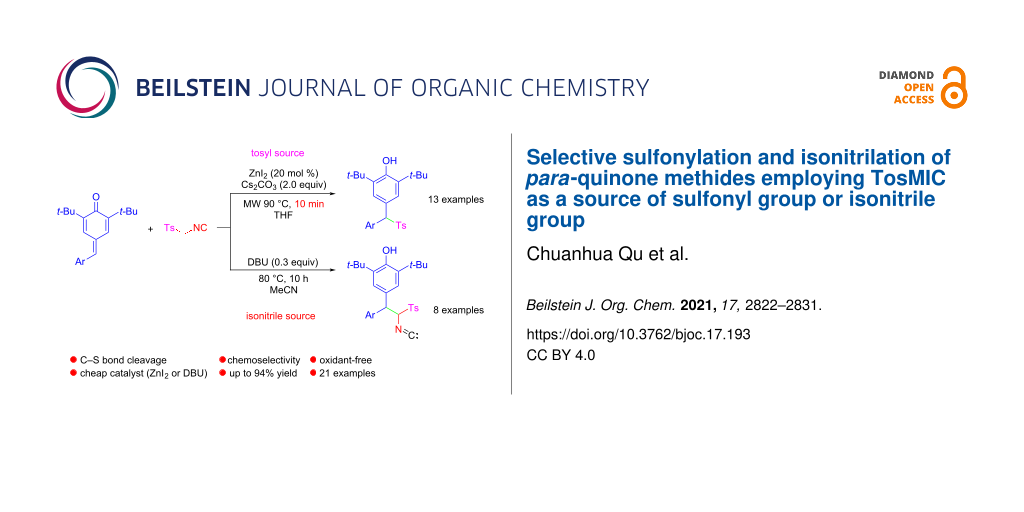
Abstract
Chemoselective sulfonylation and isonitrilation reactions for the divergent synthesis of valuable diarylmethyl sulfones and isonitrile diarylmethanes starting from easy-to-synthesize para-quinone methides (p-QMs) and commercially abundant p-toluenesulfonylmethyl isocyanide (TosMIC) by using respectively zinc iodide and 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) as catalysts were developed. The distinguishing feature of this method is that TosMIC plays a dual role from the same substrates in the reaction: as a sulfonyl source or as an isonitrile source. The synthetic utility of this protocol was also demonstrated in the synthesis of difluoroalkylated diarylmethane 5 and diarylmethane ketone derivatives 6 and 7, which are important core structures in natural products and medicines.
Graphical Abstract
Introduction
Sulfones are ubiquitous units commonly found in marketed drugs and natural products. Because of their unique electronic and structural properties, they are often used in medicinal chemistry programs to search for anti-inflammatory, anti-HIV, antimicrobial, antimalarial, and anticancer activities [1,2]. Diarylmethane motifs are widely present in natural products and pharmaceuticals that exhibit extraordinary biological activity [3,4] (Figure 1). Among them, their anticancer activity is particularly attractive, demonstrated by drugs such as letrozole [5] and entrectinib [6], with especially entrectinib showing a potent anticancer activity against a broad spectrum of human cancer cell lines. In recent decades, the construction of a hybrid system with varied biological and pharmaceutical activities has received extensive attention from medicinal chemists [7]. Therefore, merging the diarylmethane unit with the sulfonyl motif to assemble the sulfonyl diarylmethane skeleton has attractive potential application value and provides the possibility for drug discovery [8-10] (Figure 1). Consequently, the development of a rapid access to diarylmethyl sulfones is a valuable and appealing task in synthetic chemistry.
Traditionally, diarylmethyl sulfones are synthesized by transition-metal-catalyzed deoxy C–S bond-coupling reaction of sodium arylsulfinates with diarylmethanols [11], C–H functionalization of alkyl sulfones with aryl halides [12], and via a reductive strategy through nitrogen loss of sulfonyl hydrazones [13,14]. In addition, a sulfa-1,6-conjugated addition reaction [15-17] has also been developed for this purpose. Most of the reported methods are affected by long reaction times and the need for expensive metal catalysts or reagents. Therefore, there is great need to develop a more effective and rapid method for preparing diaryl methyl sulfones.
p-Toluenesulfonylmethyl isocyanide (TosMIC), a versatile synthon in organic chemistry, has been widely used to synthesize a myriad of valuable chemicals due to its high reactivity shown by the combination of acidic α-carbon atoms, isocyano groups, and sulfonyl moieties [18]. In general, TosMIC undergoes base-mediated 1,3-dipolar cycloadditions with activated alkenes to provide pyrroles as products [18] (Scheme 1A). Recently, alternative functionalizations using TosMIC as a tosyl source of arylalkenes or alkynes provided an attractive option for the synthesis of vinyl sulfones [19-23] (Scheme 1B). However, in contrast to the reaction of TosMIC as tosyl source with various aryl olefins, reports relating to reactions of TosMIC with electron-deficient olefins such as p-QMs for the preparation of highly valuable diarylmethyl sulfones are relatively scarce [24]. In addition, the important link between structural diversity and complexity with bioactivity represented by sulfones has led to the goal of developing strategies to as many these pivotal scaffolds as possible.
Scheme 1: The chemistry of TosMIC in the reactions with olefins.
Scheme 1: The chemistry of TosMIC in the reactions with olefins.
Isocyanide is an important C1 synthon. Its special reactivity, such as the ability to react with electrophilic, nucleophilic, and radical reagents [25-28], determines that it can participate in many types of reactions such as multicomponent reactions [29-32], tandem reactions [33,34], and insertion reactions [35-39], etc. In this context, the electron-rich aryl(phenol)methane isonitrile may be a new active unit, which can be seen from the previous case of p-QMs type reaction [40-51] and the above-mentioned properties of isocyanide.
