Abstract
The reported flow-batch approach enables the easy preparation of 2H-azirines and their stereoselective transformation into highly functionalized NH-aziridines, starting from vinyl azides and organolithium compounds. The protocol has been developed using cyclopentyl methyl ether (CPME) as an environmentally benign solvent, resulting into a sustainable, safe and potentially automatable method for the synthesis of interesting strained compounds.
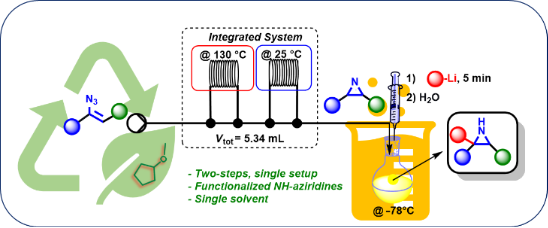
Graphical Abstract
Introduction
Since their conception in the early 1990s, Green Chemistry Principles (GCP) have been applied with increasing effort towards the design of efficient production processes [1-3]. As a result, a number of sustainable synthetic strategies has been recently developed, lowering the environmental impact and reducing the chemical hazards associated with the preparation of highly valuable compounds [4]. Among the elements that affect the sustainability of a synthetic method, the choice of the solvent is crucial [5]. In fact, chemical solvents represent most of the total amount of chemical species used in manufacturing processes, and therefore, strongly affect waste disposal requirements and process related risks [6]. Recently, a variety of sustainable solvents has been therefore identified, and their use have been combined with those of enabling technologies. In this scenario, the development of continuous flow synthetic methodologies has found its fortune in the past two decades [7-9]. Several chemical hazards can be effectively controlled through the use of microfluidic systems, because of the utilization of a reduced confined space and the exquisite control over heating and mass transfer [10]. In fact, the utilization of the microfluidic technology results in smaller temperature gradients due to the large surface–volume ratio of microreactors, and may prevent the formation of undesirable byproducts that are not avoidable under the traditional batch conditions. As a consequence, efforts have been devoted to the development of synthetic processes combining GCP and enabling technologies [11].
Within the synthetic chemistry context, the preparation of aziridines still generates interest, mostly because of their importance as source for drug prototypes and drug discovery leads [12]. Interestingly, important advances have been recently addressed in the synthesis of NH-aziridines directly from olefins [13,14]. Aziridines are otherwise accessible from a variety of acyclic precursors [15-17], even stereoselectively [18-20], and through derivatization of 2H-azirines. The reactions using 2H-azirines as electrophiles proceed with several nitrogen, oxygen and sulfur nucleophiles, enabling to access aziridines with great structural variability [21]. The reaction of azirines with Grignard and organolithium reagents has been poorly investigated, and only without using green and renewable solvents [22,23]. In turn, 2H-azirines can be smoothly obtained through intramolecular cyclization of vinyl azides, or by other strategies involving oximes, imines and oxazoles [24].
One appealing strategy for the preparation of 2H-azirines involves the use of readily available vinyl azides [25-30]. However, the batch cyclization of vinyl azides into the corresponding 2H-azirines could generate some risks, due to the explosive nature of organic azides, and possible overpressure issues caused by nitrogen generation at high temperatures. Consequently, scalability and control of this processes represents a real challenge. The exploitation of microfluidic technologies has therefore resulted in safer procedures for the preparation of 2H-azirines, offering valuable alternatives for production purposes. In 2013, Kirschning harnessed the photoinduced electrocyclization of vinyl azides in a microfluidic photoreactor yielding 2H-azirines as precursors of 1,3-dipolarophiles (Scheme 1a) [27]. Similarly, Maurya developed a microfluidic photoreactor for the synthesis of a fused β-carboline from an α-ketovinyl azide and a 1,2,3,4-tetrahydro-β-carboline (Scheme 1b) [30]. More recently, Kappe reported the generation of 2H-azirines under continuous flow conditions, and their transformation into functionalized oxazoles using acetone as the solvent (Scheme 1c) [28]. Inspired by these recent reports, we became interested in the development of an eco-friendly strategy for the safe preparation of highly functionalized NH-aziridines from acyclic precursors. Herein, we report a sustainable mixed flow-batch approach that enables the direct preparation of functionalized NH-aziridines from vinyl azides.
Scheme 1: Flow generation and transformation of 2H-azirines.
Scheme 1: Flow generation and transformation of 2H-azirines.
Results and Discussion
At the earliest stage of our research, we focused on the choice of the most suitable solvent for azide cyclization and organolithium addition reactions. Most of the previously reported flow procedures involved acetonitrile, dichloromethane and acetone as solvents, however, incompatible with the utilization of reactive alkali organometals. An exception is made for toluene, used by Kirschning for the photoinduced azirine formation [27]. Therefore, we investigated the thermally induced cyclisation of 1-(1-azidovinyl)-4-methylbenzene (1a) in refluxing 2-MeTHF and cyclopentyl methyl ether (CPME) as green solvent candidates, and compared the results with the reaction conducted in toluene (Table 1).
