Search results
Search for "cucurbit[8]uril" in Full Text gives 12 result(s) in Beilstein Journal of Organic Chemistry.
Light-responsive rotaxane-based materials: inducing motion in the solid state
- Adrian Saura-Sanmartin
Beilstein J. Org. Chem. 2023, 19, 873–880, doi:10.3762/bjoc.19.64

- the interlocked arrangement. As an illustrative example, cucurbit[8]uril-based pseudorotaxanes having a pair of styrylpyridinium threads bearing carboxylic acid groups were employed in the preparation of the uranium-organic framework U-CB[8]-MPyVB (Figure 4a) [63]. The solid structure of the MOFs

Graphical Abstract

Figure 1: a) Chemical structure of pseudorotaxanes 1; and (b) single-crystal X-ray structure of rotaxane 1a (R...

Figure 2: (a) Chemical structure of polyrotaxane 2; and (b) cartoon representation of the light-triggered deg...

Figure 3: a) Chemical structures of rotaxanes (E)-3 and (Z)-3; b) stick representation of the solid structure...

Figure 4: Stick representations of the solid structures of: (a) U-CB[8]-MPyVB showing an interlocked ligand c...
A fluorescent probe for detection of Hg2+ ions constructed by tetramethyl cucurbit[6]uril and 1,2-bis(4-pyridyl)ethene
- Xiaoqian Chen,
- Naqin Yang,
- Yue Ma,
- Xinan Yang and
- Peihua Ma
Beilstein J. Org. Chem. 2023, 19, 864–872, doi:10.3762/bjoc.19.63

- aminopropyl-1-pyrenebutanamide (PBA) was also constructed to detect Fe3+ and Ag+ ions in aqueous solution [33], which can be used as a potentially useful fluorescent sensor. Pang and co-workers found that the fluorescent probe of cucurbit[8]uril (Q[8]) and squaraine dye (SQ2) has a high selectivity for Hg2

Graphical Abstract

Figure 1: Supramolecular assembly of TMeQ[6] and 1,2-bis(4-pyridyl)ethene (G).

Figure 2: (a) UV–vis titration of G (3.0 × 10−5 mol·L−1, pH 6.5) in aqueous solution with the increase of TMe...

Figure 3: (a) Fluorescence spectra (U = 550 V, pH 6.5) of G (3.0 × 10−5 mol·L−1) in aqueous solution with inc...

Figure 4: (a) Crystal structure of complex 1; (b) and (c) the binding mode of G with TMeQ[6]; (d) the supramo...

Figure 5: (a) Fluorescence response of G (3.0 × 10−5 mol·L−1) to metal cations in aqueous solution; (b) fluor...

Figure 6: Influence of coexisting ions on Hg2+ detection by G@TMeQ[6].

Figure 7: (a) Fluorescence titration curve (U = 500 V, pH 6.5) of probe G@TMeQ[6] (3.0 × 10−5 mol·L−1) to Hg2+...

Figure 8: (A) Titration 1H NMR spectra (400 MHz) obtained for TMeQ[6] with (a) 0.00, (b) 0.50, (c) 1.00 and (...
Trichloroacetic acid fueled practical amine purifications
- Aleena Thomas,
- Baptiste Gasch,
- Enzo Olivieri and
- Adrien Quintard
Beilstein J. Org. Chem. 2022, 18, 225–231, doi:10.3762/bjoc.18.26
- from rotaxanes, cucurbit[8]uril, to supramolecular smart materials [11][12][13][14][15][16]. TCA is also known for its application in the precipitation of proteins through what is believed to be an unfolding of the proteins structures [17][18]. In this context, we hypothesized that TCA could be used to

Graphical Abstract

Scheme 1: Classical amine purification.

Scheme 2: Principle of out-of equilibrium machinery using TCA (a) and our application to amines purification ...

Scheme 3: Application of the TCA purification from a crude reaction mixture.
Host–guest interaction and properties of cucurbit[8]uril with chloramphenicol
- Lin Zhang,
- Jun Zheng,
- Guangyan Luo,
- Xiaoyue Li,
- Yunqian Zhang,
- Zhu Tao and
- Qianjun Zhang
Beilstein J. Org. Chem. 2021, 17, 2832–2839, doi:10.3762/bjoc.17.194
- Lin Zhang Jun Zheng Guangyan Luo Xiaoyue Li Yunqian Zhang Zhu Tao Qianjun Zhang Key Laboratory of Macrocyclic and Supramolecular Chemistry of Guizhou Province, Guizhou University, Guiyang 550025, China 10.3762/bjoc.17.194 Abstract The interaction between cucurbit[8]uril (Q[8]) and chloramphenicol
- inhibitory activity of CPE against E. coli. Keywords: antibacterial activity; chloramphenicol; cucurbit[8]uril; host–guest interaction; in vitro cumulative release; stability; Introduction Chloramphenicol (CPE, Figure 1A) is a broad-spectrum antibiotic resulting from the metabolism of chorismic acid in
- cucurbit[n]urils can be used as non-toxic and safe drug carriers [29][30][31], among which cucurbit[8]uril (Q[8]) [32] has a large cavity. Q[8] interacts with a variety of small drug molecules such as chrysin, oroxin A and B, baicalein, etc., which can enhance the solubility, stability, antioxidant and

Graphical Abstract

Figure 1: The structures of chloramphenicol (A) and cucurbit[n]urils (B).