Herein, we report the chemoselective sulfonylation and isonitrilation of p-QMs by changing the reaction conditions. This new general protocol allows, from the same substrates p-QMs 1 and p-tosylmethyl isocyanide (TosMIC, 2a), the formation of quite different products 3 or 4 in a simple and mild manner, which provides an efficient entry into the rapid assembly of various diarylmethyl sulfones and isonitrile diarylmethanes (Scheme 1C).
Results and Discussion
We commenced the study by using p-QMs 1a and TosMIC (2a) as model substrates to optimize the reaction conditions. As shown in Table 1, when 1a and 2a were treated with 20 mol % Ag2CO3 in THF in the presence of Cs2CO3 at 90 °C under microwave irradiation for 10 min, fortunately, the sulfonylated diarylmethane product 3a was isolated in 36% yield (Table 1, entry 2). When ZnI2 or Cu(OAc)2 was used instead of Ag2CO3, we found that the yield of 3a catalyzed by ZnI2 reached 94% (Table 1, entry 3), and the reaction with Cu2(OAc)2 as a catalyst did not proceed smoothly (Table 1, entry 1). Other bases such as CH3ONa or t-BuONa (Table 1, entries 4 and 5) were then investigated and no better result was found. In the follow-up control experiments, we studied the reaction without adding bases, and unexpectedly found Ag salts could catalyze the 1,6-conjugate addition of TosMIC (2a) and p-QM 1a to provide aryl(phenol)methane isonitrile 4a under base-free conditions (Table 1, entries 6–8). When the silver salt was removed from the reaction conditions, the reaction did not proceed (Table 1, entry 9). Then, we investigated the effect of a catalytic amount of DBU on the reaction and found that reaction efficiency did not decrease, indicating that the reaction could also be catalyzed by DBU (Table 1, entry 10). When DABCO (triethylene diamine) was used instead of DBU, the reaction also proceeded, however, the reaction efficiency was lower compared to DBU (Table 1, entry 11).
Table 1: Optimization of the reaction conditions for the sulfonylation and isonitrilation of p-quinone methides with TosMIC.a
|
|
|||||
| Entry | [M] (20 mol %) | Solvent | Base | Yield of 3a (%)b | Yield of 4a (%)b |
| 1 | Cu(OAc)2 | THF | Cs2CO3 | trace | – |
| 2 | Ag2CO3 | THF | Cs2CO3 | 36 | – |
| 3 | ZnI2 | THF | Cs2CO3 | 94 | – |
| 4 | ZnI2 | THF | CH3ONa | 61 | – |
| 5 | ZnI2 | THF | t-BuONa | 50 | – |
| 6c | Ag2CO3 | MeCN | – | – | 67 (dr = 1:1) |
| 7c | Ag2O | MeCN | – | – | 63 (dr = 1:1) |
| 8c | AgOTs | MeCN | – | – | 81 (dr = 1:1) |
| 9c | – | MeCN | – | – | trace |
| 10c,d | – | MeCN | DBU | – | 82 (dr = 1:1) |
| 11c,d | – | MeCN | DABCO | – | 61 (dr = 1:1) |
aReactions were performed on a 0.2 mmol scale of 1a using 2.0 equiv of 2a, 20 mol % [M], and 2.0 equiv of base, MW, 90 °C, 10 min; byields refer to the products isolated by column chromatography; c80 °C oil bath for 10 h was used; d0.3 equiv DBU or DABCO was used.
After identifying the optimal conditions, we first evaluated the substrate scope of the sulfonylation reaction. As shown in Scheme 2, various substituted p-QMs were readily transformed in this sulfonylation reaction, providing the corresponding sulfonylated diarylmethane derivatives with good to excellent yields. Both electron-donating groups and electron-withdrawing substituents located in the para-, ortho-, or meta-position of the p-QMs were well tolerated and furnished the desired products 3a–m in good yields (81–94% yields). It was noteworthy that p-QMs bearing functional groups, such as methyl, methoxy, tert-butyl, fluoro, chloro, bromo, and trifluoromethyl were well compatible under the optimal reaction conditions. The efficiency of this method was not affected by the pattern of substituents on the phenyl ring. In particular, p-QMs with naphthyl 1l and thienyl moieties 1m provided the products 3l and 3m in good yields (90 and 87%, respectively).
Scheme 2: ZnI2-catalyzed C–S-bond cleavage of TosMIC for the synthesis of diarylmethyl sulfones 3a–m. Reaction conditions unless otherwise specified: 1 (0.2 mmol), 2a (0.4 mmol), ZnI2 (0.04 mmol), Cs2CO3 (0.4 mmol), THF (1.0 mL), MW 90 °C, under air atmosphere for 10 min; yields are reported for the isolated products.
Scheme 2: ZnI2-catalyzed C–S-bond cleavage of TosMIC for the synthesis of diarylmethyl sulfones 3a–m. Reactio...
However, with other substituted p-QMs, such as Bpin, CN, and COOMe at C-4 position, and p-QMs bearing 2,6-diethyl or 2,6-diisopropyl, new main spots were detected by TLC, but they quickly decomposed during column chromatography so that the target products could not be obtained (Scheme 3), which might be due to the easy-to-cleave nature of the p-toluenesulfonyl group.
Scheme 3: Cases encountered by other p-QMs examinations.
Scheme 3: Cases encountered by other p-QMs examinations.
