water is very poor (4.62 × 10−6 mol·L−1). The solubility of OA increased linearly in water with the addition of Q[8]. When the concentration of Q[8] was 1.0 × 10−4 mol·L−1, the solubility of OA was increased 22.47-fold. The solubility curve equation was S = 0.01c + 0.0575, R2 = 0.9986.
![[1860-5397-16-194-6]](/bjoc/content/figures/1860-5397-16-194-6.jpg?scale=2.0&max-width=1024&background=FFFFFF)
Figure 6: The phase-solubility graph obtained for OA in a Q[8] aqueous solution at λ = 275 nm.
Figure 6: The phase-solubility graph obtained for OA in a Q[8] aqueous solution at λ = 275 nm.
Antioxidant activity
OA has strong antioxidant activity and effectively eliminates ABTS+• radicals. If the antioxidant activity of OA was decreased significantly by the formation of the OA@Q[8] complex, the medicinal value of OA would be seriously affected. Figure 7 shows the results for OA and OA@Q[8] scavenging of ABTS+• radicals in the range of 1–20 μmol·L−1. The IC50 values of OA and OA@Q[8] were 4.65 × 10−6 mol·L−1 and 4.80 × 10−6 mol·L−1, respectively, which indicates that Q[8] did not affect the antioxidant activity of OA.
![[1860-5397-16-194-7]](/bjoc/content/figures/1860-5397-16-194-7.jpg?scale=2.0&max-width=1024&background=FFFFFF)
Figure 7: The clearance rate curve of ABTS+• upon increasing the concentration of OA and the OA@Q[8] inclusion complex.
Figure 7: The clearance rate curve of ABTS+• upon increasing the concentration of OA and the OA@Q[8] inclusio...
Drug release in vitro
Figure 8 shows the cumulative release of the OA and the OA@Q[8] inclusion complex in artificial gastric juice (pH 1.2) and artificial intestinal juice (pH 6.8). It can be seen from Figure 8A that release of OA@Q[8] inclusion compound in artificial gastric juice was much higher than that of OA after 12 h. The cumulative release of OA and OA@Q[8] reached 11.25% and 27.15%, respectively, after 12 h. After 48 h, Q[8] increased the measured cumulative release of OA in artificial gastric juice by 2.3-fold. In artificial intestinal fluid (Figure 8B), the release rate of OA was faster than that of OA@Q[8]. After 12 h, the cumulative release of OA was 12.02%, while there was only 3.31% release of OA@Q[8].
![[1860-5397-16-194-8]](/bjoc/content/figures/1860-5397-16-194-8.jpg?scale=2.0&max-width=1024&background=FFFFFF)
Figure 8: The release curves of OA and OA@Q[8].
Figure 8: The release curves of OA and OA@Q[8].
Conclusion
In summary, the experimental results showed that OA and Q[8] formed a host–guest complex in a ratio of 1:1. The aglycone of OA entered the cavity of Q[8] and the glucose was located at the portal of Q[8], with a binding constant of 1.299 × 107 L·mol−1. The solubility of oroxin A was increased 22.47-fold when the concentration of the added Q[8] was 1.0 × 10−4 mol·L−1. The results of the UV absorption spectrum analysis showed that Q[8] enhanced the cumulative release of OA in artificial gastric juice by 2.3-fold, but had no effect on its antioxidant activity.
Supporting Information
| Supporting Information File 1: Apparatus, materials and methods. | ||
| Format: PDF | Size: 773.6 KB | Download |
References
-
Lagona, J.; Mukhopadhyay, P.; Chakrabarti, S.; Isaacs, L. Angew. Chem., Int. Ed. 2005, 44, 4844–4870. doi:10.1002/anie.200460675
Return to citation in text: [1] -
Isaacs, L. Chem. Commun. 2009, 619–629. doi:10.1039/b814897j
Return to citation in text: [1] -
Freeman, W. A.; Mock, W. L.; Shih, N. Y. J. Am. Chem. Soc. 1981, 103, 7367–7368. doi:10.1021/ja00414a070
Return to citation in text: [1] -
Gürbüz, S.; Idris, M.; Tuncel, D. Org. Biomol. Chem. 2015, 13, 330–347. doi:10.1039/c4ob02065k
Return to citation in text: [1] -
Wang, R.; Macartney, D. H. Org. Biomol. Chem. 2008, 6, 1955–1960. doi:10.1039/b801591k
Return to citation in text: [1] -
Wyman, I. W.; Macartney, D. H. Org. Biomol. Chem. 2010, 8, 247–252. doi:10.1039/b915694a
Return to citation in text: [1] -
Wheate, N. J. J. Inorg. Biochem. 2008, 102, 2060–2066. doi:10.1016/j.jinorgbio.2008.06.005
Return to citation in text: [1] [2] -
Ong, W.; Kaifer, A. E. J. Org. Chem. 2004, 69, 1383–1385. doi:10.1021/jo035030+
Return to citation in text: [1] -
Walker, S.; Kaur, R.; McInnes, F. J.; Wheate, N. J. Mol. Pharmaceutics 2010, 7, 2166–2172. doi:10.1021/mp100191b
Return to citation in text: [1] -
Germain, P.; Létoffé, J. M.; Merlin, M. P.; Buschmann, H. J. Thermochim. Acta 1998, 315, 87–92. doi:10.1016/s0040-6031(98)00252-4
Return to citation in text: [1] -
Wheate, N. J.; Patel, N.; Sutcliffe, O. B. Thermochim. Acta 2010, 2, 231–236.