Figure 2: (A) CPE and Q[8] structural model diagram, (B) interaction between CPE and Q[8], (C) CPE@Q[8] stack...

Figure 3: UV–vis absorption spectra of CPE with Q[8] in aqueous solution (A) or hydrochloric acid solution (C...

Figure 4: ITC data obtained for the binding of Q[8] with CPE in an aqueous solution at 25 °C.

Figure 5: 1H NMR spectra of CPE, CPE@Q[8] and Q[8] (VD2O/VDCl = 3:2).

Figure 6: IR spectra recorded for Q[8] (a), CPE (b), a physical mixture of Q[8] and CPE (c), and the CPE@Q[8]...

Figure 7: UV absorption intensity of CPE and CPE@Q[8] changes with time in the artificial gastrointestinal ju...

Figure 8: Release curve of CPE and CPE@Q[8] in artificial gastrointestinal juice (pH 1.2, pH 6.8).
Selected peptide-based fluorescent probes for biological applications
- Debabrata Maity
Beilstein J. Org. Chem. 2020, 16, 2971–2982, doi:10.3762/bjoc.16.247

- insulin Schmuck and co-workers reported a supramolecular ensemble in combination of a pyrene-tagged amphiphilic peptide beacon (6) and a macrocyclic host (cucurbit[8]uril, CB[8]) for ratiometric fluorescent detection of amino acid derivatives, specific peptides, and proteins in aqueous media (Figure 6

Graphical Abstract

Figure 1: Three different type of peptide-based fluorescent probes and their interaction with nucleic acids a...

Figure 2: A) Molecular structure of peptidic probe 1, Inset: HeLa cells incubated with peptide 1 (50 μM), sho...

Figure 3: A) Molecular structure of probe 2; B) fluorescence emission spectra for the titration of a 10 μM so...

Figure 4: A) Molecular structure of 3; B) fluorescence emission spectra for the titration of a 10 μM solution...

Figure 5: A) Molecular structure of 4 and 5; B) fluorescence spectra for the titration of a 0.5 μM solution o...

Figure 6: A) Molecular structure of 6; B) possible binding mode of pyrene termini of 6 to CB[8] according to ...

Figure 7: A) Molecular structure of peptidic probes 7 and 8; B) fluorescence emission spectra of probe 7 (5.0...

Figure 8: Top: Molecular structure of 9; bottom: A) fluorescence response of 9 (500 nM) upon addition of β-tr...

Figure 9: Top: Molecular structures of 10 and 11; bottom: A) fluorescence emission spectra of 10 (1.0 µM, λex...

Figure 10: A) Structure of two peptide amphiphiles 12 and 13; B) fluorescent spectra (λex = 400 nm) from a tit...

Figure 11: a) Molecular structure of peptide 14; b) the coordinate represents the states of sensor at differen...
Host–guest interaction of cucurbit[8]uril with oroxin A and its effect on the properties of oroxin A
- Zhishu Zeng,
- Jun Xie,
- Guangyan Luo,
- Zhu Tao and
- Qianjun Zhang
Beilstein J. Org. Chem. 2020, 16, 2332–2337, doi:10.3762/bjoc.16.194

- –guest interactions between oroxin A (OA) and cucurbit[8]uril (Q[8]) using 1H NMR, MS, UV–vis and IR spectroscopy. The results showed that OA and Q[8] formed an inclusion compound (OA@Q[8]) with a molar ratio of 1:1 and a binding constant of 1.299 × 107 L·mol−1. In addition, the effect of Q[8] on the
- the inclusion compound with Q[8]. Keywords: cucurbit[8]uril; host–guest interaction; inclusion complex; oroxin A; properties; Introduction Cucurbit[n]urils (Q[n]s) are a family of macrocyclic cage compounds synthesized by the condensation of glycoluril and formaldehyde in a strong acidic solution [1
- properties of cucurbit[8]uril Phase-solubility Phase-solubility studies were conducted to investigate the solubility of OA in the presence of Q[8]. As can be seen from Figure 6, the solubility of OA in water is very poor (4.62 × 10−6 mol·L−1). The solubility of OA increased linearly in water with the

Graphical Abstract

Figure 1: The molecular structure of OA (A) and Q[8] (B).