To further confirm the structure of the sulfonylated diarylmethanes, product 3e was chosen as a representative compound and its structure was clearly verified by single crystal X-ray diffraction analysis, as shown in Figure 2 (CCDC No. 2104242).
Figure 2: Crystal structure of diarylmethyl sulfone 3e.
Figure 2: Crystal structure of diarylmethyl sulfone 3e.
Next, the substrate scope of the 1,6-conjugate reaction of TosMIC to p-QMs was examined under optimized conditions (Table 1, entry 10). As depicted in Scheme 4, the substrate scope of p-QMs 1 was first examined. In general, the 1,6-conjugate reaction tolerated a wide range of p-QMs 1, furnishing a series of isonitrile diarylmethanes 4a–h in good to high yields (60–88%) with moderate diastereoselectivity. Substitution of the aryl ring of p-QMs 1 with functional groups such as alkyl, fluoro, bromo, trifluoromethyl, and thiophenyl was generally well tolerated (4a–g). Further, it should be noted that other activated methylene isonitriles such as methyl isocyanoacetate (2b) were also compatible with the reaction conditions providing, for example, the product 4h with a yield of 86% and excellent diastereoselectivity (dr > 19:1).
Scheme 4: DBU-catalyzed 1,6-conjugate addition for the synthesis of isonitrile diarylmethanes 4a–h. Reaction conditions unless otherwise specified: 1 (0.2 mmol), 2 (0.4 mmol), DBU (0.06 mmol), MeCN (1.0 mL), 80 °C, under air atmosphere for 10 h; yields are reported for the isolated products.
Scheme 4: DBU-catalyzed 1,6-conjugate addition for the synthesis of isonitrile diarylmethanes 4a–h. Reaction ...
To further underline the utility of this transformation, several experiments were carried out (Scheme 5). First, the model reaction is scalable. When 1b (3 mmol) and 2a (6 mmol) were mixed under the standard conditions, the desired products 3b or 4b were obtained with yields of 92% and 88%, respectively. Second, the sulfonylated diarylmethane 3b obtained through the C–S bond-cleavage sulfonylation reaction is a versatile building block for preparing diarylmethane derivatives through a nucleophilic substitution process. For example, compound 3b reacted with difluoroenolate to form the difluoroalkylated diarylmethane 5 in 83% yield via a Cu(OAc)2-catalyzed hydrodifluoroalkylation reaction [52]. Two other examples were the use of photoredox catalysis to generate acyl anions in situ from aromatic carboxylic acids via a triphenylphosphine-mediated deoxygenation process, followed by reaction with sulfonylated diarylmethane 3b to obtain diarylmethane ketone derivatives 6 and 7 [53].
Scheme 5: Synthetic applications of the synthesized compound 3b.
Scheme 5: Synthetic applications of the synthesized compound 3b.
To gain mechanistic insight into this C–S-bond cleavage sulfonylation reaction, some control experiments were conducted (Scheme 6). The reaction of 1a with TosMIC derivative 2c, bearing an aromatic ring smoothly occurred to provide product 3a; more importantly, the presence of p-chlorobenzaldehyde (I) released from 2c can be detected by separation and 1H NMR analysis (Scheme 6A). This result indicates that TosMIC may decompose to a Ts anion and formaldehyde, possibly accompanied by the formation of a cyanide ion [54]. The previous reports on the reaction mechanism of TosMIC as a source of Ts are mainly a radical mechanism [19-23]. To assess the possibility of radical intermediates, a stoichiometric amount of the radical inhibitor 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) was subjected to the model reaction system, however, the reaction was not inhibited (Scheme 6B). This result suggests that no radical pathway is involved in this transformation. Based on the above experiments, a proposed mechanism is exemplified in Scheme 6C. The sulfonylation reaction starts with ZnI2/base system mediated C–S-bond cleavage of TosMIC derivative 2c to yield Ts anion II, in which 2c acts as the sulfonyl source. Finally, the sulfonylated diarylmethane 3a is formed by a sequential addition/aromatization process.
Scheme 6: Mechanistic studies and proposed mechanism.
Scheme 6: Mechanistic studies and proposed mechanism.
Conclusion
In conclusion, we have developed a chemoselective sulfonylation and isonitrilation of p-QMs by regulating the reaction conditions. This new general protocol allows completely different products to be formed from the same substrates in a simple and gentle manner, thereby efficiently and quickly assembling various diaryl methyl sulfones and isonitrile diarylmethanes. In the follow-up study, it was found that the isonitrile diarylmethanes are versatile building blocks for the rapid assembly of a series of compounds with novel structures. We are studying this part of the work.
Experimental
General reaction procedure for synthesis of diarylmethyl sulfones 3: In an oven-dried glass tube p-QM 1 (0.2 mmol, 1.0 equiv), TosMIC (p-toluenesulfonyl isonitrile (2a, 0.4 mmol, 2.0 equiv), Cs2CO3 (0.4 mmol, 2.0 equiv), and ZnI2 (0.04 mmol, 0.2 equiv) were dissolved in THF (1 mL). The glass tube was sealed and the reaction mixture was heated under microwave irradiation at 90 °C for 10 min and monitored by TLC until starting material was consumed. Then, the reaction mixture was concentrated under reduced pressure followed by column chromatography over silica gel using petroleum/EtOAc (0 to 10%) as eluent to afford the desired product 3.
