Abstract
Substituted imidazoles are readily prepared by condensing the versatile isocyanide Asmic, anisylsulfanylmethylisocyanide, with nitrogenous π-electrophiles. Deprotonating Asmic with lithium hexamethyldisilazide effectively generates a potent nucleophile that efficiently intercepts nitrile and imine electrophiles to afford imidazoles. In situ cyclization to the imidazole is promoted by the conjugate acid, hexamethyldisilazane, which facilitates the requisite series of proton transfers. The rapid formation of imidazoles and the interchange of the anisylsulfanyl for hydrogen with Raney nickel make the method a valuable route to mono- and disubstituted imidazoles.
Graphical Abstract
Introduction
The imidazole core is the seventh most prevalent heterocycle among nitrogen-containing pharmaceuticals [1]. The privileged efficacy of imidazoles emanates from the central role of histidine in biological machinery, particularly as a base at enzymatic active sites [2]. As histidine mimics, imidazole-containing pharmaceuticals are often only N-substituted, as in the fungicides ketoconazole and econazole (Figure 1) [3], or disubstituted as illustrated by the anesthetic etomidate [4] and the antileukemia agent nilotinib [5].
Figure 1: Representative imidazole-containing pharmaceuticals.
Figure 1: Representative imidazole-containing pharmaceuticals.
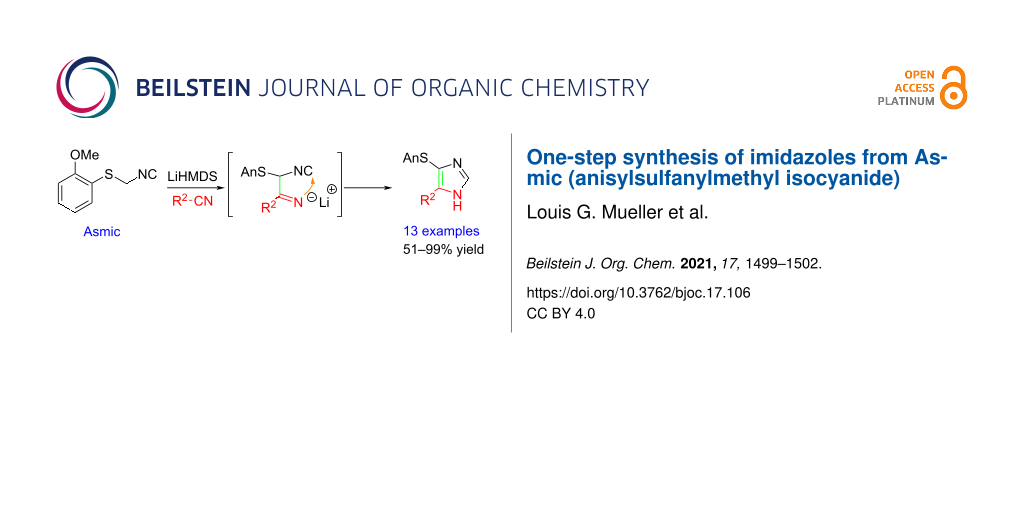
The outstanding and diverse bioactivity of imidazole-containing pharmaceuticals [6], as well as their role as ligands for transition metals [7], and organocatalysis [8], has stimulated an array of creative syntheses [9,10]. Among the numerous routes to imidazoles [11,12], the condensation of metalated isocyanides with nitrogenous π-electrophiles is distinguished by excellent efficiency and modularity. Deprotonating an isocyanide 1 affords an isocyanide-stabilized anion 2 whose condensation with an imidate or nitrile generates a transient imine 3 that readily cyclizes to afford imidazole 4 (Scheme 1). The excellent efficiency is somewhat countered by requiring isocyanides that are readily deprotonated; metalation of alkylisocyanides is challenging except for methyl isocyanide which is why most isocyanides employed in accessing imidazoles contain an adjacent electron withdrawing group (1, R1 = EWG) [13]. Installation of an electron withdrawing group adjacent to an isocyanide facilitates the deprotonation but creates weak nucleophiles 2 that are insufficiently nucleophilic to react with nitriles [14]. Described below is the use of Asmic, anisylsulfanylmethylisocyanide (5) [15], whose deprotonation affords a potent nucleophile that reacts directly with nitriles to provide an efficient, general approach to an array of imidazoles; Asmic is a crystalline, virtually odorless isocyanide with the advantage over related methods [16,17] in being readily prepared in fewer steps on at least 20 g scale [18], applicable for the synthesis of several heterocycles [19,20], and able to generate imidazoles from a broad array of nitrile and imidate electrophiles.
