Abstract
Azulene is a non-alternant, aromatic hydrocarbon with many exciting characteristics such as having a dipole moment, bright color, stimuli responsiveness, anti-Kasha photophysics, and a small HOMO–LUMO gap when compared to its isomer, naphthalene. These properties make azulene-containing polymers an intriguing entity in the field of functional polymers, especially for organic electronic applications like organic field-effect transistors (OFET) and photovoltaic (PV) cells. Since azulene has a fused five and seven-membered ring structure, it can be incorporated onto the polymer backbone through either of these rings or by involving both the rings. These azulene-connection patterns can influence the properties of the resulting polymers and the chemical synthesis in comparison to the electrochemical synthesis can be advantageous in obtaining desired patterns of substitution. Hence, this review article presents a comprehensive overview of the developments that have taken place in the last three decades in the field of chemical syntheses of azulene-containing homo- and copolymers, including brief descriptions of their key properties.
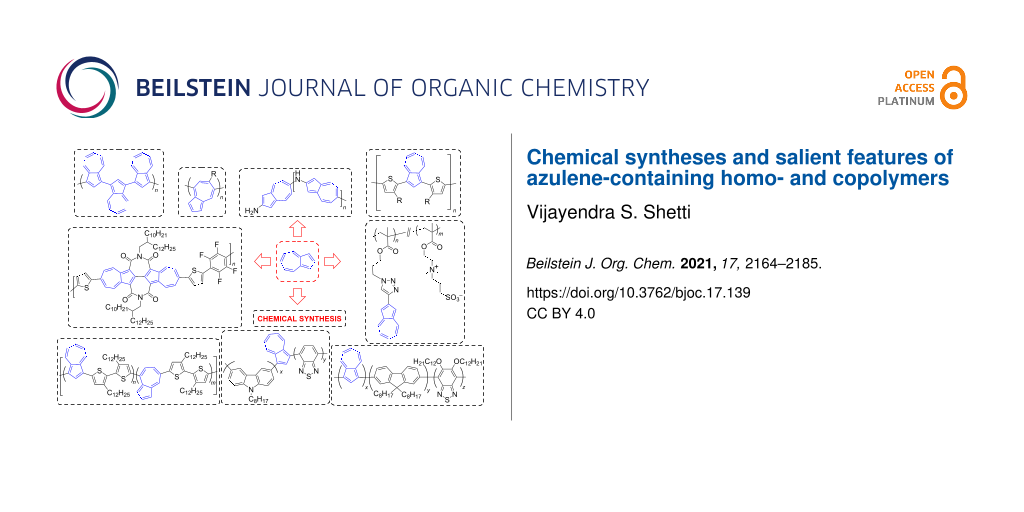
Graphical Abstract
Introduction
Azulene (C10H8) is a non-alternant, non-benzenoid, 10 π electron aromatic hydrocarbon containing a fused seven- and five-membered ring [1-5]. The electron drift from the seven-membered ring to the five-membered ring is responsible for its polarized structure which features both a 6 π electron tropylium cation and a 6 π electron cyclopentadienyl anion in the same molecule (Figure 1).
Figure 1: Chemical structure, numbering scheme, and resonance form of azulene.
Figure 1: Chemical structure, numbering scheme, and resonance form of azulene.
The striking feature of azulene is its permanent dipole moment (1.08 D) and blue color unlike its colorless isomer naphthalene [4]. Azulene possesses an unequal distribution of electron density between its highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) resulting in a relatively small electron repulsion energy in the first singlet excited state and thus, a small HOMO–LUMO (S0–S1) gap compared to naphthalene. The large energy gap between its S2 and S1 states (up to 15000 cm−1) makes internal conversion less probable, making azulene emit from the S2 state, violating Kasha’s rule [6]. These intriguing features have encouraged researchers to use azulene derivatives as functional organic molecules in the field of optoelectronics [7-14]. Employing such stimuli-responsive, non-alternant hydrocarbon with odd-membered rings in the chemical synthesis of functional polymers is also an interesting proposition and such polymers can find promising applications in the organic electronics field such as organic field-effect transistors (OFET) and photovoltaic (PV) cells [15,16]. The synthesis of azulene-containing polymers can be envisaged through chemical and electrochemical means. Through electrochemical methods, only the five-membered rings can be incorporated onto the polymer backbone, and often the polymers produced are insoluble [17]. On the other hand, chemical synthesis provides an avenue to synthesize soluble polymers where azulene can be incorporated into the backbone through either of the rings or by involving both the rings. These substitution patterns can influence the property of the resulting polymer. However, to achieve the synthesis of such homo- and copolymers, suitably tailor-made azulene monomers with specific substitution patterns are needed, and often the synthesis of such building blocks is challenging. This could be the reason why the chemical synthesis of azulene-containing polymers is only sporadically investigated. Hence, this article is intended to provide readers an overview of the developments in the area of chemical synthesis of azulene-containing homo- and copolymers that have been achieved during the last three decades.
Review
Azulene-containing homopolymers
The polyazulenes
The earliest chemical synthesis of polyazulene was reported by Neoh, Kang, and Tan in 1988 [18]. Their strategy was based on the oxidative polymerization of azulene (1) by iodine or bromine to obtain polyazulene–iodine/bromine complexes (PAz-I2/PAz-Br2) (Scheme 1).
Scheme 1: Synthesis of polyazulene-iodine (PAz-I2) and polyazulene-bromine (PAz-Br2) complexes.
Scheme 1: Synthesis of polyazulene-iodine (PAz-I2) and polyazulene-bromine (PAz-Br2) complexes.
The PAZ-I2 complex was found to be insoluble whereas PAZ-Br2 was sparingly soluble in most of the organic solvents. Although no structure was proposed for these polymer complexes, based on the elemental analysis, the authors provided the formula representation C10H6(I2)0.4 for PAZ-I2 and C10H4.9(Br2)0.72 for PAZ-Br2. The gel permeation chromatography (GPC) analysis of the tetrahydrofuran (THF)-soluble fraction of PAZ-Br2 indicated the presence of oligomeric species (average degree of polymerization of ≈7) leaving speculation about a higher degree of polymerization for the THF-insoluble component of PAZ-Br2. The UV–vis spectrum of the chloroform-soluble fraction of PAZ-Br2 displayed absorption bands in the 190–330 nm region like the azulene monomer, and a long tail with a broad peak around 690 nm supporting the oligomeric nature of the soluble fraction. The thermogravimetric analysis (TG) showed that PAZ-I2 was thermally more stable than PAZ-Br2. The electrical conductivity of PAZ-Br2 (5 × 10−3 S/cm) was far too superior compared to PAZ-I2 (10−6 S/cm).
In 1997, Kihara, Fukutomi, and Nakayama [19] reported the synthesis of what they described as ‘true polyazulene’ through a cationic polymerization reaction. Their protocol involved heating the trifluoroacetic acid (TFA) solution of azulene (1) followed by treatment with triethylamine to obtain a brown polymeric product called polyazulene (Scheme 2).
Scheme 2: Synthesis of ‘true polyazulene’ 3 or 3’ by cationic polymerization.
Scheme 2: Synthesis of ‘true polyazulene’ 3 or 3’ by cationic polymerization.
The elemental analysis of this polymer revealed that it existed as a 71:29 mixture of polymer 3 or 3’ bearing a heptafulvene structure (true polyazulene) and cycloheptatrienyl trifluoroacetate 2. The temperature and reaction time determined the overall yield and the Mn of the polyazulene formed. The maximum yield of 89% and the highest Mn = 3600 Da was obtained when azulene was heated at 100 °C for 24 hours in TFA. However, changing the reaction medium from TFA to acetic acid or methane/trifluoromethane sulfonic acid did not facilitate the polyazulene formation. The polyazulene 3 or 3’ was soluble in various organic solvents such as toluene, dichloromethane, tetrahydrofuran (THF), and N,N’-dimethylformamide (DMF). The ‘true polyazulene’ exhibited the conductivity of 5.38 × 10−8 S/cm, which was increased to 8.16 × 10−3 S/cm upon exposure to iodine atmosphere, presumably due to the formation of dehydropolyazulene via oxidative aromatization.
In 2003, Lai and co-workers [20] synthesized 1,3-polyazulene 5 from azulene in two steps as shown in Scheme 3. First, azulene (1) was dibrominated at the 1,3-positions by treatment with N-bromosuccinimide (NBS) and the consequent dehalogenative polycondensation reaction of 1,3-dibromoazulene (4) by an organonickel catalyst (Yamamoto protocol) yielded 1,3-polyazulene 5.