General reaction procedure for synthesis of isonitrile diarylmethane 4: In an oven-dried glass tube p-QM 1 (0.2 mmol, 1.0 equiv), TosMIC (p-toluenesulfonyl isonitrile (2a, 0.4 mmol, 2.0 equiv), DBU (9 µL, 0.06 mmol, 0.3 equiv) were dissolved in MeCN (1 mL) and the reaction mixture was stirred at 80 °C for 10 h and monitored by TLC. Then, the reaction mixture was concentrated under reduced pressure followed by column chromatography over silica gel using petroleum/EtOAc 10:1 to ≈5:1 as eluent to afford the desired product 4.
Supporting Information
| Supporting Information File 1: General information, characterization data, and copies of 1H and 13C NMR spectra. | ||
| Format: PDF | Size: 3.1 MB | Download |
Funding
We gratefully acknowledge the National Natural Science Foundation of China (No. 21901027, 21901029), the Science and Technology Research Program of Chongqing Municipal Education Commission (KJQN201901345 and KJQN201901346), the Chongqing Natural Science Foundation Postdoctoral Science Foundation Project (cstc2019jcyj-bshX0053) and the Scientific Research Foundation of the Chongqing University of Arts and Sciences (R2019FXY11 and R2021FYX04).
References
-
Feng, M.; Tang, B.; Liang, S. H.; Jiang, X. Curr. Top. Med. Chem. 2016, 16, 1200–1216. doi:10.2174/1568026615666150915111741
Return to citation in text: [1] -
Scott, K. A.; Njardarson, J. T. Top. Curr. Chem. 2018, 376, 5. doi:10.1007/s41061-018-0184-5
Return to citation in text: [1] -
Canel, C.; Moraes, R. M.; Dayan, F. E.; Ferreira, D. Phytochemistry 2000, 54, 115–120. doi:10.1016/s0031-9422(00)00094-7
Return to citation in text: [1] -
Abrams, P.; Freeman, R.; AnderstrÖm, C.; Mattiasson, A. Br. J. Urol. 1998, 81, 801–810. doi:10.1046/j.1464-410x.1998.00717.x
Return to citation in text: [1] -
Moradan, S.; Nikkhah, N.; Mirmohammadkhanai, M. Adv. Ther. 2017, 34, 1211–1220. doi:10.1007/s12325-017-0509-8
Return to citation in text: [1] -
Drilon, A.; Siena, S.; Dziadziuszko, R.; Barlesi, F.; Krebs, M. G.; Shaw, A. T.; de Braud, F.; Rolfo, C.; Ahn, M.-J.; Wolf, J.; Seto, T.; Cho, B. C.; Patel, M. R.; Chiu, C.-H.; John, T.; Goto, K.; Karapetis, C. S.; Arkenau, H.-T.; Kim, S.-W.; Ohe, Y.; Li, Y.-C.; Chae, Y. K.; Chung, C. H.; Otterson, G. A.; Murakami, H.; Lin, C.-C.; Tan, D. S. W.; Prenen, H.; Riehl, T.; Chow-Maneval, E.; Simmons, B.; Cui, N.; Johnson, A.; Eng, S.; Wilson, T. R.; Doebele, R. C. Lancet Oncol. 2020, 21, 261–270. doi:10.1016/s1470-2045(19)30690-4
Return to citation in text: [1] -
Mehta, G.; Singh, V. Chem. Soc. Rev. 2002, 31, 324–334. doi:10.1039/b204748a
Return to citation in text: [1] -
Gouliaev, A. H.; Slok, F. A.; Teuber, L.; Demnitz, J. Potassium channel modulators. U.S. Patent US7429618B2, Sept 30, 2008.
Return to citation in text: [1] -
Langler, R. F.; Paddock, R. L.; Thompson, D. B.; Crandall, I.; Ciach, M.; Kain, K. C. Aust. J. Chem. 2003, 56, 1127–1133. doi:10.1071/ch03073
Return to citation in text: [1] -
Ito, N.; Kurimura, M.; Yamauchi, T.; Segawa, C.; Sasaki, H.; Tai, K.; Arai, K.; Shinohara, T. In 1-position durch einen Ring substituierte benzo[1,4]diazepine zur Verwendung als Antidepressiva. Int. Pat. Appl. WO 2009/145357 A1, Dec 3, 2009.