Table 1: Thermally induced cyclization of 1-(1-azidovinyl)-4-methylbenzene (1a) under batch conditions.
|
|
||
| Solvent | Temperature | Timea |
| toluene | 110 °C | 1.5 h |
| 2-MeTHF | 80 °C | 4.0 h |
| CPME | 106 °C | 45 min |
aTime needed for complete consumption of 1a.
The reaction proceeded rapidly in CPME, while the use of 2-MeTHF resulted in longer reaction times if compared with toluene. We therefore selected CPME as the most suitable solvent for our purposes. Interestingly, besides being characterized by low toxicity, CPME has a very low affinity to water, making it suitable for moisture sensitive reactions, without previous distillation [31-33]. Subsequently, the process was examined under continuous flow conditions employing a special integrated coil reactor with two different operating temperatures (see Supporting Information File 1). A solution of 1-(1-azidovinyl)-4-methylbenzene (1a, 0.25 M in CPME) was introduced, via a high pressure syringe pump, into the coil reactor maintained at the temperature of 130 °C, and the residence time varied by adjusting the flow rate (Table 2). The reaction yield was calculated by 1H NMR analysis of the crude. In details, conversion of 1a in 2a increased from 33% to >99% by adjusting the residence time from 4 min to 16 min, respectively. The complete transformation of vinyl azide 1a was therefore achieved above the boiling point of CPME (106 °C), as enabled by the utilization of a microfluidic reactor. From a technical point of view, the pressure generated during the course of the reaction, due to nitrogen evolution, could be managed by using a high pressure pump, a stainless-steel reactor and a back-pressure regulator at 8 bar.
Under the optimized conditions, the scope of the reaction was explored on vinyl azides 1a–l that were transformed into the corresponding 2H-azirines 2a–l (Scheme 2). The methodology was found to be efficient with vinyl azides carrying aryls substituted with chlorine (2b), fluorine (2c), and bromide (2e–g). In details, ortho-, meta-, and para-bromophenyl derivatives were quantitatively transformed into the corresponding 2H-azirines 2e–g without substantial differences. Similarly, 3-(o-tolyl)-2H-azirine (2d) and 2,3-diphenyl-2H-azirine (2l) were also obtained in excellent yields. When 1-(1-azidovinyl)-2,3,4,5,6-pentafluorobenzene (1k) was reacted under optimal flow conditions, a mixture of 1k and 2H-azirine 2k (20:80 ratio) was recovered. Unfortunately, it was not possible to isolate 3-(perfluorophenyl)-2H-azirine (2k) due to its rapid polymerization in the crude mixture.
Scheme 2: Flow synthesis of 2H-azirines from vinyl azides. aThe solution of vinyl azide was re-introduced twice into the flow system to achieve full conversion.
Scheme 2: Flow synthesis of 2H-azirines from vinyl azides. aThe solution of vinyl azide was re-introduced twi...
Interestingly, aliphatic vinyl azides 1h–j were found less reactive, and the corresponding azirines 2h–j were obtained in just 74–77% yield, in mixtures with unreacted starting materials. However, when the reaction crudes were re-introduced into the flow reactor under the same conditions, azirines 2h–j were obtained quantitatively (2h, 98%; 2i, 97%; 2j, 97%).
Pursuing in our aim to develop a green approach to prepare substituted NH-aziridines from vinyl azides in a single procedure, the solution of 2a from the microfluidic system was collected in a round bottom flask cooled at −78 °C, and reacted with 1.2 equivalents of phenyllithium (PhLi) [34,35]. The mixture was stirred at the same temperature for 5 minutes, before quenching with water. To our delight, product 3a was isolated in 49% yield. Next, the reactions of several commercially available organolithium compounds were examined. As shown in Scheme 3, the reaction of 2a, generated in flow from 1a, proceeded smoothly also with hexyllithium (HexLi), n-butyllithium (n-BuLi) and isobutyllithium (iBuLi) affording the corresponding NH-aziridines 3b–d in good yields. Subsequently, other vinyl azides were tested for this flow-batch two-step procedure (Scheme 3).
Scheme 3: Mixed flow-batch approach for the preparation of functionalized NH-aziridines from vinyl azides.
Scheme 3: Mixed flow-batch approach for the preparation of functionalized NH-aziridines from vinyl azides.
Starting from vinyl azide 1d, the corresponding 3-(o-tolyl)-2H-azirine (2d) was generated under flow conditions, and reacted with PhLi, HexLi and BuLi, furnishing NH-azirdines 3e–g. Aliphatic vinyl azides 1i and 1j were also subjected to this mixed flow-batch protocol, generating NH-aziridines 3h,i in good yields (Scheme 3). The reaction was found to be efficient when PhLi was added to the collected solution of 2,3-diphenyl-2H-azirine (2m), affording 2,2,3-triphenylaziridine (3j) in 62% yield. Moreover, the reaction was found fully diastereoselective when HexLi or iBuLi were added to 2m. In fact, only products deriving from the attack of the organolithium on the less hindered face (i.e., anti with respect to the phenyl substituent at C3), leading to 3k (52%), and 3l (45%), were observed. The relative stereochemistry was assigned by NOESY experiments (see Supporting Information File 1). Unfortunately, when the protocol was applied to 1-(1-azidovinyl)-2,3,4,5,6-pentafluorobenzene (1k), only a complex mixture was recovered likely because of the instability of the corresponding 2H-azirine 2k (vide infra).