Scheme 3: Synthesis of 1,3-polyazulene 5 by Yamamoto protocol.
Scheme 3: Synthesis of 1,3-polyazulene 5 by Yamamoto protocol.
The polymer was partially soluble in many organic solvents such as chloroform, THF, xylenes, DMF, N-methylpyrrolidone (NMP), and also in Brønsted acids like TFA and conc. H2SO4. The molecular weight (Mn) and polydispersity (PD) of 1,3-polyazulene 5 were 16,400 and 1.15, respectively, as determined by GPC in THF. The presence of resonance signals in the region δ 7.1–8.6 ppm of the 1H NMR spectrum and the close IR spectral resemblance to azulene confirmed the integrity of the azulene units in 1,3-polyazulene 5. The robustness of the 1,3-polyazulene backbone was evidenced by TG analysis where it retained over 60% of its mass even after heating to 1000 °C. The absorption spectrum recorded in chloroform revealed a considerable red shift in the absorption band of 1,3-polyazulene 5 (417 nm) compared to azulene (1, 341 nm) supporting the presence of extended conjugation prevailing in the polymer. The absorption and EPR spectral patterns of 1,3-polyazulene 5 recorded in TFA and H2SO4 were contrasting to each other inferring the varied degree of protonation caused by these acids on the polymer backbone. The direct current (DC) conductivity of protonated and iodine-doped 1,3-polyazulene 5 (0.74 and 1.22 S/cm respectively) was significantly higher than its neutral form (<10−11 S/cm). The increased conductivity of 1,3-polyazulene 5 upon protonation can be attributed to the better stabilization of cation radicals, di-, and polycations by the unique dipolar nature of azulene, and in the case of iodine doping, it was attributed to the strengthened spin–spin interaction arising due to a high radical concentration. Recently, it was also established that the water-dispersible poly(1,3-azulene)-polystyrenesulfonate (PSS) polymer can display an overall conductivity up to 0.1 S/cm under ambient conditions and an ion Seebeck coefficient value as high as 4.5 mV K−1 [21].
In 2012, Hawker and co-workers [22] reported the synthesis of 4,7-polyazulenes 17–20 having backbone connectivity through the seven-membered ring of azulene. The key precursors required in this synthesis, 4,7-dibromo-6-(n-alkyl)azulenes 12–14 were synthesized by treating 2,5-dibromo-3-alkylthiophenes 6–8 with HOF·CH3CN followed by reaction with dimethylaminofulvene (Scheme 4).
Scheme 4: Synthesis of 4,7-dibromo-6-(n-alkyl)azulenes 12–14.
Scheme 4: Synthesis of 4,7-dibromo-6-(n-alkyl)azulenes 12–14.
The synthesis of 4,7-diethynyl-6-(n-dodecyl)azulene (16) is shown in Scheme 5A. The Sonogashira cross-coupling reaction between 4,7-dibromo-6-(n-dodecyl)azulene (13) and 4,7-diethynyl-6-(n-dodecyl)azulene (16) yielded 4,7-polyazulene 17 linked through ethynyl bridges (Scheme 5B). Similarly, the Yamamoto cross-coupling reaction carried out on 12–14 yielded the directly connected 4,7-polyazulenes 18–20 in 60–79% yields (Scheme 6).
Scheme 5: Synthesis of (A) 4,7-diethynyl-6-(n-dodecyl)azulene (16) and (B) 4,7-polyazulene 17 containing an ethynyl spacer.
Scheme 5: Synthesis of (A) 4,7-diethynyl-6-(n-dodecyl)azulene (16) and (B) 4,7-polyazulene 17 containing an e...
Scheme 6: Synthesis of directly connected 4,7-polyazulenes 18–20.
Scheme 6: Synthesis of directly connected 4,7-polyazulenes 18–20.
The majority of these polymers were soluble in common organic solvents and the GPC data obtained in chloroform showed their Mn to be in the range of 2.4 to 7.2 KDa. These polymers possessed high thermal stability as well. The absorption maxima displayed by polymer 18 (409 nm) in dichloromethane solution was comparable to the above-discussed 1,3-polyazulene 5 (404 nm), however, the long alkyl substituents present at the 6-position of 19 and 20 were disrupting the effective π-conjugation along the polymer backbone, resulting in the blue shift of absorption bands (λmax around 350 nm). Interestingly, the ethynyl linker in 17 was assisting the effective delocalization despite the presence of the n-alkyl group at position 6, as evidenced by its large red-shifted absorption at 456 nm compared to 19. The protonation of 17 and 18 by TFA resulted in a large red-shifted broad absorption band in the 500–900 nm region due to the formation of azulenium cations in the polymer backbone unlike the 1,3-polyazulene 5, which had no significant effect on its absorption spectrum upon protonation. The HOMO–LUMO gap for the protonated 17 (1.32 eV) was also reduced compared to its neutral form (1.49 eV). These polymers, unlike 1,3-polyazulene 5, displayed reversible acid–base chemistry. With the help of absorption spectroscopy and EPR analysis, the authors could establish the fact that effective stabilization of 6 π-electron tropylium cations can be achieved by incorporating the seven-membered rings of azulene in the polymer backbone. The enhanced stability of these 4,7-polyazulenes 17–20 in acids and their stimuli-responsible behavior make them potential materials for photonic device applications.
Poly[2,6-aminoazulene]
In order to construct polyazulenes with the head-to-tail alignment of dipoles, azulene should be functionalized at the 2,6-positions, which are diagonally opposite to each other. This will lead to an alternate arrangement of seven and five-membered rings along the polymer backbone and can facilitate effective electron delocalization along the C2 axis (C2v symmetry) passing through 2,6-positions of azulene [23-25]. The first chemical synthesis of such polyazulene called poly[2,6-aminoazulene] 31 was reported by Luh and co-workers [26] in 2017 and achieved by using the Buchwald–Hartwig reaction protocol (Scheme 7). The synthesis of the key building blocks 2-aminoazulene (24), 2-amino-6-bromoazulene (26), and its corresponding carbamate, tert-butyl N-(6-bromoazulen-2-yl)carbamate (27), used in their synthesis is presented in Scheme 7A. The carbamate 27 was subjected to a Buchwald–Hartwig reaction by using [Pd(allyl)Cl]2 and JackiePhos as a ligand to obtain the polymer 30 in 52% yield. The deprotection of the N-Boc functionality led to the formation of poly[2,6-aminoazulene] 31 in excellent yields (Scheme 7C).
Scheme 7: Synthesis of (A) tert-butyl N-(6-bromoazulen-2-yl)carbamate (27), (B) dimeric aminoazulene 29, and (C) poly[2,6-aminoazulene] 31.
Scheme 7: Synthesis of (A) tert-butyl N-(6-bromoazulen-2-yl)carbamate (27), (B) dimeric aminoazulene 29, and ...
The N-Boc-protected polymer 30 possessed good solubility in organic solvents and its Mn and PDI were 2900 Da and 1.22, respectively. The aminoazulene dimer 29 was also synthesized (Scheme 7B) in this study as a reference compound. When compared to the dimeric aminoazulene 29 (481 nm) and 2-aminoazulene (24, 394 nm), the polyaminoazulene 31 exhibited a far red-shifted broad absorption band spanning 400–800 nm in NMP solution with a maximum at 591 nm. Accordingly, the optical energy gap for the polymer 31 was the lowest (1.65 eV) in comparison to dimer 29 (2.15 eV) and monomer, 2-aminoazulene (24, 3.15 eV) emphasizing the advantage associated with inducing a 2,6-connectivity along the polymer backbone.
The iminium zwitterion resonance structure (Figure 2) can contribute in extending the conjugation along the backbone of poly[2(6)-aminoazulene] 31 and this property of 31 makes it very different from the well-known polyaniline. The absorption peak positions of the protonated forms of monomer 24, dimer 29, and polyazulene 31 were further red-shifted and their respective energy gaps were also reduced compared to their neutral forms. The oxidation potential for the neutral (−0.23 V) and protonated forms of polyaminoazulene 31 (0.70 V) was significantly different unlike the case of protonated emeraldine. The Nafion membrane doped with protonated polyaminoazulene 31 displayed good proton conductivity and reduced methanol permeability, making it suitable for methanol fuel cell applications.
Figure 2: Iminium zwitterionic resonance forms of poly[2(6)-aminoazulene] 31.