Return to citation in text: [1] -
Reddy, M. A.; Reddy, P. S.; Sreedhar, B. Adv. Synth. Catal. 2010, 352, 1861–1869. doi:10.1002/adsc.200900905
Return to citation in text: [1] -
Zhou, G.; Ting, P. C.; Aslanian, R. G. Tetrahedron Lett. 2010, 51, 939–941. doi:10.1016/j.tetlet.2009.12.035
Return to citation in text: [1] -
Feng, X.-W.; Wang, J.; Zhang, J.; Yang, J.; Wang, N.; Yu, X.-Q. Org. Lett. 2010, 12, 4408–4411. doi:10.1021/ol101955x
Return to citation in text: [1] -
Zhao, J.-L.; Guo, S.-H.; Qiu, J.; Gou, X.-F.; Hua, C.-W.; Chen, B. Tetrahedron Lett. 2016, 57, 2375–2378. doi:10.1016/j.tetlet.2016.04.044
Return to citation in text: [1] -
Liu, T.; Liu, J.; Xia, S.; Meng, J.; Shen, X.; Zhu, X.; Chen, W.; Sun, C.; Cheng, F. ACS Omega 2018, 3, 1409–1415. doi:10.1021/acsomega.7b01745
Return to citation in text: [1] -
Guan, X.-Y.; Zhang, L.-D.; You, P.-S.; Liu, S.-S.; Liu, Z.-Q. Tetrahedron Lett. 2019, 60, 244–247. doi:10.1016/j.tetlet.2018.12.023
Return to citation in text: [1] -
Liu, Z.-Q.; You, P.-S.; Zhang, L.-D.; Liu, D.-Q.; Liu, S.-S.; Guan, X.-Y. Molecules 2020, 25, 539. doi:10.3390/molecules25030539
Return to citation in text: [1] -
Kumar, K. ChemistrySelect 2020, 5, 10298–10328. doi:10.1002/slct.202001344
Return to citation in text: [1] [2] -
Phanindrudu, M.; Tiwari, D. K.; Sridhar, B.; Likhar, P. R.; Tiwari, D. K. Org. Chem. Front. 2016, 3, 795–798. doi:10.1039/c6qo00063k
Return to citation in text: [1] [2] -
Kadari, L.; Palakodety, R. K.; Yallapragada, L. P. Org. Lett. 2017, 19, 2580–2583. doi:10.1021/acs.orglett.7b00896
Return to citation in text: [1] [2] -
Chu, X.-Q.; Ge, D.; Loh, T.-P.; Shen, Z.-L. Org. Chem. Front. 2019, 6, 835–840. doi:10.1039/c8qo01346b
Return to citation in text: [1] [2] -
Bounar, H.; Liu, Z.; Zhang, L.; Guan, X.; Yang, Z.; Liao, P.; Bi, X.; Li, X. Org. Biomol. Chem. 2015, 13, 8723–8728. doi:10.1039/c5ob01129a
Return to citation in text: [1] [2] -
Phanindrudu, M.; Jaya, P.; Likhar, P. R.; Tiwari, D. K. Tetrahedron 2020, 76, 131263. doi:10.1016/j.tet.2020.131263
Return to citation in text: [1] [2] -
Kumar, P.; Kale, S. B.; Gonnade, R. G.; Das, U. ChemistrySelect 2021, 6, 7158–7161. doi:10.1002/slct.202102272
Return to citation in text: [1] -
Cai, Z.; Ren, Y.; Li, X.; Shi, J.; Tong, B.; Dong, Y. Acc. Chem. Res. 2020, 53, 2879–2891. doi:10.1021/acs.accounts.0c00514
Return to citation in text: [1] -
Song, B.; Xu, B. Chem. Soc. Rev. 2017, 46, 1103–1123. doi:10.1039/c6cs00384b
Return to citation in text: [1] -
Giustiniano, M.; Basso, A.; Mercalli, V.; Massarotti, A.; Novellino, E.; Tron, G. C.; Zhu, J. Chem. Soc. Rev. 2017, 46, 1295–1357. doi:10.1039/c6cs00444j
Return to citation in text: [1] -
Wilson, R. M.; Stockdill, J. L.; Wu, X.; Li, X.; Vadola, P. A.; Park, P. K.; Wang, P.; Danishefsky, S. J. Angew. Chem., Int. Ed. 2012, 51, 2834–2848. doi:10.1002/anie.201106628
Return to citation in text: [1] -
Cioc, R. C.; Ruijter, E.; Orru, R. V. A. Green Chem. 2014, 16, 2958–2975. doi:10.1039/c4gc00013g
Return to citation in text: [1] -
Dömling, A. Chem. Rev. 2006, 106, 17–89. doi:10.1021/cr0505728
Return to citation in text: [1] -
Slobbe, P.; Ruijter, E.; Orru, R. V. A. Med. Chem. Commun. 2012, 3, 1189–1218. doi:10.1039/c2md20089a
Return to citation in text: [1] -
Sadjadi, S.; Heravi, M. M.; Nazari, N. RSC Adv. 2016, 6, 53203–53272. doi:10.1039/c6ra02143c
Return to citation in text: [1] -
Lygin, A. V.; de Meijere, A. Angew. Chem., Int. Ed. 2010, 49, 9094–9124. doi:10.1002/anie.201000723
Return to citation in text: [1] -
Nair, V.; Rajesh, C.; Vinod, A. U.; Bindu, S.; Sreekanth, A. R.; Mathen, J. S.; Balagopal, L. Acc. Chem. Res. 2003, 36, 899–907. doi:10.1021/ar020258p
Return to citation in text: [1] -