Scheme 1: Asmic-condensation approach to imidazoles.
Scheme 1: Asmic-condensation approach to imidazoles.
Results and Discussion
Exploratory deprotonation of Asmic (5) with BuLi followed by addition of butyronitrile afforded an essentially quantitative conversion to imidazole 7a (cf. Table 1, entry 1). An analogous reaction with benzonitrile gave a significantly lower yield of imidazole 7f (cf. Table 1, entry 6) suggesting that the cleaner reaction profile with butyronitrile might have benefited from the acidic methylene protons of butyronitrile functioning as a proton shuttle during the cyclization cascade. Screening weaker bases with stronger conjugate acids to facilitate the requisite proton transfers identified LiHMDS as optimal; the LiHMDS-promoted condensation of Asmic with benzonitrile afforded imidazole 7f in 93% yield (Table 1, entry 6). Presumably the cyclization of 6 is followed by protonation at the former isocyanide carbon by HMDS (the emerging C-2 of the imidazole) with the reformed LiHMDS deprotonating C-4 to form the imidazole ring.
Table 1: Asmic condensations to imidazoles.
|
|
|||
| Entry | Imidazole | Entry | Imidazole |
| 1 |
7a, (99%) |
7 |
7g, (77%) |
| 2 |
7b, (76%) |
8 |
7h, (65%) |
| 3 |
7c, (88%) |
9 |
7i, X = F (60%) 7j, X = F (80%) 7k, X = F (61%) |
| 4 |
7d, (67%) |
10 |
7l, (78%) |
| 5 |
7e, (57%) |
||
| 6 |
7f, (93%) |
||
aPrepared by trapping with methyl N-phenylformimidate.
Identifying LiHMDS as the optimal base allowed the scope of the Asmic-based imidazole synthesis to be explored (Table 1). Aliphatic nitriles including cyclopropanecarbonitrile, cyclohexanecarbonitrile, and the sterically demanding adamantanecarbonitrile efficiently gave the corresponding imidazoles (7a–e) (Table 1, entries 1–5). Aryl nitriles with electron-donating or withdrawing substituents were competent reaction partners, providing a range of aryl-substituted imidazoles (Table 1, entries 6–10). Most reactions were complete in under one hour, though the less electrophilic p-methoxybenzonitrile required 2.5 h to provide imidazole 7h (Table 1, entry 8). Trapping lithiated Asmic with 1-methyl-1H-indole-3-carbonitrile afforded indole 7l whereas trapping with ethyl N-phenylformimidate afforded the selectively N-1 protected imidazole 7m (Scheme 2) [21]. Collectively, the condensations of lithiated Asmic with nitriles or an imidate provides an efficient route to substituted imidazoles.
Scheme 2: Asmic condensation with methyl N-phenylformimidate.
Scheme 2: Asmic condensation with methyl N-phenylformimidate.
Raney nickel hydrogenolysis was effective in interchanging the C4 anisylsulfanyl group for hydrogen (Scheme 3); attempted lithium–anisylsulfanyl exchange [19] or palladium- [22] or nickel- [23] anisylsulfanyl cross coupling was not successful. Raney nickel reduction of 7f and 7m afforded the monosubsituted imidazoles 8f and 8m, respectively.
Scheme 3: Anisylsulfanylimidazole reduction to monosubstituted imidazoles.
Scheme 3: Anisylsulfanylimidazole reduction to monosubstituted imidazoles.
Conclusion
Deprotonating Asmic with LiHMDS and trapping with nitriles or imidate electrophiles provides a robust, efficient synthesis of imidazoles. The method is rapid, modular and efficient. The anisylsulfanyl substituent serves as a valuable handle to the corresponding C-4 unsubstituted imidazoles providing an efficient route to diverse monosubstituted imidazoles.
References
-
Vitaku, E.; Smith, D. T.; Njardarson, J. T. J. Med. Chem. 2014, 57, 10257–10274. doi:10.1021/jm501100b
Return to citation in text: [1] -
Rebek, J., Jr. Struct. Chem. 1990, 1, 129–131. doi:10.1007/bf00675792
Return to citation in text: [1] -
Shalini, K.; Sharma, P. K.; Kumar, N. Chem. Sin. 2010, 1, 36–47.