Figure 2: 1H NMR titration of OA with Q[8] were performed in D2O containing 10% DMSO by volume, OA (500 μmol·L...

Figure 3: (A) The UV–vis absorption spectra recorded for OA in the presence of Q[8] (c(Q[8]), labeled a–k: 0,...

Figure 4: IR spectra recorded for (a) Q[8], (b) OA, (c) a physical mixture of Q[8] and OA, and (d) the OA@Q[8...

Figure 5: The possible interaction mode for OA@Q[8].

Figure 6: The phase-solubility graph obtained for OA in a Q[8] aqueous solution at λ = 275 nm.

Figure 7: The clearance rate curve of ABTS+• upon increasing the concentration of OA and the OA@Q[8] inclusio...

Figure 8: The release curves of OA and OA@Q[8].
The interaction between cucurbit[8]uril and baicalein and the effect on baicalein properties
- Xiaodong Zhang,
- Jun Xie,
- Zhiling Xu,
- Zhu Tao and
- Qianjun Zhang
Beilstein J. Org. Chem. 2020, 16, 71–77, doi:10.3762/bjoc.16.9
- Xiaodong Zhang Jun Xie Zhiling Xu Zhu Tao Qianjun Zhang Key Laboratory of Macrocyclic and Supramolecular Chemistry of Guizhou Province, Guizhou University, Guiyang 550025, China 10.3762/bjoc.16.9 Abstract The host–guest interactions between baicalein (BALE) and cucurbit[8]uril (Q[8]) and the
- corresponding properties of the inclusion complex were studied using 1H NMR, IR and UV–vis spectroscopy and DTA. The results showed that BALE forms an inclusion compound (1:1) with Q[8], and the properties of baicalein are changed by cucurbit[8]uril. Keywords: baicalein; cucurbit[8]uril; host–guest interaction
- ] inclusion complex had an obvious melting point at 448.7 °C and the melting point and endothermic peaks of BALE and Q[8] disappeared. The results showed that a new material appeared, which improved the thermal stability of BALE (Figure 6d). The effect of cucurbit[8]uril on baicalein properties Stability

Graphical Abstract

Figure 1: Chemical structures of baicalein (left), cucurbit[8]uril (right).

Figure 2: The possible interaction model for Q[8] and baicalein.

Figure 3: 1H NMR spectra (400 MHz) of Q[8] (a), baicalein (b) and BALE–Q[8] (1:1) (c) recorded in DCl.

Figure 4: The absorption spectra of BALE upon the addition of Q[8] under different conditions [10 mol·L−1 HCl...

Figure 5: IR spectra of Q[8] (a), BALE (b), a physical mixture of Q[8]-BALE (NQ[8]/NBALE = 1:1) (c) and the B...

Figure 6: DTA spectra of BALE (a), Q[8] (b), a physical mixture Q[8]-BALE (NQ[8]/NBALE = 1:1) (c) and the BAL...

Figure 7: The stability curve of UV–vis absorption obtained for an isoconcentration of BALE and the BALE–Q[8]...

Figure 8: The phase solubility graph obtained for BALE in Q[8] at λ = 270 nm.

Figure 9: The clearance rate curve (A) and clearance time curve (B) of ABTS+· upon increasing the concentrati...

Figure 10: The release curves of BALE and BALE–Q[8].
Complexation of chiral amines by resorcin[4]arene sulfonic acids in polar media – circular dichroism and diffusion studies of chirality transfer and solvent dependence
- Bartosz Setner and
- Agnieszka Szumna
Beilstein J. Org. Chem. 2019, 15, 1913–1924, doi:10.3762/bjoc.15.187
- , Supporting Information File 1). It should be noted that chirality induction can be exploited to design chirality sensing ensembles [17]. This strategy was successfully implemented by the Nau group for sensing of chiral aromatic compounds. Ternary complexes between cucurbit[8]uril, dicationic dyes, and chiral

Graphical Abstract

Figure 1: Structures of the compounds used in this study and labelling scheme for NMR spectra.

Figure 2: Spectra of complexes [1(LysOMe)2], [1(ArgOMe)2], [1(HisOMe)2]: 1H NMR (a–g) and ROESY (h–j) in meth...

Figure 3: CD (a) and UV (b) spectra of complexes [1(LysOMe)2], [1(PheOMe)2], [1(ValOMe)2], [1(ArgOMe)2], and [...

Figure 4: DOSY spectra of 1 (a), [1(LysOMe)2] (b), [1(ArgOMe)2] (c), [1(HisOMe)2] (d) and [LysOMe + 1(LysOMe)2...

Figure 5: 1H NMR spectra of 1 (a), LysOMe (b), 1H NMR and DOSY spectra of [1(LysOMe)2] (insets show the shape...