Boyarskiy, V. P.; Bokach, N. A.; Luzyanin, K. V.; Kukushkin, V. Y. Chem. Rev. 2015, 115, 2698–2779. doi:10.1021/cr500380d
Return to citation in text: [1] -
Vlaar, T.; Ruijter, E.; Maes, B. U. W.; Orru, R. V. A. Angew. Chem., Int. Ed. 2013, 52, 7084–7097. doi:10.1002/anie.201300942
Return to citation in text: [1] -
Qiu, G.; Ding, Q.; Wu, J. Chem. Soc. Rev. 2013, 42, 5257–5269. doi:10.1039/c3cs35507a
Return to citation in text: [1] -
Collet, J. W.; Roose, T. R.; Ruijter, E.; Maes, B. U. W.; Orru, R. V. A. Angew. Chem., Int. Ed. 2020, 59, 540–558. doi:10.1002/anie.201905838
Return to citation in text: [1] -
Zhang, B.; Studer, A. Chem. Soc. Rev. 2015, 44, 3505–3521. doi:10.1039/c5cs00083a
Return to citation in text: [1] -
Gai, K.; Fang, X.; Li, X.; Xu, J.; Wu, X.; Lin, A.; Yao, H. Chem. Commun. 2015, 51, 15831–15834. doi:10.1039/c5cc06287j
Return to citation in text: [1] -
Yuan, Z.; Fang, X.; Li, X.; Wu, J.; Yao, H.; Lin, A. J. Org. Chem. 2015, 80, 11123–11130. doi:10.1021/acs.joc.5b01793
Return to citation in text: [1] -
Zhang, X.-Z.; Du, J.-Y.; Deng, Y.-H.; Chu, W.-D.; Yan, X.; Yu, K.-Y.; Fan, C.-A. J. Org. Chem. 2016, 81, 2598–2606. doi:10.1021/acs.joc.5b02725
Return to citation in text: [1] -
Su, Y.; Zhao, Y.; Chang, B.; Ling, Q.; Feng, Y.; Zhao, X.; Huang, D.; Wang, K.-H.; Huo, C.; Hu, Y. Org. Biomol. Chem. 2020, 18, 4257–4266. doi:10.1039/d0ob00778a
Return to citation in text: [1] -
You, Y.; Quan, B.-X.; Wang, Z.-H.; Zhao, J.-Q.; Yuan, W.-C. Org. Biomol. Chem. 2020, 18, 4560–4565. doi:10.1039/d0ob00979b
Return to citation in text: [1] -
Roiser, L.; Waser, M. Org. Lett. 2017, 19, 2338–2341. doi:10.1021/acs.orglett.7b00869
Return to citation in text: [1] -
Winter, M.; Schütz, R.; Eitzinger, A.; Ofial, A. R.; Waser, M. Eur. J. Org. Chem. 2020, 3812–3817. doi:10.1002/ejoc.202000295
Return to citation in text: [1] -
Zhao, S.; Zhu, Y.; Zhang, M.; Song, X.; Chang, J. Synthesis 2019, 51, 2136–2148. doi:10.1055/s-0037-1610691
Return to citation in text: [1] -
Zhang, J.-R.; Jin, H.-S.; Sun, J.; Wang, J.; Zhao, L.-M. Eur. J. Org. Chem. 2020, 4988–4994. doi:10.1002/ejoc.202000830
Return to citation in text: [1] -
Kale, S. B.; Jori, P. K.; Thatikonda, T.; Gonnade, R. G.; Das, U. Org. Lett. 2019, 21, 7736–7740. doi:10.1021/acs.orglett.9b02641
Return to citation in text: [1] -
Yuan, Z.; Gai, K.; Wu, Y.; Wu, J.; Lin, A.; Yao, H. Chem. Commun. 2017, 53, 3485–3488. doi:10.1039/c7cc00677b
Return to citation in text: [1] -
Deng, Y.-H.; Chu, W.-D.; Shang, Y.-H.; Yu, K.-Y.; Jia, Z.-L.; Fan, C.-A. Org. Lett. 2020, 22, 8376–8381. doi:10.1021/acs.orglett.0c02998
Return to citation in text: [1] -
Qu, C.-H.; Song, G.-T.; Tang, D.-Y.; Shao, J.-W.; Li, H.-y.; Xu, Z.-G.; Chen, Z.-Z. J. Org. Chem. 2020, 85, 12785–12796. doi:10.1021/acs.joc.0c01686
Return to citation in text: [1] -
Rossi-Ashton, J. A.; Clarke, A. K.; Unsworth, W. P.; Taylor, R. J. K. ACS Catal. 2020, 10, 7250–7261. doi:10.1021/acscatal.0c01923
Return to citation in text: [1] -
Liu, J.; Liu, Z.; Liao, P.; Bi, X. Org. Lett. 2014, 16, 6204–6207. doi:10.1021/ol5031316
Return to citation in text: [1]
| 40. | Gai, K.; Fang, X.; Li, X.; Xu, J.; Wu, X.; Lin, A.; Yao, H. Chem. Commun. 2015, 51, 15831–15834. doi:10.1039/c5cc06287j |
| 41. | Yuan, Z.; Fang, X.; Li, X.; Wu, J.; Yao, H.; Lin, A. J. Org. Chem. 2015, 80, 11123–11130. doi:10.1021/acs.joc.5b01793 |
| 42. | Zhang, X.-Z.; Du, J.-Y.; Deng, Y.-H.; Chu, W.-D.; Yan, X.; Yu, K.-Y.; Fan, C.-A. J. Org. Chem. 2016, 81, 2598–2606. doi:10.1021/acs.joc.5b02725 |
| 43. | Su, Y.; Zhao, Y.; Chang, B.; Ling, Q.; Feng, Y.; Zhao, X.; Huang, D.; Wang, K.-H.; Huo, C.; Hu, Y. Org. Biomol. Chem. 2020, 18, 4257–4266. doi:10.1039/d0ob00778a |