Return to citation in text: [1] -
McGrath, M.; Raines, D. E. Methods Enzymol. 2018, 603, 153–169. doi:10.1016/bs.mie.2018.01.026
Return to citation in text: [1] -
García-Ferrer, M.; Wojnicz, A.; Mejía, G.; Koller, D.; Zubiaur, P.; Abad-Santos, F. Clin. Ther. 2019, 41, 2558–2570.e7. doi:10.1016/j.clinthera.2019.10.009
Return to citation in text: [1] -
Rani, N.; Kumar, P.; Singh, R.; de Sousa, D. P.; Sharma, P. Curr. Drug Targets 2020, 21, 1130–1155. doi:10.2174/1389450121666200530203247
Return to citation in text: [1] -
Zhu, L.; Cheng, L.; Zhang, Y.; Xie, R.; You, J. J. Org. Chem. 2007, 72, 2737–2743. doi:10.1021/jo062059f
Return to citation in text: [1] -
Flanigan, D. M.; Romanov-Michailidis, F.; White, N. A.; Rovis, T. Chem. Rev. 2015, 115, 9307–9387. doi:10.1021/acs.chemrev.5b00060
Return to citation in text: [1] -
Soni, J.; Sethiya, A.; Sahiba, N.; Agarwal, D. K.; Agarwal, S. Curr. Org. Synth. 2019, 16, 1078–1104. doi:10.2174/1570179416666191007092548
Return to citation in text: [1] -
Rani, N.; Sharma, A.; Singh, R. Mini-Rev. Org. Chem. 2015, 12, 34–65. doi:10.2174/1570193x11666141028235010
Return to citation in text: [1] -
Lygin, A. V.; de Meijere, A. Angew. Chem., Int. Ed. 2010, 49, 9094–9124. doi:10.1002/anie.201000723
Return to citation in text: [1] -
Katritzky, A. R.; Cheng, D.; Musgrave, R. P. Heterocycles 1997, 44, 67–70. doi:10.3987/com-95-s5
Return to citation in text: [1] -
Altundas, B.; Marrazzo, J.-P. R.; Fleming, F. F. Org. Biomol. Chem. 2020, 18, 6467–6482. doi:10.1039/d0ob01340d
Return to citation in text: [1] -
van Leusen, A. M. An approach with Tosmic has been to form a dianion from 1 which facilitates condensation with nitriles. In The Effect of Sulfur-Substituents on the Chemistry of Alkyl Isocyanides in Perspectives in the Organic Chemistry of Sulfur; Zwanenburg, B.; Klunder, A. J. H., Eds.; Elsevier, 1986; pp 119–144.
Return to citation in text: [1] -
Fleming, F. F.; Altundas, B. Asmic, Anisylsulfanylmethylisocyanide. Encyclopedia of Reagents for Organic Synthesis; 2020. doi:10.1002/047084289x.rn02298
Return to citation in text: [1] -
Ranganathan, S.; Singh, W. P. Tetrahedron Lett. 1988, 29, 1435–1436. doi:10.1016/s0040-4039(00)80317-2
Return to citation in text: [1] -
van Leusen, A. M.; Schut, J. Tetrahedron Lett. 1976, 17, 285–288. doi:10.1016/s0040-4039(00)93710-9
Return to citation in text: [1] -
Alwedi, E.; Altundas, B.; Chao, A.; Ziminsky, Z. L.; Natrayan, M.; Fleming, F. F. Org. Synth. 2021, 98, 147–170. doi:10.15227/orgsyn.098.0147
Return to citation in text: [1] -
Mueller, L. G., Jr.; Chao, A.; Alwedi, E.; Natrajan, M.; Fleming, F. F. Org. Lett. 2021, 23, 1500–1503. doi:10.1021/acs.orglett.1c00288
Return to citation in text: [1] [2] -
Kumar, C. V. S.; Holyoke, C. W., Jr.; Keller, T. M.; Fleming, F. F. J. Org. Chem. 2020, 85, 9153–9160. doi:10.1021/acs.joc.0c01119
Return to citation in text: [1] -
Attempts to trap lithiated Asmic with methyl N-tosylbenzimidate or (Z)-N-benzylbenzimidoyl chloride afforded the corresponding imidazoles in low yield accompanied by numerous side products.