Figure 6: 1H NMR spectra of (R)-2 (a); [1((R)-2] (b); [1 + 1((R)-2] (c) (insets show the shape of signals f, ...

Figure 7: 1H NMR and DOSY spectra of (R)-2 (a); [1(R)-2] (b) (inset show the shape signals f, DMSO-d6, 298K, ...
Host–guest interactions in nor-seco-cucurbit[10]uril: novel guest-dependent molecular recognition and stereoisomerism
- Xiaodong Zhang,
- Wei Wu,
- Zhu Tao and
- Xin-Long Ni
Beilstein J. Org. Chem. 2019, 15, 1705–1711, doi:10.3762/bjoc.15.166
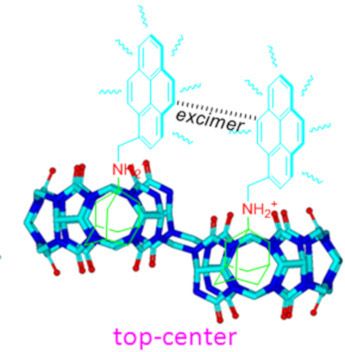
- because of their varying cavity rigidity and larger portal sizes as compared with those of other macrocyclic hosts [4][5][6][7][8][9][10][11][12][13]. For example, cucurbit[8]uril (Q[8] or CB[8]), a large homologue of the Q[n] family, is unique because of its ability to bind two hetero- and homo-aromatic

Graphical Abstract

Scheme 1: Chemical structures of host-1, host-2, G1, and G2.

Figure 1: 1H NMR spectra of G1 (1.0 mmol, D2O, pD = 2.0) in the presence of host-1 at different concentration...

Figure 2: 1H NMR spectra of G1 (1.0 mmol, D2O, pD = 2.0) in the presence of host-2 at different concentration...

Scheme 2: Plausible diastereomers showing the fluorescence response of G2 with host-1.

Figure 3: Fluorescence spectral changes of G2 (10.0 μM) in the presence of host-1 (a) and host-2 (b) at diffe...

Figure 4: 1H NMR spectra of G2 (1.0 mmol, D2O, pD = 2.0) in the presence of different concentrations of host-1...
Tetrathiafulvalene – a redox-switchable building block to control motion in mechanically interlocked molecules
- Hendrik V. Schröder and
- Christoph A. Schalley
Beilstein J. Org. Chem. 2018, 14, 2163–2185, doi:10.3762/bjoc.14.190

- host molecule cucurbit[8]uril (9), which is enlarged by an additional glycoluril unit in comparison to 7, provides sufficient space to accommodate two planar molecules with cofacial orientation [68]. When the water-soluble TTF derivative 10 gets oxidized to its radical cation 10●+, a 2:1 complex is

Graphical Abstract

Figure 1: The two one-electron oxidation reactions of tetrathiafulvalene (TTF, 1) and the corresponding prope...

Figure 2: UV–vis spectra and photographs of TTF 2 in its three stable oxidation states (black line = 2, orang...

Figure 3: Structure and conformations of two TTF dimers in solution, the mixed-valence and the radical-cation...

Figure 4: (a) The isomerism problem of TTF. (b)–(d) Major synthetic breakthroughs for the construction of TTF...

Figure 5: (a) Host–guest equilibrium between π-electron-poor cyclophane 3 and different TTFs with their corre...

Figure 6: TTF complexes with different host molecules.

Figure 7: Stable TTF (a) radical-cation and (b) mixed-valence dimers in confined molecular spaces.

Figure 8: A “three-pole supramolecular switch”: Controlled by its oxidation state, TTF (1) jumps back and for...

Figure 9: Redox-controlled closing and opening motion of the artificial molecular lasso 12.

Figure 10: Graphical illustration how a non-degenerate TTF-based shuttle works under electrochemical operation....

Figure 11: The first TTF-based rotaxane 13.

Figure 12: A redox-switchable bistable molecular shuttle 14.

Figure 13: The redox-switchable cyclodextrin-based rotaxane 15.

Figure 14: The redox-switchable non-ionic rotaxane 16 with a pyromellitic diimide macrocycle.

Figure 15: The redox-switchable TTF rotaxane 17 based on a crown/ammonium binding motif.

Figure 16: Structure and operation of the electro- and photochemically switchable rotaxane 18 which acts as po...

Figure 17: (a) The redox-switchable rotaxane 19 with a donor–acceptor pair which is stable in five different s...

Figure 18: Schematic representation of a molecular electronic memory based on a bistable TTF-based rotaxane. (...

Figure 19: Schematic representation of bending motion of a microcantilever beam with gold surface induced by o...

Figure 20: TTF-dimer interactions in a redox-switchable tripodal [4]rotaxane 22.

Figure 21: (a) A molecular friction clutch 23 which can be operated by electrochemical stimuli. (b) Schematic ...