| 44. | You, Y.; Quan, B.-X.; Wang, Z.-H.; Zhao, J.-Q.; Yuan, W.-C. Org. Biomol. Chem. 2020, 18, 4560–4565. doi:10.1039/d0ob00979b |
| 45. | Roiser, L.; Waser, M. Org. Lett. 2017, 19, 2338–2341. doi:10.1021/acs.orglett.7b00869 |
| 46. | Winter, M.; Schütz, R.; Eitzinger, A.; Ofial, A. R.; Waser, M. Eur. J. Org. Chem. 2020, 3812–3817. doi:10.1002/ejoc.202000295 |
| 47. | Zhao, S.; Zhu, Y.; Zhang, M.; Song, X.; Chang, J. Synthesis 2019, 51, 2136–2148. doi:10.1055/s-0037-1610691 |
| 48. | Zhang, J.-R.; Jin, H.-S.; Sun, J.; Wang, J.; Zhao, L.-M. Eur. J. Org. Chem. 2020, 4988–4994. doi:10.1002/ejoc.202000830 |
| 49. | Kale, S. B.; Jori, P. K.; Thatikonda, T.; Gonnade, R. G.; Das, U. Org. Lett. 2019, 21, 7736–7740. doi:10.1021/acs.orglett.9b02641 |
| 50. | Yuan, Z.; Gai, K.; Wu, Y.; Wu, J.; Lin, A.; Yao, H. Chem. Commun. 2017, 53, 3485–3488. doi:10.1039/c7cc00677b |
| 51. | Deng, Y.-H.; Chu, W.-D.; Shang, Y.-H.; Yu, K.-Y.; Jia, Z.-L.; Fan, C.-A. Org. Lett. 2020, 22, 8376–8381. doi:10.1021/acs.orglett.0c02998 |
| 33. | Lygin, A. V.; de Meijere, A. Angew. Chem., Int. Ed. 2010, 49, 9094–9124. doi:10.1002/anie.201000723 |
| 34. | Nair, V.; Rajesh, C.; Vinod, A. U.; Bindu, S.; Sreekanth, A. R.; Mathen, J. S.; Balagopal, L. Acc. Chem. Res. 2003, 36, 899–907. doi:10.1021/ar020258p |
| 35. | Boyarskiy, V. P.; Bokach, N. A.; Luzyanin, K. V.; Kukushkin, V. Y. Chem. Rev. 2015, 115, 2698–2779. doi:10.1021/cr500380d |
| 36. | Vlaar, T.; Ruijter, E.; Maes, B. U. W.; Orru, R. V. A. Angew. Chem., Int. Ed. 2013, 52, 7084–7097. doi:10.1002/anie.201300942 |
| 37. | Qiu, G.; Ding, Q.; Wu, J. Chem. Soc. Rev. 2013, 42, 5257–5269. doi:10.1039/c3cs35507a |
| 38. | Collet, J. W.; Roose, T. R.; Ruijter, E.; Maes, B. U. W.; Orru, R. V. A. Angew. Chem., Int. Ed. 2020, 59, 540–558. doi:10.1002/anie.201905838 |
| 39. | Zhang, B.; Studer, A. Chem. Soc. Rev. 2015, 44, 3505–3521. doi:10.1039/c5cs00083a |
| 1. | Feng, M.; Tang, B.; Liang, S. H.; Jiang, X. Curr. Top. Med. Chem. 2016, 16, 1200–1216. doi:10.2174/1568026615666150915111741 |
| 2. | Scott, K. A.; Njardarson, J. T. Top. Curr. Chem. 2018, 376, 5. doi:10.1007/s41061-018-0184-5 |
| 25. | Cai, Z.; Ren, Y.; Li, X.; Shi, J.; Tong, B.; Dong, Y. Acc. Chem. Res. 2020, 53, 2879–2891. doi:10.1021/acs.accounts.0c00514 |
| 26. | Song, B.; Xu, B. Chem. Soc. Rev. 2017, 46, 1103–1123. doi:10.1039/c6cs00384b |
| 27. | Giustiniano, M.; Basso, A.; Mercalli, V.; Massarotti, A.; Novellino, E.; Tron, G. C.; Zhu, J. Chem. Soc. Rev. 2017, 46, 1295–1357. doi:10.1039/c6cs00444j |
| 28. | Wilson, R. M.; Stockdill, J. L.; Wu, X.; Li, X.; Vadola, P. A.; Park, P. K.; Wang, P.; Danishefsky, S. J. Angew. Chem., Int. Ed. 2012, 51, 2834–2848. doi:10.1002/anie.201106628 |
| 6. | Drilon, A.; Siena, S.; Dziadziuszko, R.; Barlesi, F.; Krebs, M. G.; Shaw, A. T.; de Braud, F.; Rolfo, C.; Ahn, M.-J.; Wolf, J.; Seto, T.; Cho, B. C.; Patel, M. R.; Chiu, C.-H.; John, T.; Goto, K.; Karapetis, C. S.; Arkenau, H.-T.; Kim, S.-W.; Ohe, Y.; Li, Y.-C.; Chae, Y. K.; Chung, C. H.; Otterson, G. A.; Murakami, H.; Lin, C.-C.; Tan, D. S. W.; Prenen, H.; Riehl, T.; Chow-Maneval, E.; Simmons, B.; Cui, N.; Johnson, A.; Eng, S.; Wilson, T. R.; Doebele, R. C. Lancet Oncol. 2020, 21, 261–270. doi:10.1016/s1470-2045(19)30690-4 |
| 29. | Cioc, R. C.; Ruijter, E.; Orru, R. V. A. Green Chem. 2014, 16, 2958–2975. doi:10.1039/c4gc00013g |
| 30. | Dömling, A. Chem. Rev. 2006, 106, 17–89. doi:10.1021/cr0505728 |
| 31. | Slobbe, P.; Ruijter, E.; Orru, R. V. A. Med. Chem. Commun. 2012, 3, 1189–1218. doi:10.1039/c2md20089a |
| 32. | Sadjadi, S.; Heravi, M. M.; Nazari, N. RSC Adv. 2016, 6, 53203–53272. doi:10.1039/c6ra02143c |
| 5. | Moradan, S.; Nikkhah, N.; Mirmohammadkhanai, M. Adv. Ther. 2017, 34, 1211–1220. doi:10.1007/s12325-017-0509-8 |