Return to citation in text: [1] -
Uzunova, V. D.; Cullinane, C.; Brix, K.; Nau, W. M.; Day, A. I. Org. Biomol. Chem. 2010, 8, 2037–2042. doi:10.1039/b925555a
Return to citation in text: [1] -
Hettiarachchi, G.; Nguyen, D.; Wu, J.; Lucas, D.; Ma, D.; Isaacs, L.; Briken, V. PLoS One 2010, 5, e10514. doi:10.1371/journal.pone.0010514
Return to citation in text: [1] -
Rekharsky, M. V.; Mori, T.; Yang, C.; Ko, Y. H.; Selvapalam, N.; Kim, H.; Sobransingh, D.; Kaifer, A. E.; Liu, S.; Isaacs, L.; Chen, W.; Moghaddam, S.; Gilson, M. K.; Kim, K.; Inoue, Y. Proc. Natl. Acad. Sci. U. S. A. 2007, 104, 20737–20742. doi:10.1073/pnas.0706407105
Return to citation in text: [1] -
Angelos, S.; Yang, Y.-W.; Patel, K.; Stoddart, J. F.; Zink, J. I. Angew. Chem., Int. Ed. 2008, 47, 2222–2226. doi:10.1002/anie.200705211
Return to citation in text: [1] -
Walker, S.; Oun, R.; McInnes, F. J.; Wheate, N. J. Isr. J. Chem. 2011, 51, 616–624. doi:10.1002/ijch.201100033
Return to citation in text: [1] -
Kennedy, A. R.; Florence, A. J.; McInnes, F. J.; Wheate, N. J. Dalton Trans. 2009, 7695–7700. doi:10.1039/b907917c
Return to citation in text: [1] -
McInnes, F. J.; Anthony, N. G.; Kennedy, A. R.; Wheate, N. J. Org. Biomol. Chem. 2010, 8, 765–773. doi:10.1039/b918372h
Return to citation in text: [1] -
Zhao, Y.; Buck, D. P.; Morris, D. L.; Pourgholami, M. H.; Day, A. I.; Collins, J. G. Org. Biomol. Chem. 2008, 6, 4509–4515. doi:10.1039/b813759e
Return to citation in text: [1] -
Zhao, Y.; Pourgholami, M. H.; Morris, D. L.; Collins, J. G.; Day, A. I. Org. Biomol. Chem. 2010, 8, 3328–3337. doi:10.1039/c003732j
Return to citation in text: [1] -
Na’il, S.; Marieh, B.; Handawi, A.; Leena, A.; Liaquat, A.; Salman, A.; Thies, T.; Bassam, H.; Mohammed, M. Int. J. Pharm. 2014, 460, 53–62. doi:10.1016/j.ijpharm.2013.11.010
Return to citation in text: [1] -
Wanying, L.; Hang, Y.; David, B.; Jianbo, X.; Ying, Z.; Ruibing, W. Food Chem. Toxicol. 2017, 109, 923–929.
Return to citation in text: [1] -
Harminder; Singh, V.; Chaudhary, A. K. Indian J. Pharm. Sci. 2011, 73, 483.
Return to citation in text: [1] -
Chen, L.-J.; Games, D. E.; Jones, J. J. Chromatogr. A 2003, 988, 95–105. doi:10.1016/s0021-9673(02)01954-4
Return to citation in text: [1] -
Santanu, S.; Upal, K. M.; Arijit, M.; Dilipkumar, P.; Silpi, L. M.; Souvik, R. Nat. Prod. Commun. 2010, 5, 1239–1242.