Figure 22: Fusion between rotaxane and catenane: a [3]rotacatenane 24 which can stabilize TTF dimers.

Figure 23: The first TTF-based catenane 25.

Figure 24: Electrochemically controlled circumrotation of the bistable catenane 26.

Figure 25: A tristable switch based on the redox-active [2]catenane 27 with three different stations.

Figure 26: Structure of catenane-functionalized MOF NU-1000 [108] with structural representation of subcomponents. ...

Figure 27: (a) [3]Catenanes 29 and 30 which can stabilize mixed-valence or radical-cation dimers of TTF. (b) S...
Supramolecular chemistry: from aromatic foldamers to solution-phase supramolecular organic frameworks
- Zhan-Ting Li
Beilstein J. Org. Chem. 2015, 11, 2057–2071, doi:10.3762/bjoc.11.222

- cavity of cucurbit[8]uril (CB[8]) are highlighted. Keywords: donor–acceptor interaction; foldamer; hydrogen bond; radical cation dimerization; supramolecular organic framework; Review Childhood and growing up I was born on July 23rd, 1966 in the small, remote village of Fang-Liu (a combination of two
- good solubility in water (Scheme 17). Shortly after, Kangda revealed that the three phenyl-bipyridine units strongly stacked in the two-dimensional space when mixing with 1.5 equiv of cucurbit[8]uril (CB[8]), which could encapsulate the stacked bipyridine dimers [82][83]. In collaboration with Yi Liu

Graphical Abstract

Scheme 1: Proposed structures of complexes between 1a and 1b with 2.

Scheme 2: The formation of catenanes 6a–c.

Scheme 3: The structures of cantenanes 7a–c.

Scheme 4: The structures of dimer 8·8 and compounds 9 and 10.

Figure 1: X-ray structure of 10 showing a quadruple hydrogen-bonded dimeric motif [17].

Scheme 5: The structures of compounds 11a–g.

Figure 2: Zipper-featured folding motif of δ-peptides 11a–g driven by the cooperative donor–acceptor interact...

Scheme 6: The structures of compounds 12a–g and the formation of the helical conformation by the longer oligo...

Scheme 7: The structures of compounds 13a,b, 14, and 15a–d.

Scheme 8:
The structures of complex C60 ![[Graphic 1]](/bjoc/content/inline/1860-5397-11-222-i19.png?max-width=637&scale=1.18182) 16 and dynamic [2]catenane formed by compounds 17–19.
16 and dynamic [2]catenane formed by compounds 17–19.

Scheme 9: The structure of homodimers 20a·20a and 20b·20b.

Scheme 10: The structures of foldamers 21 and 22a–c.

Scheme 11: Complexation-promoted hydrolysis of foldamer 23.

Scheme 12: The structure of foldamer 24.

Scheme 13: The structures of foldamers 24a–c.

Scheme 14: Proposed structures of heterodimers 25·28, 26·28, and 27·28.

Scheme 15: Proposed structure of complex formed by 29 and 30.

Scheme 16: The structures of polymers P31a,b and P32a–d.

Scheme 17: The structure of compound 33.

Scheme 18: The structure of compound 34.
Molecular recognition of organic ammonium ions in solution using synthetic receptors
- Andreas Späth and
- Burkhard König
Beilstein J. Org. Chem. 2010, 6, No. 32, doi:10.3762/bjoc.6.32


Graphical Abstract

Figure 1: Biologically important amines and quaternary ammonium salts: histamine (1), dopamine (2) and acetyl...

Figure 2: Crown ether 18-crown-6.

Figure 3: Conformations of 18-crown-6 (4) in solvents of different polarity.

Figure 4: Binding topologies of the ammonium ion depending on the crown ring size.

Figure 5: A “pseudorotaxane” structure consisting of 24-crown-8 and a secondary ammonium ion (5); R = Ph.

Figure 6: Typical examples of azacrown ethers, cryptands and related aza macrocycles.

Figure 7: Binding of ammonium to azacrown ethers and cryptands [111-113].

Figure 8: A 19-crown-6-ether with decalino blocking groups (11) and a thiazole-dibenzo-18-crown-6-ether (12).

Figure 9: 1,3-Bis(6-oxopyridazin-1-yl)propane derivatives 13 and 14 by Campayo et al.

Figure 10: Fluorescent azacrown-PET-sensors based on coumarin.

Figure 11: Two different pyridino-cryptands (17 and 18) compared to a pyridino-crown (19); chiral ammonium ion...

Figure 12: Pyridino-18-crown-6 ligand (21), a similar acridino-18-crown-6 ligand (22) and a structurally relat...

Figure 13: Ciral pyridine-azacrown ether receptors 24.

Figure 14: Chiral 15-crown-5 receptors 26 and an analogue 18-crown-6 ligand 27 derived from amino alcohols.