Figure 2: Iminium zwitterionic resonance forms of poly[2(6)-aminoazulene] 31.
Azulene-containing copolymers
Azulene-thiophene/bithiophene copolymers
Lai and co-workers [27,28], in 2004, reported the synthesis of conjugated polymers containing azulene and bithiophene units, called poly{1,3-bis[2-(3-alkylthienyl)]azulene} 33–38. The 1,3-bis(3-alkylthienyl)azulene 32, synthesized by the Grignard reaction of 1,3-dibromoazulene (4) with 2-bromo-3-alkylthiophene, was subjected to the oxidative polymerization by using ferric chloride to obtain polymers 33–38 in 42–58% yields as shown in Scheme 8.
Scheme 8: Synthesis of poly{1,3-bis[2-(3-alkylthienyl)]azulene} 33–38.
Scheme 8: Synthesis of poly{1,3-bis[2-(3-alkylthienyl)]azulene} 33–38.
These polymers possessed good thermal stability (Td > 400 °C) and most of them were soluble in organic solvents such as THF, chloroform, dichloromethane, xylene, and toluene. The number-average molecular weights (Mn) of polymers 33–38 were in the range 16,000 to 41,000 Da (determined by GPC in THF) and the degree of polymerization was 40–60. The conjugation between the azulene-bithiophene units along the polymer backbone resulted in the red-shifted absorption bands compared to their corresponding monomers and, upon protonation, the absorption bands were even extended to the NIR region [29]. The conductivity studies performed on either iodine-doped (p-doping or oxidative doping) or protonated polymers (by using TFA) showed higher conductivities in comparison to most of the poly(thiophene-arylene) copolymers [30,31], emphasizing the role of the azulene units in stabilizing the polarons or bipolarons formed during the doping process. In particular, among the iodine-doped polymers, 38 displayed a maximum conductivity of 2.23 S/cm with 45% iodine uptake, and the protonated polymer 36 recorded a conductivity value of 1.10 S/cm. The electrochemical impedance spectroscopy (EIS) studies revealed that these polymers could be used as coatings in corrosion control applications as their conductivity was significantly enhanced (103–104-fold) when kept in contact with the aqueous acid solution.
Further, the coordination of polymers 37 and 39 to a multinuclear Ru cluster was investigated by the same group [32]. The organometallic complexes 40–43 were synthesized by treating polymers 37 and 39 with Ru3(CO)12 in refluxing xylene (Scheme 9).
Scheme 9: Synthesis of polymer ruthenium complexes 40–43.
Scheme 9: Synthesis of polymer ruthenium complexes 40–43.
The ruthenium content in these complexes can be varied by changing the ratio of reactants during the reaction. The 1H NMR chemical shift values of the azulene unit were used as a tool to determine the extent of ruthenium coordination to the azulene units in the polymer backbone as the coordinated azulene displays upfield-shifted resonance signals when compared to free azulenes. The absorption and electrochemical studies conducted on these complexes 40–43 revealed that their properties could be tuned by varying the ruthenium content in the polymer. A higher ruthenium content was inducing a larger blue-shift of the absorption bands and larger cathodic shift of the oxidation potential in these polymers compared to their metal-free counterparts.
In 2012, Hawker and co-workers [22], along with 17–20, also synthesized a polymer 45 which had azulene and thiophene units connected via the 4,7-positions of azulene. The reaction of 4,7-dibromo-6-(n-dodecyl)azulene (13) with 2,5-bis(trimethylstannyl)thiophene (44) under Stille reaction conditions produced the polymer 45 in 62% yield (Scheme 10).
Scheme 10: Synthesis of 4,7-polyazulenes 45 containing a thienyl linker.
Scheme 10: Synthesis of 4,7-polyazulenes 45 containing a thienyl linker.
The Mn and PDI for polymer 45 were 5100 Da and 1.4, respectively and its thermal stability was excellent (Td = 404 °C). Polymer 45 exhibited reversible acid–base response, which was evident from its absorption and EPR studies. The optical HOMO–LUMO gap for 45 was smaller in the protonated form (1.50 eV) compared to its neutral form (1.62 eV).
In 2014, Wang, He, and co-workers [33,34] synthesized the conjugated polymers containing poly(thienyl-azulene) units capable of absorbing in the near IR region (1.0 to 2.5 μm). Their synthetic strategy involved employing 1,3-dibromo-[2-(3-dodecylthien-2-yl)]azulene (46) as a key precursor and Suzuki and Stille cross-coupling reactions as polymerization tools. The polymer 50 was synthesized from the 1,3-dibromo-[2-(3-dodecylthien-2-yl)]azulene (46) by using two successive Suzuki coupling reactions with thiophene boron ester 47 and biphenyl-4,4’-diboronic acid bis(pinacol) ester 49 (Scheme 11), whereas the Suzuki coupling of 46 with 2,1,3-benzothiadiazole-4,7-bis(boronic acid pinacol ester) 51 yielded the polymer 52 in good yields (Scheme 11). The other two polymers 54 and 56 were synthesized through Stille coupling reactions of 46 with 2,6-bis(trimethylstannyl)-4,8-bis(2-ethylhexyloxy)benzo[1,2-b:4,5-b’]dithiophene (53) and tin agent 55, respectively, as shown in the Scheme 12.
Scheme 11: Synthesis of azulene-bithiophene 48 and azulene-benzothiadiazole 52 copolymers. Conditions: (a): (i) Na2CO3, Aliquat 336, Pd(PPh3)4, 100 °C, toluene, 90% (ii) NBS, CHCl3/HOAc, −10 °C, 83%, (b): Na2CO3, Aliquat 336, Pd(PPh3)4, toluene, 110 °C, 9%, (c): Na2CO3, Aliquat 336, Pd(PPh3)4, toluene, 110 °C, 12%.
Scheme 11: Synthesis of azulene-bithiophene 48 and azulene-benzothiadiazole 52 copolymers. Conditions: (a): (i...
Scheme 12: Synthesis of azulene-benzodithiophene copolymer 54 and azulene-bithiophene copolymer 56.
Scheme 12: Synthesis of azulene-benzodithiophene copolymer 54 and azulene-bithiophene copolymer 56.
These polymers 50, 52, 54, and 56 displayed good solubility in most of the commonly used organic solvents and GPC analysis revealed their number-average molecular weight (Mn) to be in the range of 25800 to 48600 Da with polydispersity in the range 1.29–1.9. They also exhibited excellent thermal stability as indicated by their decomposition temperature, which was over 350 °C. The polymers 50, 54, and 56 exhibited absorption bands in the 400–450 nm range due to π–π* transition. However, polymer 52, which contains a benzothiadiazole ring was an exception, as it showed a highly red-shifted band at 550 nm. The important feature of these polymers worth highlighting here is the gradual bleaching of bands due to π–π* transition and display of absorption bands in the near-IR region upon protonation with TFA. The protonated forms of these polymers displayed absorption in the region 1900–2500 nm, i.e., almost covering the entire near-IR region of the spectrum, the largest shift of 2500 nm was displayed by the polymer 56. The origin of such absorption bands in the near-IR region can be attributed to an intramolecular charge transfer (ICT) process leading to the lowering of the HOMO–LUMO bandgap [34]. The azulene moiety plays a pivotal role here as it is polar resonance stabilized and protonation leads to a drift in its electron density from the seven-membered ring to the five-membered ring generating a stable tropylium cation making it a strong electron acceptor during the intramolecular charge transfer (ICT) process. These protonated polymers were very stable in solution for a long period of time. All these polymers exhibited good chemical and electrochemical stability and easy film-forming properties, important attributes for fabricating near-IR-based optoelectronic devices.
In 2014, Hawker, Robb, and co-workers [35] reported the synthesis of azulene-bithiophene copolymers 61–65 in which varied ratios of different regioisomers of azulene (1,3- and 4,7-connected) were present (Scheme 13). The synthesis of the key building block 5,5’-bis(trimethylstannyl)-3,3’-didodecyl-2,2’-bithiophene (60) is shown in Scheme 13A. The reaction of the bis-stannylated bithiophene 60 with varying ratios of dibromoazulenes 4 and 12 under Stille reaction conditions furnished the azulene-bithiophene copolymers 61–65 in decent yields (Scheme 13B).
Scheme 13: Synthesis of (A) 5,5’-bis(trimethylstannyl)-3,3’-didodecyl-2,2’-bithiophene (60) and (B) azulene-bithiophene copolymers 61–65 containing varied ratios of different regioisomers of azulene.