| 19. | Phanindrudu, M.; Tiwari, D. K.; Sridhar, B.; Likhar, P. R.; Tiwari, D. K. Org. Chem. Front. 2016, 3, 795–798. doi:10.1039/c6qo00063k |
| 20. | Kadari, L.; Palakodety, R. K.; Yallapragada, L. P. Org. Lett. 2017, 19, 2580–2583. doi:10.1021/acs.orglett.7b00896 |
| 21. | Chu, X.-Q.; Ge, D.; Loh, T.-P.; Shen, Z.-L. Org. Chem. Front. 2019, 6, 835–840. doi:10.1039/c8qo01346b |
| 22. | Bounar, H.; Liu, Z.; Zhang, L.; Guan, X.; Yang, Z.; Liao, P.; Bi, X.; Li, X. Org. Biomol. Chem. 2015, 13, 8723–8728. doi:10.1039/c5ob01129a |
| 23. | Phanindrudu, M.; Jaya, P.; Likhar, P. R.; Tiwari, D. K. Tetrahedron 2020, 76, 131263. doi:10.1016/j.tet.2020.131263 |
| 3. | Canel, C.; Moraes, R. M.; Dayan, F. E.; Ferreira, D. Phytochemistry 2000, 54, 115–120. doi:10.1016/s0031-9422(00)00094-7 |
| 4. | Abrams, P.; Freeman, R.; AnderstrÖm, C.; Mattiasson, A. Br. J. Urol. 1998, 81, 801–810. doi:10.1046/j.1464-410x.1998.00717.x |
| 24. | Kumar, P.; Kale, S. B.; Gonnade, R. G.; Das, U. ChemistrySelect 2021, 6, 7158–7161. doi:10.1002/slct.202102272 |
| 13. | Feng, X.-W.; Wang, J.; Zhang, J.; Yang, J.; Wang, N.; Yu, X.-Q. Org. Lett. 2010, 12, 4408–4411. doi:10.1021/ol101955x |
| 14. | Zhao, J.-L.; Guo, S.-H.; Qiu, J.; Gou, X.-F.; Hua, C.-W.; Chen, B. Tetrahedron Lett. 2016, 57, 2375–2378. doi:10.1016/j.tetlet.2016.04.044 |
| 54. | Liu, J.; Liu, Z.; Liao, P.; Bi, X. Org. Lett. 2014, 16, 6204–6207. doi:10.1021/ol5031316 |
| 12. | Zhou, G.; Ting, P. C.; Aslanian, R. G. Tetrahedron Lett. 2010, 51, 939–941. doi:10.1016/j.tetlet.2009.12.035 |
| 19. | Phanindrudu, M.; Tiwari, D. K.; Sridhar, B.; Likhar, P. R.; Tiwari, D. K. Org. Chem. Front. 2016, 3, 795–798. doi:10.1039/c6qo00063k |
| 20. | Kadari, L.; Palakodety, R. K.; Yallapragada, L. P. Org. Lett. 2017, 19, 2580–2583. doi:10.1021/acs.orglett.7b00896 |
| 21. | Chu, X.-Q.; Ge, D.; Loh, T.-P.; Shen, Z.-L. Org. Chem. Front. 2019, 6, 835–840. doi:10.1039/c8qo01346b |
| 22. | Bounar, H.; Liu, Z.; Zhang, L.; Guan, X.; Yang, Z.; Liao, P.; Bi, X.; Li, X. Org. Biomol. Chem. 2015, 13, 8723–8728. doi:10.1039/c5ob01129a |
| 23. | Phanindrudu, M.; Jaya, P.; Likhar, P. R.; Tiwari, D. K. Tetrahedron 2020, 76, 131263. doi:10.1016/j.tet.2020.131263 |
| 11. | Reddy, M. A.; Reddy, P. S.; Sreedhar, B. Adv. Synth. Catal. 2010, 352, 1861–1869. doi:10.1002/adsc.200900905 |
| 52. | Qu, C.-H.; Song, G.-T.; Tang, D.-Y.; Shao, J.-W.; Li, H.-y.; Xu, Z.-G.; Chen, Z.-Z. J. Org. Chem. 2020, 85, 12785–12796. doi:10.1021/acs.joc.0c01686 |
| 8. | Gouliaev, A. H.; Slok, F. A.; Teuber, L.; Demnitz, J. Potassium channel modulators. U.S. Patent US7429618B2, Sept 30, 2008. |
| 9. | Langler, R. F.; Paddock, R. L.; Thompson, D. B.; Crandall, I.; Ciach, M.; Kain, K. C. Aust. J. Chem. 2003, 56, 1127–1133. doi:10.1071/ch03073 |
| 10. | Ito, N.; Kurimura, M.; Yamauchi, T.; Segawa, C.; Sasaki, H.; Tai, K.; Arai, K.; Shinohara, T. In 1-position durch einen Ring substituierte benzo[1,4]diazepine zur Verwendung als Antidepressiva. Int. Pat. Appl. WO 2009/145357 A1, Dec 3, 2009. |
| 15. | Liu, T.; Liu, J.; Xia, S.; Meng, J.; Shen, X.; Zhu, X.; Chen, W.; Sun, C.; Cheng, F. ACS Omega 2018, 3, 1409–1415. doi:10.1021/acsomega.7b01745 |
| 16. | Guan, X.-Y.; Zhang, L.-D.; You, P.-S.; Liu, S.-S.; Liu, Z.-Q. Tetrahedron Lett. 2019, 60, 244–247. doi:10.1016/j.tetlet.2018.12.023 |
| 17. | Liu, Z.-Q.; You, P.-S.; Zhang, L.-D.; Liu, D.-Q.; Liu, S.-S.; Guan, X.-Y. Molecules 2020, 25, 539. doi:10.3390/molecules25030539 |
| 53. | Rossi-Ashton, J. A.; Clarke, A. K.; Unsworth, W. P.; Taylor, R. J. K. ACS Catal. 2020, 10, 7250–7261. doi:10.1021/acscatal.0c01923 |
© 2021 Qu et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.