Figure 15: C2-symmetric chiral 18-crown-6 amino alcohol derivatives 28 and related macrocycles.

Figure 16: Macrocycles with diamide-diester groups (30).

Figure 17: C2-symmetric chiral aza-18-crown-6 ethers (31) with phenethylamine residues.

Figure 18: Chiral C-pivot p-methoxy-phenoxy-lariat ethers.

Figure 19: Chiral lariat crown ether 34.

Figure 20: Sucrose-based chiral crown ether receptors 36.

Figure 21: Permethylated fructooligosaccharide 37 showing induced-fit chiral recognition.

Figure 22: Biphenanthryl-18-crown-6 derivative 38.

Figure 23: Chiral lariat crown ethers derived from binol by Fuji et al.

Figure 24: Chiral phenolic crown ether 41 with “aryl chiral barriers” and guest amines.

Figure 25: Chiral bis-crown receptor 43 with a meso-ternaphthalene backbone.

Figure 26: Chromogenic pH-dependent bis-crown chemosensor 44 for diamines.

Figure 27: Triamine guests for binding to receptor 44.

Figure 28: Chiral bis-crown phenolphthalein chemosensors 46.

Figure 29: Crown ether amino acid 47.

Figure 30: Luminescent receptor 48 for bis-alkylammonium guests.

Figure 31: Luminescent CEAA (49a), a bis-CEAA receptor for amino acids (49b) and the structure of lysine bindi...

Figure 32: Luminescent CEAA tripeptide for binding small peptides.

Figure 33: Bis crown ether 51a self assembles co-operatively with C60-ammonium ion 51b.

Figure 34: Triptycene-based macrotricyclic dibenzo-[24]-crown-8 ether host 52 and guests.

Figure 35: Copper imido diacetic acid azacrown receptor 53a and the suggested His-Lys binding motif; a copper ...

Figure 36: Urea (54) and thiourea (55) benzo crown receptor for transport and extraction of amino acids.

Figure 37: Crown pyryliums ion receptors 56 for amino acids.

Figure 38: Ditopic sulfonamide bridged crown ether receptor 57.

Figure 39: Luminescent peptide receptor 58.

Figure 40: Luminescent receptor 59 for the detection of D-glucosamine hydrochloride in water/ethanol and lumin...

Figure 41: Guanidinium azacrown receptor 61 for simple amino acids and ditopic receptor 62 with crown ether an...

Figure 42: Chiral bicyclic guanidinium azacrown receptor 63 and similar receptor 64 for the enantioselective t...

Figure 43: Receptors for zwitterionic species based on luminescent CEAAs.

Figure 44: 1,10-Azacrown ethers with sugar podand arms and the anticancer agent busulfan.

Figure 45: Benzo-18-crown-6 modified β-cyclodextrin 69 and β-cyclodextrin functionalized with diaza-18-crown-6...

Figure 46: Receptors for colorimetric detection of primary and secondary ammonium ions.

Figure 47: Porphyrine-crown-receptors 72.

Figure 48: Porphyrin-crown ether conjugate 73 and fullerene-ammonium ion guest 74.

Figure 49: Calix[4]arene (75a), homooxocalix[4]arene (75b) and resorcin[4]arene (75c) compared (R = H, alkyl c...

Figure 50: Calix[4]arene and ammonium ion guest (R = H, alkyl, OAcyl etc.), possible binding sites; A: co-ordi...

Figure 51: Typical guests for studies with calixarenes and related molecules.

Figure 52: Lower rim modified p-tert-butylcalix[5]arenes 82.

Figure 53: The first example of a water soluble calixarene.

Figure 54: Sulfonated water soluble calix[n]arenes that bind ammonium ions.

Figure 55: Displacement assay for acetylcholine (3) with a sulfonato-calix[6]arene (84b).

Figure 56: Amino acid inclusion in p-sulfonatocalix[4]arene (84a).

Figure 57: Calixarene receptor family 86 with upper and lower rim functionalization.

Figure 58: Calix[6]arenes 87 with one carboxylic acid functionality.

Figure 59: Sulfonated calix[n]arenes with mono-substitution at the lower rim systematically studied on their r...

Figure 60: Cyclotetrachromotropylene host (91) and its binding to lysine (81c).

Figure 61: Calixarenes 92 and 93 with phosphonic acids groups.

Figure 62: Calix[4]arene tetraphosphonic acid (94a) and a double bridged analogue (94b).

Figure 63: Calix[4]arene tetraphosphonic acid ester (92c) for surface recognition experiments.

Figure 64: Calixarene receptors 95 with α-aminophosphonate groups.

Figure 65: A bridged homocalix[3]arene 95 and a distally bridged homocalix[4]crown 96.

Figure 66: Homocalix[3]arene ammonium ion receptor 97a and the Reichardt’s dye (97b) for colorimetric assays.