Scheme 13: Synthesis of (A) 5,5’-bis(trimethylstannyl)-3,3’-didodecyl-2,2’-bithiophene (60) and (B) azulene-bi...
The final composition of the 1,3- and 4,7-regioisomers of azulene in the polymer chain was determined by 1H NMR spectroscopy. All these polymers 61–65 were soluble in common organic solvents like THF, dichloromethane, and chloroform and had Mn in the range 6000 to 21000 Da with PDI of 1.3–1.6. The polymers 61–65 displayed stimuli-responsive behavior as evidenced by shifting of their absorption bands from the UV region to the near-IR region upon protonation. This property was dependent on the composition of the azulene units and their connection pattern along the polymer backbone. For example, the protonated polymer 62 with 71% 1,3-disubstituted azulene displayed the highest absorption in the near-IR region. Also, the protonated polymer 61 showed strong fluorescence at 480 nm with a large Stokes shift of 155 nm.
In 2015, Zhang, Liu, and co-workers [36] reported the synthesis of conjugated donor–acceptor (D–A)-type azulene-dithienyldiketopyrrolopyrrole (DPP) polymers 69, 71, and 72. The synthesis of one of the key building blocks, 1,3-bisborylated azulene 67 is shown in the Scheme 14. The azulene-DPP polymers containing azulene units connected via 1,3- and 4,7-positions are synthesized in decent yields by using appropriately functionalized azulene and DPP monomers under Suzuki reaction conditions as delineated in Scheme 15.
Scheme 14: Synthesis of 1,3-bisborylated azulene 67.
Scheme 14: Synthesis of 1,3-bisborylated azulene 67.
Scheme 15: Synthesis of D–A-type azulene-DPP copolymers 69, 71, and 72. Conditions: (a) Pd(PPh3)4, K2CO3, Aliquat 336, toluene, 90 °C.
Scheme 15: Synthesis of D–A-type azulene-DPP copolymers 69, 71, and 72. Conditions: (a) Pd(PPh3)4, K2CO3, Aliq...
All these polymers were soluble in organic solvents such as toluene, chloroform, tetrachloroethane, and the Mw for 69, 71, and 72 were 41700, 38100, and 49400 Da with PDI being 3.4, 2.7, and 3.3, respectively. The polymers were thermally stable with Td above 300 °C. The absorption spectra of 69, 71, and 72 in chloroform displayed strong absorption at 667, 670, and 627 nm, and in the thin-film form they were further red-shifted. The optical energy gaps for 69, 71, and 72 were 1.33, 1.38, and 1.23 eV, respectively. Thin-films of 69 and 71, in which azulene was incorporated onto the polymer backbone in a 1,3-fashion, exhibited p-type semiconducting behavior, whereas the polymer 72, in which a 4,7-connectivity pattern of azulene is present, was ambipolar with hole and electron mobilities of 0.062 and 0.021 cm2 V−1 s−1, respectively. The polymer 69 behaved like an electron donor for organic PV cells and the blend of thin-film 69 with PC71BM showed a power conversion efficiency (PCE) of 2.04%.
In 2018, Gao and co-workers [37] reported the synthesis of conjugated polymers containing 2,6-connected azulene units in the polymer backbone. The key monomer, N,N’-bis(2-decyltetradecyl)-6,6’-di(thiophen-2-yl)-[2,2’-biazulene]-1,1’3,3’-tetracarboxdiimide (TBAzDI) 79 to make these polymers was synthesized as shown in the Scheme 16.
Scheme 16: Synthesis of the key precursor TBAzDI 79.
Scheme 16: Synthesis of the key precursor TBAzDI 79.
The monomer 79 was reacted with N-dodecylthieno[3,4-c]pyrrole-4,6-dione (TPD, 80) and 1,2,4,5-tetrafluorobenzene (TFB, 82) via a palladium-catalyzed direct C–H arylation strategy to obtain the polymers 81 and 83, respectively, in good yields (Scheme 17).
Scheme 17: Synthesis of TBAzDI-based polymers 81 and 83. Conditions: (a) P(o-tol)3, Pd2(dba)3, PivOH, Cs2CO3, THF, 80 °C, 68%; (b) P(o-tol)3, Hermann's catalyst, Cs2CO3, THF, 120 °C, 77%.
Scheme 17: Synthesis of TBAzDI-based polymers 81 and 83. Conditions: (a) P(o-tol)3, Pd2(dba)3, PivOH, Cs2CO3, ...
Both polymers 81 and 83 were soluble in solvents such as chloroform and chlorobenzene and their Mn and PDI were found to be 22100 and 13500 Da, and 3.68 and 3.26, respectively. Their thermal stability was excellent as they had Td above 400 °C. The solution-state absorption spectra of the polymers 81 and 83 in o-dichlorobenzene displayed a strong absorption band at 661 and 516 nm, respectively. The thin-film transistors made out of these polymers displayed electron mobility of 0.42 cm2 V−1 s−1 for 81 and 0.24 cm2 V−1 s−1 for 83. The polymer 81 with an electron mobility of 0.42 cm2 V−1 s−1 represents one of the best unipolar n-type polymers for OFET applications. These polymers were also tested as electron acceptors for all-polymer solar cell (PSC) devices, and 81 in particular, showed a power conversion efficiency (PCE) value of 1.82%. These results reiterated the importance of the 2,6-connectivity pattern of azulene units along the polymer backbone.
Very recently, Xu and Png [38] reported azulene-thiophene copolymers, poly(2-arylazulene-alt-thiophene) 99–101, in which the 2-position of the azulene was substituted by an aryl group. To achieve the synthesis of these polymers, 1,3-dibromo-2-arylazulenes 92–98, were first synthesized from azulene-1-carboxylic acid (84) in two steps as shown in Scheme 18A [39]. The treatment of dibromo monomers 96–98 with bis(trimethylstannyl)thiophene (44) under Stille reaction conditions yielded poly(2-arylazulene-alt-thiophene) 99–101 in good yields (Scheme 18B).
Scheme 18: Synthesis of (A) 1,3-dibromo-2-arylazulene 92–98 and (B) 2-arylazulene-thiophene copolymers 99–101.
Scheme 18: Synthesis of (A) 1,3-dibromo-2-arylazulene 92–98 and (B) 2-arylazulene-thiophene copolymers 99–101.
All three polymers possessed good thermal stability and their Mn was in the range of 6900 to 12000 Da. Their absorption maxima were around 350 nm in chloroform solution and were red-shifted (up to 1000 nm) on protonation. The electrochemical HOMO–LUMO gap for polymers 99–101 was in the range 1.2–1.35 eV. The polymers 99 and 100 having an electron-donating aryl group at the 2-position were most sensitive to acid (TFA) and changed their colors drastically. This enhanced acid sensitivity could be attributed to the resonance stabilization of the positive charge on the five-membered ring of the azulene by the electron-donating groups at the 2-position. These polymers were quite robust as they underwent several cycles of electro-cycling before degradation.
Azulene-fluorene copolymers
In 2009, Xu and co-workers [40] synthesized various examples of azulene-fluorene conjugated polymers by using Suzuki cross-coupling reactions. The first set of polymers, poly[2,7-(9,9-dialkylfluorenyl)-alt-(1’,3’-azulenyl)] 106–109 was prepared by the reaction of 1,3-dibromoazulene (4) with 9,9-dialkylfluorene-2,7-bis(trimethyleneborate) 102–105 under Suzuki reaction conditions (Scheme 19A). To make the other two polymers, poly{[1,3-bis(9,9’-dihexylfluoren-2’-yl)azulenyl]-alt-[2”,7”-(9”,9”-dialkylfluorenyl]}, 115 and 116, a key azulene-containing building block, 1,3-bis(7-bromo-9,9-dihexylfluoren-2-yl)azulene (114) was required, and its synthesis was achieved from 2-bromofluorene (110) by following the protocol presented in Scheme 19B. The reaction of 114 with fluorene borates 102 and 104 under Suzuki conditions yielded polymers 115 and 116, respectively (Scheme 19C).
Scheme 19: Synthesis of (A) poly[2,7-(9,9-dialkylfluorenyl)-alt-(1’,3’-azulenyl)] 106–109, (B) 1,3-bis(7-bromo-9,9-dihexylfluoren-2-yl)azulene (114) and (C) poly{[1,3-bis(9,9’-dihexylfluoren-2’-yl)azulenyl]-alt-[2”,7”-(9”,9”-dialkylfluorenyl]} 115 and 116.
