Graphical Abstract
The stereochemistry of some alkene products (2i–k) in Scheme 4 of the original publication was misattributed. The corrected structures are shown in Scheme 1.
Scheme 1: Corrected Scheme 4 of the original article. Scope of the Et3B and the DICAB-initiated SF5Cl additions on alkynes. Unless noted otherwise, isolated yields are reported. aYield estimated by 19F NMR analysis of the crude mixture using 2-fluoro-4-nitrotoluene as an internal standard.
Scheme 1: Corrected Scheme 4 of the original article. Scope of the Et3B and the DICAB-initiated SF5Cl additio...
A corrected version of Supporting Information File 1 is also part of this Correction. The new Supporting Information File 1 is the complete file with the corrections marked in yellow color.
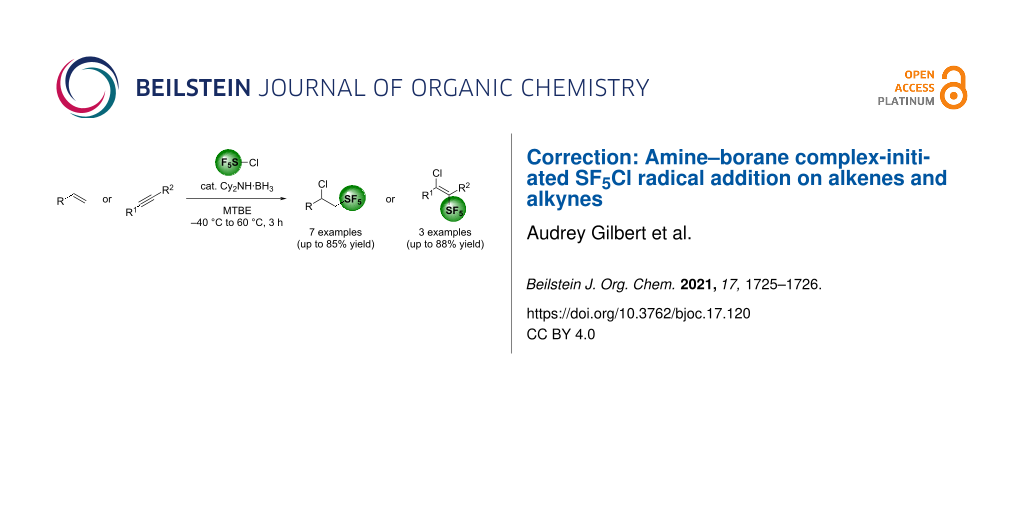
Finally, the Table of Content graphic was also corrected. The corrected version of the original graphical abstract is shown in Scheme 2.
Scheme 2: Corrected graphical abstract of the original publication.
Scheme 2: Corrected graphical abstract of the original publication.
We apologize for any inconvenience caused.
Supporting Information
| Supporting Information File 1: General information, synthetic procedures, additional optimization results, NMR spectra for known compounds (1H, 19F) and full characterization of all new compounds. | ||
| Format: PDF | Size: 2.6 MB | Download |
© 2021 Gilbert et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0). Please note that the reuse, redistribution and reproduction in particular requires that the author(s) and source are credited and that individual graphics may be subject to special legal provisions.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (https://www.beilstein-journals.org/bjoc/terms)