Figure 67: Chromogenic diazo-bridged calix[4]arene 98.

Figure 68: Calixarene receptor 99 by Huang et al.

Figure 69: Calixarenes 100 reported by Parisi et al.

Figure 70: Guest molecules for inclusion in calixarenes 100: DAP × 2 HCl (101a), APA (101b) and Lys-OMe × 2 HC...

Figure 71: Different N-linked peptido-calixarenes open and with glycol chain bridges.

Figure 72: (S)-1,1′-Bi-2-naphthol calixarene derivative 104 published by Kubo et al.

Figure 73: A chiral ammonium-ion receptor 105 based on the calix[4]arene skeleton.

Figure 74: R-/S-phenylalaninol functionalized calix[6]arenes 106a and 106b.

Figure 75: Capped homocalix[3]arene ammonium ion receptor 107.

Figure 76: Two C3 symmetric capped calix[6]arenes 108 and 109.

Figure 77: Phosphorous-containing rigidified calix[6]arene 110.

Figure 78: Calix[6]azacryptand 111.

Figure 79: Further substituted calix[6]azacryptands 112.

Figure 80: Resorcin[4]arene (75c) and the cavitands (113).

Figure 81: Tetrasulfonatomethylcalix[4]resorcinarene (114).

Figure 82: Resorcin[4]arenes (115a/b) and pyrogallo[4]arenes (115c, 116).

Figure 83: Displacement assay for acetylcholine (3) with tetracyanoresorcin[4]arene (117).

Figure 84: Tetramethoxy resorcinarene mono-crown-5 (118).

Figure 85: Components of a resorcinarene based displacement assay for ammonium ions.

Figure 86: Chiral basket resorcin[4]arenas 121.

Figure 87: Resorcinarenes with deeper cavitand structure (122).

Figure 88: Resorcinarene with partially open deeper cavitand structure (123).

Figure 89: Water-stabilized deep cavitands with partially structure (124, 125).

Figure 90: Charged cavitands 126 for tetralkylammonium ions.

Figure 91: Ditopic calix[4]arene receptor 127 capped with glycol chains.

Figure 92: A calix[5]arene dimer for diammonium salt recognition.

Figure 93: Calixarene parts 92c and 129 for the formation molecular capsules.

Figure 94: Encapsulation of a quaternary ammonium cation by two resorcin[4]arene molecules (NMe4+@[75c]2 × Cl−...

Figure 95: Encapsulation of a quaternary ammonium cation by six resorcin[4]arene molecules (NMe3D+@[130]6 × Cl−...

Figure 96: Structure and schematic of cucurbit[6]uril (CB[6], 131a).

Figure 97: Cyclohexanocucurbit[6]uril (CB′[6], 132) and the guest molecule spermine (133).

Figure 98: α,α,δ,δ-Tetramethylcucurbit[6]uril (134).

Figure 99: Structure of the cucurbituril-phthalhydrazide analogue 135.

Figure 100: Organic cavities for the displacement assay for amine differentiation.

Figure 101: Displacement assay methodology for diammonium- and related guests involving cucurbiturils and some ...

Figure 102: Nor-seco-Cucurbituril (±)-bis-ns-CB[6] (140) and guest molecules.

Figure 103: The cucurbit[6]uril based complexes 141 for chiral discrimination.

Figure 104: Cucurbit[7]uril (131c) and its ferrocene guests (142) opposed.

Figure 105: Cucurbit[7]uril (131c) guest inclusion and representative guests.

Figure 106: Cucurbit[7]uril (131c) binding to succinylcholine (145) and different bis-ammonium and bis-phosphon...

Figure 107: Paraquat-cucurbit[8]uril complex 149.

Figure 108: Gluconuril-based ammonium receptors 150.

Figure 109: Examples of clefts (151a), tweezers (151b, 151c, 151d) and clips (151e).

Figure 110: Kemp’s triacid (152a), on example of Rebek’s receptors (152b) and guests.

Figure 111: Amino acid receptor (154) by Rebek et al.

Figure 112: Hexagonal lattice designed hosts by Bell et al.

Figure 113: Bell’s amidinium receptor (156) and the amidinium ion (157).

Figure 114: Aromatic phosphonic acids.

Figure 115: Xylene phosphonates 159 and 160a/b for recognition of amines and amino alcohols.

Figure 116: Bisphosphonate recognition motif 161 for a colorimetric assay with alizarin complexone (163) for ca...

Figure 117: Bisphosphonate/phosphate clip 164 and bisphosphonate cleft 165.

Figure 118: N-Methylpyrazine 166a, N-methylnicotinamide iodide (166b) and NAD+ (166c).

Figure 119: Bisphosphate cavitands.

Figure 120: Bisphosphonate 167 of Schrader and Finocchiaro.

Figure 121: Tweezer 168 for noradrenaline (80b).