Scheme 19: Synthesis of (A) poly[2,7-(9,9-dialkylfluorenyl)-alt-(1’,3’-azulenyl)] 106–109, (B) 1,3-bis(7-bromo...
The solubility of all polymers was good in organic solvents like THF, dichloromethane, chloroform, and toluene, and the thermal data reflected on their high thermal stability (Td 408–434 °C). The number average molecular weight Mn (GPC in THF) and polydispersity of these polymers were in the range of 6800–12500 Da and 1.29–2.21, respectively. The UV–vis absorption features of polymers 115 and 116 were altered upon protonation due to the formation of azulenium cations in the polymer backbone and they displayed a significant color change on protonation. None of these polymers were fluorescent in the neutral form, however, 115 and 116 displayed fluorescence emission at 385 nm in the protonated state. The electrochemical bandgap of these polymers was in the impressive range of 1.57–1.62 eV.
In 2014, Hawker, Robb, and co-workers [35] also reported the synthesis of azulene-fluorene copolymers 117–121, containing varying ratios of 1,3-and 4,7-connected azulene units along the polymer chain (Scheme 20). The key building blocks used in this synthesis were 1,3-dibromoazulene (4), 4,7-dibromoazulene (12), and 9,9-dioctylfluorene-2,7-diboronate 103. The synthesis of the polymers 117–121 were carried out by reacting 9,9-dioctylfluorene-2,7-diboronate 103 with varying ratios of 1,3-dibromoazulene (4) and 4,7-dibromoazulene (12) under Suzuki reaction conditions (Scheme 20).
Scheme 20: Synthesis of azulene-fluorene copolymers 117–121 containing varying ratios of 1,3- and 4,7-connected azulene units.
Scheme 20: Synthesis of azulene-fluorene copolymers 117–121 containing varying ratios of 1,3- and 4,7-connecte...
As stated above in the case of azulene-thiophene polymers 61–65, the final composition of 1,3- and 4,7-regioisomers of azulene in the polymer chain was determined by 1H NMR spectroscopy. All these polymers were soluble in common organic solvents like THF, dichloromethane, and chloroform, and had Mn in the range 9800 to 34300 Da with PDI of 1.6–2.2. The absorption studies conducted on these polymers revealed their stimuli-responsive nature in protonated states and the influence of the substitution pattern of the azulene units on this behavior. The polymer containing entirely 1,3-substituted azulene, 117, exhibited a strong fluorescence at 420 nm in the protonated state and the attenuation of the fluorescence signals took place with the increasing population of 4,7-disubstitued azulene units in the polymer chain. The thin film made up of polymer 121, containing entirely 4,7-disubstituted azulene, showed a reversible and rapid color switching in an acidic environment. This paper presented an interesting strategy of tuning the property of a polymer by incorporating a varying degree of regioisomers of same building block.
Further, in 2019, Gao and co-workers [41] disclosed the synthesis of azulene-fluorene conjugated polymers connected via 2,6-positions of the azulene ring (Scheme 21). The key starting material to make these polymers was 2,6-dibromoazulene (125), which was synthesized from tropolone (21) in five steps (Scheme 21A). The Suzuki–Miyaura coupling reaction of 2,6-dibromoazulene (125) with 2,2’-(9,9-dioctyl-9H-flourene-2,7-diyl)di(1,3,2-dioxaborolane) (103) furnished the polymer 126 in 31% yield (Scheme 21B). The other polymer 129 was obtained from 2,6-dibromoazulene (125) in two steps as presented in Scheme 21C.
Scheme 21: Synthesis of (A) 2,6-dibromoazulene (125), (B) azulene-fluorene copolymer 126, and (C) azulene-fluorene copolymer 129.
Scheme 21: Synthesis of (A) 2,6-dibromoazulene (125), (B) azulene-fluorene copolymer 126, and (C) azulene-fluo...
The two polymers 126 and 129 possessed good solubility in organic solvents and high thermal stability (Td: 418 and 432 °C for 126 and 129, respectively in a nitrogen atmosphere). The Mn and PDI for 126 was 40400 Da and 2.08 and for 129 58300 Da and 1.73, respectively. The response to protonation (TFA) of these polymers was noteworthy as they exhibited rapid and reversible color changes both in solution and thin-film state. The direct current (DC) conductivity values recorded for the thin films of the protonated polymers 126 and 129 were 2.94 and 0.32 S/cm, respectively, far larger than the value noticed for its protonated 1,3-connected counterpart (10−3 S/cm). This is presumably due to the ease of protonation of the 5-membered ring of azulene rings in the polymer as they have vacant 1,3-positions available for protonation.
Xu and Png [38], along with the above-mentioned poly(2-arylazulene-alt-thiophene) 99–101, also reported the synthesis of the next generation of azulene-fluorene conjugated polymers, 131–134 in which the 2-position of azulene was substituted by different aryl groups (Scheme 22). The 1,3-dibromo-2-arylazulenes 92–95 upon reaction with fluorene diboronic acid 130 under Suzuki reaction conditions yielded polymers 131–134 in 51–65% yields (Scheme 22).
Scheme 22: Synthesis of 2-arylazulene-fluorene copolymers 131–134.
Scheme 22: Synthesis of 2-arylazulene-fluorene copolymers 131–134.
These polymers 131–134 were thermally very stable and exhibited Mn and PDI in the range 10300–15100 Da and 1.4–2.3, respectively. Like their predecessors 106–109, their electrochemical bandgap was in the range 1.25–1.60 eV. However, the chemical nature of the aryl substituents situated at the 2-position dictated their ease of protonation with TFA. It was observed that electron-donating substituents present at the 2-position facilitated the protonation whereas electron-withdrawing substituents discouraged the protonation.
In 2013, Xu and co-workers [42] reported the synthesis of azulene-fluorene-benzothiadiazole terpolymers 136–138, containing varying degrees of azulene and benzothiadiazole units in good yields by adopting the Suzuki coupling protocol (Scheme 23).
Scheme 23: Synthesis of azulene-fluorene-benzothiadiazole terpolymers 136–138.
Scheme 23: Synthesis of azulene-fluorene-benzothiadiazole terpolymers 136–138.
The polymers 136–138 were of relatively high molecular weight (Mn in the range 17000–30900 Da) with PDI 1.45–2.03 and had a good thermal stability (Td > 340 °C in N2). The solution-state absorption profiles (in THF solution) of these polymers displayed two bands in the region 328–349 and 392–407 nm and these peak positions were red-shifted in comparison to azulene-fluorene polymers because of the electron transfer from the azulene to benzothiadiazole units. The optical (2.19–2.38 eV) and electrochemical (2.25–2.40 eV) band gaps for the polymers 136–138 were in good agreement with each other and the gap decreased with an increase in the percentage of azulene in the polymer backbone. The thin films of these polymers exhibited electrochromism, where the color changed from yellowish green (neutral and reduced state) to greyish brown (oxidized state) with electrochromic contrasts of 17 and 13% for polymers 137 and 138, respectively, in the NIR region. In addition, the azulene-2,1,3-benzothiadiazole containing D–A-type copolymers have also been used in photovoltaic device applications [43].
Azulene-carbazole copolymers
The same group [44] also synthesized the azulene-carbazole-conjugated polymer 140 and a set of terpolymers 141–144 containing azulene-carbazole-benzothiadiazole, with varying composition of N-alkyl carbazole and benzothiadiazole units by a Suzuki protocol (Scheme 24).
Scheme 24: Synthesis of azulene-carbazole-benzothiadiazole-conjugated polymers 140–144.
Scheme 24: Synthesis of azulene-carbazole-benzothiadiazole-conjugated polymers 140–144.
These polymers exhibited Mn in the range 4200–7200 Da with PDI 1.14–1.38. The oxidation potential of the benzothiadiazole-containing polymers 141–144 was low compared to all-azulene-carbazole polymer 140 due to the electron transfer from azulene to benzothiadiazole and, due to this, they exhibited better electrochromism. An electrochromic device (ECD) constructed with polymer 143 exhibited black to transmissive electrochromism with high contrast.