Return to citation in text: [1] -
Liebeskind, L. S.; Srogl, J. Org. Lett. 2002, 4, 979–981. doi:10.1021/ol0200091
Return to citation in text: [1] -
Takei, H.; Miura, M.; Sugimura, H.; Okamura, H. Chem. Lett. 1979, 8, 1447–1450. doi:10.1246/cl.1979.1447
Return to citation in text: [1]
| 22. | Liebeskind, L. S.; Srogl, J. Org. Lett. 2002, 4, 979–981. doi:10.1021/ol0200091 |
| 21. | Attempts to trap lithiated Asmic with methyl N-tosylbenzimidate or (Z)-N-benzylbenzimidoyl chloride afforded the corresponding imidazoles in low yield accompanied by numerous side products. |
| 19. | Mueller, L. G., Jr.; Chao, A.; Alwedi, E.; Natrajan, M.; Fleming, F. F. Org. Lett. 2021, 23, 1500–1503. doi:10.1021/acs.orglett.1c00288 |
| 1. | Vitaku, E.; Smith, D. T.; Njardarson, J. T. J. Med. Chem. 2014, 57, 10257–10274. doi:10.1021/jm501100b |
| 5. | García-Ferrer, M.; Wojnicz, A.; Mejía, G.; Koller, D.; Zubiaur, P.; Abad-Santos, F. Clin. Ther. 2019, 41, 2558–2570.e7. doi:10.1016/j.clinthera.2019.10.009 |
| 18. | Alwedi, E.; Altundas, B.; Chao, A.; Ziminsky, Z. L.; Natrayan, M.; Fleming, F. F. Org. Synth. 2021, 98, 147–170. doi:10.15227/orgsyn.098.0147 |
| 4. | McGrath, M.; Raines, D. E. Methods Enzymol. 2018, 603, 153–169. doi:10.1016/bs.mie.2018.01.026 |
| 19. | Mueller, L. G., Jr.; Chao, A.; Alwedi, E.; Natrajan, M.; Fleming, F. F. Org. Lett. 2021, 23, 1500–1503. doi:10.1021/acs.orglett.1c00288 |
| 20. | Kumar, C. V. S.; Holyoke, C. W., Jr.; Keller, T. M.; Fleming, F. F. J. Org. Chem. 2020, 85, 9153–9160. doi:10.1021/acs.joc.0c01119 |
| 15. | Fleming, F. F.; Altundas, B. Asmic, Anisylsulfanylmethylisocyanide. Encyclopedia of Reagents for Organic Synthesis; 2020. doi:10.1002/047084289x.rn02298 |
| 16. | Ranganathan, S.; Singh, W. P. Tetrahedron Lett. 1988, 29, 1435–1436. doi:10.1016/s0040-4039(00)80317-2 |
| 17. | van Leusen, A. M.; Schut, J. Tetrahedron Lett. 1976, 17, 285–288. doi:10.1016/s0040-4039(00)93710-9 |
| 9. | Soni, J.; Sethiya, A.; Sahiba, N.; Agarwal, D. K.; Agarwal, S. Curr. Org. Synth. 2019, 16, 1078–1104. doi:10.2174/1570179416666191007092548 |
| 10. | Rani, N.; Sharma, A.; Singh, R. Mini-Rev. Org. Chem. 2015, 12, 34–65. doi:10.2174/1570193x11666141028235010 |
| 13. | Altundas, B.; Marrazzo, J.-P. R.; Fleming, F. F. Org. Biomol. Chem. 2020, 18, 6467–6482. doi:10.1039/d0ob01340d |
| 8. | Flanigan, D. M.; Romanov-Michailidis, F.; White, N. A.; Rovis, T. Chem. Rev. 2015, 115, 9307–9387. doi:10.1021/acs.chemrev.5b00060 |
| 14. | van Leusen, A. M. An approach with Tosmic has been to form a dianion from 1 which facilitates condensation with nitriles. In The Effect of Sulfur-Substituents on the Chemistry of Alkyl Isocyanides in Perspectives in the Organic Chemistry of Sulfur; Zwanenburg, B.; Klunder, A. J. H., Eds.; Elsevier, 1986; pp 119–144. |
| 7. | Zhu, L.; Cheng, L.; Zhang, Y.; Xie, R.; You, J. J. Org. Chem. 2007, 72, 2737–2743. doi:10.1021/jo062059f |
| 23. | Takei, H.; Miura, M.; Sugimura, H.; Okamura, H. Chem. Lett. 1979, 8, 1447–1450. doi:10.1246/cl.1979.1447 |
| 6. | Rani, N.; Kumar, P.; Singh, R.; de Sousa, D. P.; Sharma, P. Curr. Drug Targets 2020, 21, 1130–1155. doi:10.2174/1389450121666200530203247 |
| 11. | Lygin, A. V.; de Meijere, A. Angew. Chem., Int. Ed. 2010, 49, 9094–9124. doi:10.1002/anie.201000723 |
| 12. | Katritzky, A. R.; Cheng, D.; Musgrave, R. P. Heterocycles 1997, 44, 67–70. doi:10.3987/com-95-s5 |
© 2021 Mueller et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0). Please note that the reuse, redistribution and reproduction in particular requires that the author(s) and source are credited and that individual graphics may be subject to special legal provisions.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (https://www.beilstein-journals.org/bjoc/terms)