Figure 122: Different tripods and heparin (170).

Figure 123: Squaramide based receptors 172.

Figure 124: Cage like NH4+ receptor 173 of Kim et al.

Figure 125: Ammonium receptors 174 of Chin et al.

Figure 126: 2-Oxazolin-based ammonium receptors 175a–d and 176 by Ahn et al.

Figure 127: Racemic guest molecules 177.

Figure 128: Tripods based on a imidazole containing macrocycle (178) and the guest molecules employed in the st...

Figure 129: Ammonium ion receptor 180.

Figure 130: Tetraoxa[3.3.3.3]paracyclophanes 181 and a cyclophanic tetraester (182).

Figure 131: Peptidic bridged paraquat-cyclophane.

Figure 132: Shape-selective noradrenaline host.

Figure 133: Receptor 185 for binding of noradrenaline on surface layers from Schrader et al.

Figure 134: Tetraphosphonate receptor for binding of noradrenaline.

Figure 135: Tetraphosphonate 187 of Schrader and Finocchiaro.

Figure 136: Zinc-Porphyrin ammonium-ion receptors 188 and 189 of Mizutani et al.

Figure 137: Zinc porphyrin receptor 190.

Figure 138: Zinc porphyrin receptors 191 capable of amino acid binding.

Figure 139: Zinc-porphyrins with amino acid side chains for stereoinduction.

Figure 140: Bis-zinc-bis-porphyrin based on Tröger’s base 193.

Figure 141: BINAP-zinc-prophyrin derivative 194 and it’s guests.

Figure 142: Bisaryl-linked-zinc-porphyrin receptors.

Figure 143: Bis-zinc-porphyrin 199 for diamine recognition and guests.

Figure 144: Bis-zinc-porphyrin crown ether 201.

Figure 145: Bis-zinc-porphyrin 202 for stereodiscrimination (L = large substituent; S = small substituent).

Figure 146: Bis-zinc-porphyrin[3]rotaxane and its copper complex and guests.

Figure 147: Dien-bipyridyl ligand 206 for co-ordination of two metal atoms.

Figure 148: The ligand and corresponding tetradentate co-complex 207 serving as enantioselective receptor for a...

Figure 149: Bis(oxazoline)–copper(II) complex 208 for the recognition of amino acids in aqueous solution.

Figure 150: Zinc-salen-complexes 209 for the recognition tertiary amines.

Figure 151: Bis(oxazoline)–copper(II) 211 for the recognition of amino acids in aqueous solution.

Figure 152: Zn(II)-complex of a C2 terpyridine crown ether.

Figure 153: Displacement assay and receptor for aspartate over glutamate.

Figure 154: Chiral complex 214 for a colorimetric displacement assay for amino acids.

Figure 155: Metal complex receptor 215 with tripeptide side arms.

Figure 156: A sandwich complex 216 and its displaceable dye 217.

Figure 157: Lanthanide complexes 218–220 for amino acid recognition.

Figure 158: Nonactin (221), valinomycin (222) and vancomycin (223).

Figure 159: Monesin (224a) and a chiral analogue for enantiodiscrimination of ammonium guests (224b).

Figure 160: Chiral podands (226) compared to pentaglyme-dimethylether (225) and 18-crown-6 (4).

Figure 161: Lasalocid A (228).

Figure 162: Lasalocid derivatives (230) of Sessler et al.

Figure 163: The Coporphyrin I tetraanion (231).

Figure 164: Linear and cyclic peptides for ammonium ion recognition.

Figure 165: Cyclic and bicyclic depsipeptides for ammonium ion recognition.

Figure 166: α-Cyclodextrin (136a) and novocaine (236).

Figure 167: Helical diol receptor 237 by Reetz and Sostmann.

Figure 168: Ammonium binding spherand by Cram et al. (238a) and the cyclic[6]metaphenylacetylene 238b in compar...

Figure 169: Receptor for peptide backbone and ammonium binding (239).

Figure 170: Anion sensor principle with 3-hydroxy-2-naphthanilide of Jiang et al.

Figure 171: 7-bromo-3-hydroxy-N-(2-hydroxyphenyl)naphthalene 2-carboxamide (241) and its amine binding.

Figure 172: Naturally occurring catechins with affinity to quaternary ammonium ions.

Figure 173: Spiropyran (244) and merocyanine form (244a) of the amino acid receptors of Fuji et al.

Figure 174: Coumarin aldehyde (245) and its iminium species with amino acid bound (245a) by Glass et al.

Figure 175: Coumarin aldehyde appended with boronic acid.

Figure 176: Quinolone aldehyde dimers by Glass et al.

Figure 177: Chromogenic ammonium ion receptors with trifluoroacetophenone recognition motifs.

Figure 178: Chromogenic ammonium ion receptor with trifluoroacetophenone recognition motif bound on different m...