Azulene-methacrylate copolymers
Emrick and co-workers [45] reported the synthesis of azulene-substituted methacrylate polymers derived from a free radical polymerization strategy, where azulenes were used as pendants. The key starting points to make these polymers were azulene-2-yl methacrylate (146) and triazole-containing azulene methacrylate 150, whose synthesis is outlined in Scheme 25. The azulene-2-yl methacrylate (146) was synthesized in 75% yield from 2-hydroxyazulene (145) by treating it with methacryloyl chloride in chloroform (Scheme 25A). The 2-hydroxyazulene (145) was converted to 2-bromoazulene (147) and subsequently to the TMS acetylene derivative 148 suitable for the ‘click’ reaction [46] to obtain the triazole 149, which was eventually transformed into the triazole-containing azulene methacrylate monomer 150 (Scheme 25B).
Scheme 25: Synthesis of (A) azulene-2-yl methacrylate (146) and (B) the triazole-containing azulene methacrylate 150.
Scheme 25: Synthesis of (A) azulene-2-yl methacrylate (146) and (B) the triazole-containing azulene methacryla...
The monomers 146 and 150 were then subjected to free radical polymerization by using azobis(isobutyronitrile) (AIBN) to obtain the polymers 151 and 152 in 73 and 82% yields, respectively (Scheme 26A and B). The Mn and PDI for these polymers 151 and 152 were 13500 Da, 2.5 and 13600 Da, 2.2, respectively, and their solubility was good in organic solvents. In order to obtain polymers containing a varying degree of pendant azulene units, such as 154, 155, 157, and 158, the authors performed the free radical copolymerization of the monomers 146 and 150 with methyl methacrylate (153) (Scheme 27A and B) and sulfobetaine methacrylate (156) (Scheme 27C and D) in the presence of AIBN.
Scheme 26: Synthesis of (A) azulene methacrylate polymer 151 and (B) triazole-containing azulene methacrylate polymer 152.
Scheme 26: Synthesis of (A) azulene methacrylate polymer 151 and (B) triazole-containing azulene methacrylate ...
Scheme 27: Synthesis of azulene methyl methacrylate polymers 154, 155 (A and B) and azulene-sulfobetaine methacrylate polymers 157, 158 (C and D).
Scheme 27: Synthesis of azulene methyl methacrylate polymers 154, 155 (A and B) and azulene-sulfobetaine metha...
The Mn for the 154 and 155 series was in the range of 13900–22000 Da with a PDI range of 1.7–2.6. Likewise, for the 157 and 158 series the Mn range was 18900–38900 Da with PDI 1.5–1.7. The composition of azulene in the polymers 154 and 155 was 20–40 mol % azulene, whereas the polymers 157 and 158 were containing 25–75 mol % azulene. The photophysical studies carried out on the neutral/protonated polymer series 155 and 158 were supportive of the fact that the optoelectronic property, electronic interactions, and exciton migration can be tuned by varying the density of pendant azulene units along the polymer backbone. The polymers with increasing density of pendant azulene in the backbone displayed improved device performance in comparison to the control devices featuring only poly(sulfobetainemethacrylate) interlayers. The polymer 158 containing the highest azulene density (75 mol % azulene) was found to be an effective cathode modification layer in a bulk-heterojunction solar cell with a power conversion efficiency of 7.9%.
Conclusion
This review has described the chemical syntheses and key features of azulene-containing polymers reported in the last three decades. Azulene-containing homopolymers (polyazulenes) and copolymers incorporating thiophene, fluorene, benzothiadiazole, and carbazole units along the polymer backbone can be synthesized by utilizing cross-coupling strategies such as Suzuki, Sonogashira, Stille, Yamamoto, and Buchwald–Hartwig reactions. Azulene can be incorporated onto the polymer backbone by involving its five-membered rings via a 1,3-fashion, the seven-membered rings via a 4,7-fashion and, both the rings can be incorporated via 2,6-fashion, and these patterns can influence the properties of the resulting polymer. By and large, the reported azulene-containing homo- and copolymers have proven to be efficient functional materials for organic field-effect transistor (OFET), all-polymer solar cell (PSC) applications, and they can also exhibit NIR absorption and electrochromism, and electrical conductivity. However, the reports on polymers containing azulene with a 2,6-connectivity pattern are scarce in the literature. As this mode of connectivity pattern has the advantage of involving both the dipoles of azulene onto the polymer backbone, warrants further investigation. The reported 2,6-polyaminoazulene 31 behaved distinctively to polyanilines and can be a useful proton-conducting membrane material in methanol fuel cells. Also, as azulenes are known to contravene Kasha’s rule, it is intriguing to explore, how their presence in the polymers can favor the energy utilization from higher excited states. This may lead to a new dimension in photoluminescent materials research. It can be concluded that the research field of azulene-containing functional polymers is still in its infancy and there is a lot of scope for chemists and material scientists to design and chemically synthesize exotic azulene-containing polymers having improved characteristics. The author hopes that this review might stimulate such research efforts in the future.
References
-
Gordon, M. Chem. Rev. 1952, 50, 127–200. doi:10.1021/cr60155a004
Return to citation in text: [1] -
Hafner, K. Justus Liebigs Ann. Chem. 1957, 606, 79–89. doi:10.1002/jlac.19576060109
Return to citation in text: [1] -
Lemal, D. M.; Goldman, G. D. J. Chem. Educ. 1988, 65, 923–925. doi:10.1021/ed065p923
Return to citation in text: [1] -
Salter, C.; Foresman, J. B. J. Chem. Educ. 1998, 75, 1341–1345. doi:10.1021/ed075p1341
Return to citation in text: [1] [2] -
Liu, R. S. H. J. Chem. Educ. 2002, 79, 183. doi:10.1021/ed079p183
Return to citation in text: [1] -
Demchenko, A. P.; Tomin, V. I.; Chou, P.-T. Chem. Rev. 2017, 117, 13353–13381. doi:10.1021/acs.chemrev.7b00110
Return to citation in text: [1] -
Xin, H.; Gao, X. ChemPlusChem 2017, 82, 945–956. doi:10.1002/cplu.201700039
Return to citation in text: [1] -