Return to citation in text: [1] -
Qiu, J.; Wang, D.; Zhang, Y.; Dong, J.; Wang, J.; Niu, X. PLoS One 2013, 8, e80197. doi:10.1371/journal.pone.0080197
Return to citation in text: [1] -
Li, D.-q.; Zhao, J.; Li, S.-p.; Zhang, Q.-w. Anal. Bioanal. Chem. 2014, 406, 1975–1984. doi:10.1007/s00216-013-7612-8
Return to citation in text: [1] -
Yan, R.; Cao, Y.; Yang, B. Molecules 2014, 19, 4409–4417. doi:10.3390/molecules19044409
Return to citation in text: [1] -
Chen, H.; He, G.; Li, C.; Dong, L.; Xie, X.; Wu, J.; Gao, Y.; Zhou, J. RSC Adv. 2014, 4, 45151–45154. doi:10.1039/c4ra08573f
Return to citation in text: [1] -
He, J.; Du, L.; Bao, M.; Zhang, B.; Qian, H.; Zhou, Q.; Cao, Z. Anti-Cancer Drugs 2016, 27, 204–215. doi:10.1097/cad.0000000000000318
Return to citation in text: [1] -
Xie, J.; Zeng, Z.; Tao, Z.; Zhang, Q. Chem. Res. Chin. Univ. 2020. doi:10.1007/s40242-020-0005-6
Return to citation in text: [1] -
Zhang, X.; Xie, J.; Xu, Z.; Tao, Z.; Zhang, Q. Beilstein J. Org. Chem. 2020, 16, 71–77. doi:10.3762/bjoc.16.9
Return to citation in text: [1]
| 1. | Lagona, J.; Mukhopadhyay, P.; Chakrabarti, S.; Isaacs, L. Angew. Chem., Int. Ed. 2005, 44, 4844–4870. doi:10.1002/anie.200460675 |
| 2. | Isaacs, L. Chem. Commun. 2009, 619–629. doi:10.1039/b814897j |
| 3. | Freeman, W. A.; Mock, W. L.; Shih, N. Y. J. Am. Chem. Soc. 1981, 103, 7367–7368. doi:10.1021/ja00414a070 |
| 10. | Germain, P.; Létoffé, J. M.; Merlin, M. P.; Buschmann, H. J. Thermochim. Acta 1998, 315, 87–92. doi:10.1016/s0040-6031(98)00252-4 |
| 25. | Santanu, S.; Upal, K. M.; Arijit, M.; Dilipkumar, P.; Silpi, L. M.; Souvik, R. Nat. Prod. Commun. 2010, 5, 1239–1242. |
| 26. | Qiu, J.; Wang, D.; Zhang, Y.; Dong, J.; Wang, J.; Niu, X. PLoS One 2013, 8, e80197. doi:10.1371/journal.pone.0080197 |
| 27. | Li, D.-q.; Zhao, J.; Li, S.-p.; Zhang, Q.-w. Anal. Bioanal. Chem. 2014, 406, 1975–1984. doi:10.1007/s00216-013-7612-8 |
| 28. | Yan, R.; Cao, Y.; Yang, B. Molecules 2014, 19, 4409–4417. doi:10.3390/molecules19044409 |
| 29. | Chen, H.; He, G.; Li, C.; Dong, L.; Xie, X.; Wu, J.; Gao, Y.; Zhou, J. RSC Adv. 2014, 4, 45151–45154. doi:10.1039/c4ra08573f |
| 30. | He, J.; Du, L.; Bao, M.; Zhang, B.; Qian, H.; Zhou, Q.; Cao, Z. Anti-Cancer Drugs 2016, 27, 204–215. doi:10.1097/cad.0000000000000318 |
| 7. | Wheate, N. J. J. Inorg. Biochem. 2008, 102, 2060–2066. doi:10.1016/j.jinorgbio.2008.06.005 |
| 8. | Ong, W.; Kaifer, A. E. J. Org. Chem. 2004, 69, 1383–1385. doi:10.1021/jo035030+ |
| 9. | Walker, S.; Kaur, R.; McInnes, F. J.; Wheate, N. J. Mol. Pharmaceutics 2010, 7, 2166–2172. doi:10.1021/mp100191b |
| 31. | Xie, J.; Zeng, Z.; Tao, Z.; Zhang, Q. Chem. Res. Chin. Univ. 2020. doi:10.1007/s40242-020-0005-6 |