Dong, J.-X.; Zhang, H.-L. Chin. Chem. Lett. 2016, 27, 1097–1104. doi:10.1016/j.cclet.2016.05.005
Return to citation in text: [1] -
Yamaguchi, Y.; Ogawa, K.; Nakayama, K.-i.; Ohba, Y.; Katagiri, H. J. Am. Chem. Soc. 2013, 135, 19095–19098. doi:10.1021/ja410696j
Return to citation in text: [1] -
Wang, F.; Lin, T. T.; He, C.; Chi, H.; Tang, T.; Lai, Y.-H. J. Mater. Chem. 2012, 22, 10448–10451. doi:10.1039/c2jm31098h
Return to citation in text: [1] -
Yamaguchi, Y.; Maruya, Y.; Katagiri, H.; Nakayama, K.-i.; Ohba, Y. Org. Lett. 2012, 14, 2316–2319. doi:10.1021/ol3007327
Return to citation in text: [1] -
Amir, E.; Amir, R. J.; Campos, L. M.; Hawker, C. J. J. Am. Chem. Soc. 2011, 133, 10046–10049. doi:10.1021/ja203267g
Return to citation in text: [1] -
Murai, M.; Ku, S.-Y.; Treat, N. D.; Robb, M. J.; Chabinyc, M. L.; Hawker, C. J. Chem. Sci. 2014, 5, 3753–3760. doi:10.1039/c4sc01623h
Return to citation in text: [1] -
Xin, H.; Hou, B.; Gao, X. Acc. Chem. Res. 2021, 54, 1737–1753. doi:10.1021/acs.accounts.0c00893
Return to citation in text: [1] -
Huang, J.; Huang, S.; Zhao, Y.; Feng, B.; Jiang, K.; Sun, S.; Ke, C.; Kymakis, E.; Zhuang, X. Small Methods 2020, 4, 2000628. doi:10.1002/smtd.202000628
Return to citation in text: [1] -
Zeng, H. N.; Png, Z. M.; Xu, J. Chem. – Asian J. 2020, 15, 1904–1915. doi:10.1002/asia.202000444
Return to citation in text: [1] -
Tourillon, G.; Garnier, F. J. Electroanal. Chem. Interfacial Electrochem. 1982, 135, 173–178. doi:10.1016/0022-0728(82)90015-8
Return to citation in text: [1] -
Neoh, K. G.; Kang, E. T.; Tan, T. C. Polym. Bull. 1988, 19, 325–331. doi:10.1007/bf00263932
Return to citation in text: [1] -
Kihara, N.; Nakayama, H.; Fukutomi, T. Macromolecules 1997, 30, 6385–6387. doi:10.1021/ma970510m
Return to citation in text: [1] -
Wang, F.; Lai, Y.-H.; Kocherginsky, N. M.; Kosteski, Y. Y. Org. Lett. 2003, 5, 995–998. doi:10.1021/ol0274615
Return to citation in text: [1] -
Tang, T.; Kyaw, A. K. K.; Zhu, Q.; Xu, J. Chem. Commun. 2020, 56, 9388–9391. doi:10.1039/d0cc03840g
Return to citation in text: [1] -
Murai, M.; Amir, E.; Amir, R. J.; Hawker, C. J. Chem. Sci. 2012, 3, 2721–2725. doi:10.1039/c2sc20615c
Return to citation in text: [1] [2] -
Dutta, S.; Lakshmi, S.; Pati, S. K. Bull. Mater. Sci. 2008, 31, 353–358. doi:10.1007/s12034-008-0055-8
Return to citation in text: [1] -
Dias, J. R. J. Phys. Org. Chem. 2007, 20, 395–409. doi:10.1002/poc.1159
Return to citation in text: [1] -
Koch, M.; Blacque, O.; Venkatesan, K. J. Mater. Chem. C 2013, 1, 7400–7408. doi:10.1039/c3tc31610f
Return to citation in text: [1] -
Hou, I. C.-Y.; Shetti, V.; Huang, S.-L.; Liu, K.-L.; Chao, C.-Y.; Lin, S.-C.; Lin, Y.-J.; Chen, L.-Y.; Luh, T.-Y. Org. Chem. Front. 2017, 4, 773–778. doi:10.1039/c7qo00087a
Return to citation in text: [1] -
Wang, F.; Lai, Y.-H. Macromolecules 2003, 36, 536–538. doi:10.1021/ma025662i
Return to citation in text: [1] -
Wang, F.; Lai, Y.-H.; Han, M.-Y. Macromolecules 2004, 37, 3222–3230. doi:10.1021/ma035335q
Return to citation in text: [1] -
Tang, T.; Ding, G.; Lin, T.; Chi, H.; Liu, C.; Lu, X.; Wang, F.; He, C. Macromol. Rapid Commun. 2013, 34, 431–436. doi:10.1002/marc.201200695
Return to citation in text: [1] -
Yamamoto, T.; Morita, A.; Miyazaki, Y.; Maruyama, T.; Wakayama, H.; Zhou, Z.-h.; Nakamura, Y.; Kanbara, T.; Sasaki, S.; Kubota, K. Macromolecules 1992, 25, 1214–1223. doi:10.1021/ma00030a003
Return to citation in text: [1] -
Ruiz, J. P.; Dharia, J. R.; Reynolds, J. R.; Buckley, L. J. Macromolecules 1992, 25, 849–860. doi:10.1021/ma00028a053
Return to citation in text: [1] -
Wang, F.; Lai, Y.-H.; Han, M. Y. Org. Lett. 2003, 5, 4791–4794. doi:10.1021/ol0357346
Return to citation in text: [1] -
Tang, T.; Lin, T.; Wang, F.; He, C. Polym. Chem. 2014, 5, 2980–2989. doi:10.1039/c3py01609a
Return to citation in text: [1] -
Tang, T.; Lin, T.; Wang, F.; He, C. J. Phys. Chem. B 2015, 119, 8176–8183. doi:10.1021/acs.jpcb.5b01613
Return to citation in text: [1] [2] -
Tsurui, K.; Murai, M.; Ku, S.-Y.; Hawker, C. J.; Robb, M. J. Adv. Funct. Mater. 2014, 24, 7338–7347. doi:10.1002/adfm.201402554
Return to citation in text: [1] [2] -
Yao, J.; Cai, Z.; Liu, Z.; Yu, C.; Luo, H.; Yang, Y.; Yang, S.; Zhang, G.; Zhang, D. Macromolecules 2015, 48, 2039–2047. doi:10.1021/acs.macromol.5b00158
Return to citation in text: [1] -
Xin, H.; Ge, C.; Jiao, X.; Yang, X.; Rundel, K.; McNeill, C. R.; Gao, X. Angew. Chem., Int. Ed. 2018, 57, 1322–1326. doi:10.1002/anie.201711802
Return to citation in text: [1] -
Mao Png, Z.; Xu, J. Chem. – Asian J. 2020, 15, 2505–2512. doi:10.1002/asia.202000627
Return to citation in text: [1] [2] -
Png, Z. M.; Tam, T. L. D.; Xu, J. Org. Lett. 2020, 22, 5009–5013. doi:10.1021/acs.orglett.0c01576
Return to citation in text: [1] -
Wang, X.; Ng, J. K.-P.; Jia, P.; Lin, T.; Cho, C. M.; Xu, J.; Lu, X.; He, C. Macromolecules 2009, 42, 5534–5544. doi:10.1021/ma900847r
Return to citation in text: [1] -
Gao, H.; Ge, C.; Hou, B.; Xin, H.; Gao, X. ACS Macro Lett. 2019, 8, 1360–1364. doi:10.1021/acsmacrolett.9b00657
Return to citation in text: [1] -
Lim, S. Z. H.; Neo, W. T.; Cho, C. M.; Wang, X.; Tan, A. Y. X.; Chan, H. S. O.; Xu, J. Aust. J. Chem. 2013, 66, 1048–1056. doi:10.1071/ch13147
Return to citation in text: [1] -
Umeyama, T.; Watanabe, Y.; Miyata, T.; Imahori, H. Chem. Lett. 2015, 44, 47–49. doi:10.1246/cl.140904
Return to citation in text: [1] -
Ding, G.; Cho, C. M.; Chen, C.; Zhou, D.; Wang, X.; Tan, A. Y. X.; Xu, J.; Lu, X. Org. Electron. 2013, 14, 2748–2755. doi:10.1016/j.orgel.2013.07.037
Return to citation in text: [1] -
Puodziukynaite, E.; Wang, H.-W.; Lawrence, J.; Wise, A. J.; Russell, T. P.; Barnes, M. D.; Emrick, T. J. Am. Chem. Soc. 2014, 136, 11043–11049. doi:10.1021/ja504670k
Return to citation in text: [1] -
Kolb, H. C.; Finn, M. G.; Sharpless, K. B. Angew. Chem., Int. Ed. 2001, 40, 2004–2021. doi:10.1002/1521-3773(20010601)40:11<2004::aid-anie2004>3.0.co;2-5
Return to citation in text: [1]
| 41. | Gao, H.; Ge, C.; Hou, B.; Xin, H.; Gao, X. ACS Macro Lett. 2019, 8, 1360–1364. doi:10.1021/acsmacrolett.9b00657 |
| 38. | Mao Png, Z.; Xu, J. Chem. – Asian J. 2020, 15, 2505–2512. doi:10.1002/asia.202000627 |
| 42. | Lim, S. Z. H.; Neo, W. T.; Cho, C. M.; Wang, X.; Tan, A. Y. X.; Chan, H. S. O.; Xu, J. Aust. J. Chem. 2013, 66, 1048–1056. doi:10.1071/ch13147 |