| 32. | Zhang, X.; Xie, J.; Xu, Z.; Tao, Z.; Zhang, Q. Beilstein J. Org. Chem. 2020, 16, 71–77. doi:10.3762/bjoc.16.9 |
| 5. | Wang, R.; Macartney, D. H. Org. Biomol. Chem. 2008, 6, 1955–1960. doi:10.1039/b801591k |
| 6. | Wyman, I. W.; Macartney, D. H. Org. Biomol. Chem. 2010, 8, 247–252. doi:10.1039/b915694a |
| 7. | Wheate, N. J. J. Inorg. Biochem. 2008, 102, 2060–2066. doi:10.1016/j.jinorgbio.2008.06.005 |
| 22. | Wanying, L.; Hang, Y.; David, B.; Jianbo, X.; Ying, Z.; Ruibing, W. Food Chem. Toxicol. 2017, 109, 923–929. |
| 4. | Gürbüz, S.; Idris, M.; Tuncel, D. Org. Biomol. Chem. 2015, 13, 330–347. doi:10.1039/c4ob02065k |
| 23. | Harminder; Singh, V.; Chaudhary, A. K. Indian J. Pharm. Sci. 2011, 73, 483. |
| 24. | Chen, L.-J.; Games, D. E.; Jones, J. J. Chromatogr. A 2003, 988, 95–105. doi:10.1016/s0021-9673(02)01954-4 |
| 15. | Angelos, S.; Yang, Y.-W.; Patel, K.; Stoddart, J. F.; Zink, J. I. Angew. Chem., Int. Ed. 2008, 47, 2222–2226. doi:10.1002/anie.200705211 |
| 16. | Walker, S.; Oun, R.; McInnes, F. J.; Wheate, N. J. Isr. J. Chem. 2011, 51, 616–624. doi:10.1002/ijch.201100033 |
| 19. | Zhao, Y.; Buck, D. P.; Morris, D. L.; Pourgholami, M. H.; Day, A. I.; Collins, J. G. Org. Biomol. Chem. 2008, 6, 4509–4515. doi:10.1039/b813759e |
| 20. | Zhao, Y.; Pourgholami, M. H.; Morris, D. L.; Collins, J. G.; Day, A. I. Org. Biomol. Chem. 2010, 8, 3328–3337. doi:10.1039/c003732j |
| 14. | Rekharsky, M. V.; Mori, T.; Yang, C.; Ko, Y. H.; Selvapalam, N.; Kim, H.; Sobransingh, D.; Kaifer, A. E.; Liu, S.; Isaacs, L.; Chen, W.; Moghaddam, S.; Gilson, M. K.; Kim, K.; Inoue, Y. Proc. Natl. Acad. Sci. U. S. A. 2007, 104, 20737–20742. doi:10.1073/pnas.0706407105 |
| 21. | Na’il, S.; Marieh, B.; Handawi, A.; Leena, A.; Liaquat, A.; Salman, A.; Thies, T.; Bassam, H.; Mohammed, M. Int. J. Pharm. 2014, 460, 53–62. doi:10.1016/j.ijpharm.2013.11.010 |
| 12. | Uzunova, V. D.; Cullinane, C.; Brix, K.; Nau, W. M.; Day, A. I. Org. Biomol. Chem. 2010, 8, 2037–2042. doi:10.1039/b925555a |
| 13. | Hettiarachchi, G.; Nguyen, D.; Wu, J.; Lucas, D.; Ma, D.; Isaacs, L.; Briken, V. PLoS One 2010, 5, e10514. doi:10.1371/journal.pone.0010514 |
| 11. | Wheate, N. J.; Patel, N.; Sutcliffe, O. B. Thermochim. Acta 2010, 2, 231–236. |
| 17. | Kennedy, A. R.; Florence, A. J.; McInnes, F. J.; Wheate, N. J. Dalton Trans. 2009, 7695–7700. doi:10.1039/b907917c |
| 18. | McInnes, F. J.; Anthony, N. G.; Kennedy, A. R.; Wheate, N. J. Org. Biomol. Chem. 2010, 8, 765–773. doi:10.1039/b918372h |
© 2020 Zeng et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0). Please note that the reuse, redistribution and reproduction in particular requires that the authors and source are credited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (https://www.beilstein-journals.org/bjoc)