| 1. | Gordon, M. Chem. Rev. 1952, 50, 127–200. doi:10.1021/cr60155a004 |
| 2. | Hafner, K. Justus Liebigs Ann. Chem. 1957, 606, 79–89. doi:10.1002/jlac.19576060109 |
| 3. | Lemal, D. M.; Goldman, G. D. J. Chem. Educ. 1988, 65, 923–925. doi:10.1021/ed065p923 |
| 4. | Salter, C.; Foresman, J. B. J. Chem. Educ. 1998, 75, 1341–1345. doi:10.1021/ed075p1341 |
| 5. | Liu, R. S. H. J. Chem. Educ. 2002, 79, 183. doi:10.1021/ed079p183 |
| 15. | Huang, J.; Huang, S.; Zhao, Y.; Feng, B.; Jiang, K.; Sun, S.; Ke, C.; Kymakis, E.; Zhuang, X. Small Methods 2020, 4, 2000628. doi:10.1002/smtd.202000628 |
| 16. | Zeng, H. N.; Png, Z. M.; Xu, J. Chem. – Asian J. 2020, 15, 1904–1915. doi:10.1002/asia.202000444 |
| 29. | Tang, T.; Ding, G.; Lin, T.; Chi, H.; Liu, C.; Lu, X.; Wang, F.; He, C. Macromol. Rapid Commun. 2013, 34, 431–436. doi:10.1002/marc.201200695 |
| 7. | Xin, H.; Gao, X. ChemPlusChem 2017, 82, 945–956. doi:10.1002/cplu.201700039 |
| 8. | Dong, J.-X.; Zhang, H.-L. Chin. Chem. Lett. 2016, 27, 1097–1104. doi:10.1016/j.cclet.2016.05.005 |
| 9. | Yamaguchi, Y.; Ogawa, K.; Nakayama, K.-i.; Ohba, Y.; Katagiri, H. J. Am. Chem. Soc. 2013, 135, 19095–19098. doi:10.1021/ja410696j |
| 10. | Wang, F.; Lin, T. T.; He, C.; Chi, H.; Tang, T.; Lai, Y.-H. J. Mater. Chem. 2012, 22, 10448–10451. doi:10.1039/c2jm31098h |
| 11. | Yamaguchi, Y.; Maruya, Y.; Katagiri, H.; Nakayama, K.-i.; Ohba, Y. Org. Lett. 2012, 14, 2316–2319. doi:10.1021/ol3007327 |
| 12. | Amir, E.; Amir, R. J.; Campos, L. M.; Hawker, C. J. J. Am. Chem. Soc. 2011, 133, 10046–10049. doi:10.1021/ja203267g |
| 13. | Murai, M.; Ku, S.-Y.; Treat, N. D.; Robb, M. J.; Chabinyc, M. L.; Hawker, C. J. Chem. Sci. 2014, 5, 3753–3760. doi:10.1039/c4sc01623h |
| 14. | Xin, H.; Hou, B.; Gao, X. Acc. Chem. Res. 2021, 54, 1737–1753. doi:10.1021/acs.accounts.0c00893 |
| 30. | Yamamoto, T.; Morita, A.; Miyazaki, Y.; Maruyama, T.; Wakayama, H.; Zhou, Z.-h.; Nakamura, Y.; Kanbara, T.; Sasaki, S.; Kubota, K. Macromolecules 1992, 25, 1214–1223. doi:10.1021/ma00030a003 |
| 31. | Ruiz, J. P.; Dharia, J. R.; Reynolds, J. R.; Buckley, L. J. Macromolecules 1992, 25, 849–860. doi:10.1021/ma00028a053 |
| 6. | Demchenko, A. P.; Tomin, V. I.; Chou, P.-T. Chem. Rev. 2017, 117, 13353–13381. doi:10.1021/acs.chemrev.7b00110 |
| 26. | Hou, I. C.-Y.; Shetti, V.; Huang, S.-L.; Liu, K.-L.; Chao, C.-Y.; Lin, S.-C.; Lin, Y.-J.; Chen, L.-Y.; Luh, T.-Y. Org. Chem. Front. 2017, 4, 773–778. doi:10.1039/c7qo00087a |
| 4. | Salter, C.; Foresman, J. B. J. Chem. Educ. 1998, 75, 1341–1345. doi:10.1021/ed075p1341 |
| 27. | Wang, F.; Lai, Y.-H. Macromolecules 2003, 36, 536–538. doi:10.1021/ma025662i |
| 28. | Wang, F.; Lai, Y.-H.; Han, M.-Y. Macromolecules 2004, 37, 3222–3230. doi:10.1021/ma035335q |
| 20. | Wang, F.; Lai, Y.-H.; Kocherginsky, N. M.; Kosteski, Y. Y. Org. Lett. 2003, 5, 995–998. doi:10.1021/ol0274615 |
| 22. | Murai, M.; Amir, E.; Amir, R. J.; Hawker, C. J. Chem. Sci. 2012, 3, 2721–2725. doi:10.1039/c2sc20615c |
| 45. | Puodziukynaite, E.; Wang, H.-W.; Lawrence, J.; Wise, A. J.; Russell, T. P.; Barnes, M. D.; Emrick, T. J. Am. Chem. Soc. 2014, 136, 11043–11049. doi:10.1021/ja504670k |
| 19. | Kihara, N.; Nakayama, H.; Fukutomi, T. Macromolecules 1997, 30, 6385–6387. doi:10.1021/ma970510m |
| 23. | Dutta, S.; Lakshmi, S.; Pati, S. K. Bull. Mater. Sci. 2008, 31, 353–358. doi:10.1007/s12034-008-0055-8 |
| 24. | Dias, J. R. J. Phys. Org. Chem. 2007, 20, 395–409. doi:10.1002/poc.1159 |
| 25. | Koch, M.; Blacque, O.; Venkatesan, K. J. Mater. Chem. C 2013, 1, 7400–7408. doi:10.1039/c3tc31610f |
| 46. | Kolb, H. C.; Finn, M. G.; Sharpless, K. B. Angew. Chem., Int. Ed. 2001, 40, 2004–2021. doi:10.1002/1521-3773(20010601)40:11<2004::aid-anie2004>3.0.co;2-5 |
| 18. | Neoh, K. G.; Kang, E. T.; Tan, T. C. Polym. Bull. 1988, 19, 325–331. doi:10.1007/bf00263932 |
| 43. | Umeyama, T.; Watanabe, Y.; Miyata, T.; Imahori, H. Chem. Lett. 2015, 44, 47–49. doi:10.1246/cl.140904 |
| 17. | Tourillon, G.; Garnier, F. J. Electroanal. Chem. Interfacial Electrochem. 1982, 135, 173–178. doi:10.1016/0022-0728(82)90015-8 |
| 21. | Tang, T.; Kyaw, A. K. K.; Zhu, Q.; Xu, J. Chem. Commun. 2020, 56, 9388–9391. doi:10.1039/d0cc03840g |
| 44. | Ding, G.; Cho, C. M.; Chen, C.; Zhou, D.; Wang, X.; Tan, A. Y. X.; Xu, J.; Lu, X. Org. Electron. 2013, 14, 2748–2755. doi:10.1016/j.orgel.2013.07.037 |
| 33. | Tang, T.; Lin, T.; Wang, F.; He, C. Polym. Chem. 2014, 5, 2980–2989. doi:10.1039/c3py01609a |
| 34. | Tang, T.; Lin, T.; Wang, F.; He, C. J. Phys. Chem. B 2015, 119, 8176–8183. doi:10.1021/acs.jpcb.5b01613 |
| 32. | Wang, F.; Lai, Y.-H.; Han, M. Y. Org. Lett. 2003, 5, 4791–4794. doi:10.1021/ol0357346 |
| 22. | Murai, M.; Amir, E.; Amir, R. J.; Hawker, C. J. Chem. Sci. 2012, 3, 2721–2725. doi:10.1039/c2sc20615c |
| 40. | Wang, X.; Ng, J. K.-P.; Jia, P.; Lin, T.; Cho, C. M.; Xu, J.; Lu, X.; He, C. Macromolecules 2009, 42, 5534–5544. doi:10.1021/ma900847r |
| 35. | Tsurui, K.; Murai, M.; Ku, S.-Y.; Hawker, C. J.; Robb, M. J. Adv. Funct. Mater. 2014, 24, 7338–7347. doi:10.1002/adfm.201402554 |
| 38. | Mao Png, Z.; Xu, J. Chem. – Asian J. 2020, 15, 2505–2512. doi:10.1002/asia.202000627 |
| 39. | Png, Z. M.; Tam, T. L. D.; Xu, J. Org. Lett. 2020, 22, 5009–5013. doi:10.1021/acs.orglett.0c01576 |
| 36. | Yao, J.; Cai, Z.; Liu, Z.; Yu, C.; Luo, H.; Yang, Y.; Yang, S.; Zhang, G.; Zhang, D. Macromolecules 2015, 48, 2039–2047. doi:10.1021/acs.macromol.5b00158 |
| 37. | Xin, H.; Ge, C.; Jiao, X.; Yang, X.; Rundel, K.; McNeill, C. R.; Gao, X. Angew. Chem., Int. Ed. 2018, 57, 1322–1326. doi:10.1002/anie.201711802 |
| 34. | Tang, T.; Lin, T.; Wang, F.; He, C. J. Phys. Chem. B 2015, 119, 8176–8183. doi:10.1021/acs.jpcb.5b01613 |
| 35. | Tsurui, K.; Murai, M.; Ku, S.-Y.; Hawker, C. J.; Robb, M. J. Adv. Funct. Mater. 2014, 24, 7338–7347. doi:10.1002/adfm.201402554 |
© 2021 Shetti; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0). Please note that the reuse, redistribution and reproduction in particular requires that the author(s) and source are credited and that individual graphics may be subject to special legal provisions.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (https://www.beilstein-journals.org/bjoc/terms)