Conclusion
In summary, we have developed a sustainable mixed flow-batch approach for the synthesis of functionalized NH-aziridines starting from vinyl azides using a single green solvent for two reaction steps. Several vinyl azides have been quantitatively transformed into the corresponding 2H-azirines in a microfluidic reactor, overcoming the hazards associated with this transformation under batch conditions. A small library of functionalized aryl and alkyl-substituted NH-aziridines has been created under operationally simple conditions. Notably, the addition reaction was found to proceed stereoselectively when organolithium compounds were added to 2,3-diphenyl-2H-azirine. This is a fast, safe, green and convenient method to access this interesting structural motif without requiring protection/deprotection steps or long synthetic pathways.
Supporting Information
| Supporting Information File 1: Description of general methods, general procedures, characterization data for all compounds and copies of 1H,13C,19F, NOESY spectra. | ||
| Format: PDF | Size: 5.0 MB | Download |
References
-
Chen, T.-L.; Kim, H.; Pan, S.-Y.; Tseng, P.-C.; Lin, Y.-P.; Chiang, P.-C. Sci. Total Environ. 2020, 716, 136998. doi:10.1016/j.scitotenv.2020.136998
Return to citation in text: [1] -
Ratti, R. SN Appl. Sci. 2020, 2, No. 263. doi:10.1007/s42452-020-2019-6
Return to citation in text: [1] -
Loste, N.; Roldán, E.; Giner, B. Environ. Sci. Pollut. Res. 2020, 27, 6215–6227. doi:10.1007/s11356-019-07177-5
Return to citation in text: [1] -
Sharma, S.; Das, J.; Braje, W. M.; Dash, A. K.; Handa, S. ChemSusChem 2020, 13, 2859–2875. doi:10.1002/cssc.202000317
Return to citation in text: [1] -
Osei Akoto, C. Industrial Applications of Green Solvents in Organic and Drug Synthesis for Sustainable Development of Chemical Process and Technologies. In Applications of Nanotechnology for Green Synthesis; Inamuddin, A. A., Ed.; Nanotechnology in the Life Sciences; Springer: Cham, Switzerland, 2020; pp 19–40. doi:10.1007/978-3-030-44176-0_2
Return to citation in text: [1] -
Häckl, K.; Kunz, W. C. R. Chim. 2018, 21, 572–580. doi:10.1016/j.crci.2018.03.010
Return to citation in text: [1] -
Fanelli, F.; Parisi, G.; Degennaro, L.; Luisi, R. Beilstein J. Org. Chem. 2017, 13, 520–542. doi:10.3762/bjoc.13.51
Return to citation in text: [1] -
Porta, R.; Benaglia, M.; Puglisi, A. Org. Process Res. Dev. 2016, 20, 2–25. doi:10.1021/acs.oprd.5b00325
Return to citation in text: [1] -
Degennaro, L.; Carlucci, C.; De Angelis, S.; Luisi, R. J. Flow Chem. 2016, 6, 136–166. doi:10.1556/1846.2016.00014
Return to citation in text: [1] -
Plutschack, M. B.; Pieber, B.; Gilmore, K.; Seeberger, P. H. Chem. Rev. 2017, 117, 11796–11893. doi:10.1021/acs.chemrev.7b00183
Return to citation in text: [1] -
Bogdan, A. R.; Dombrowski, A. W. J. Med. Chem. 2019, 62, 6422–6468. doi:10.1021/acs.jmedchem.8b01760
Return to citation in text: [1] -
Dembitsky, V. M.; Terent’ev, A. O.; Levitsky, D. O. Aziridine Alkaloids: Origin, Chemistry and Activity. Natural Products; Springer: Berlin, Heidelberg, Germany, 2013; pp 977–1006. doi:10.1007/978-3-642-22144-6_93
Return to citation in text: [1] -
Jat, J. L.; Paudyal, M. P.; Gao, H.; Xu, Q.-L.; Yousufuddin, M.; Devarajan, D.; Ess, D. H.; Kürti, L.; Falck, J. R. Science 2014, 343, 61–65. doi:10.1126/science.1245727
Return to citation in text: [1] -
Oseka, M.; Laudadio, G.; van Leest, N. P.; Dyga, M.; de Andrade Bartolomeu, A.; Goossen, L.; de Bruin, B.; Thiago de Oliveira, K.; Noel, T. ChemRxiv 2020. doi:10.26434/chemrxiv.12824135.v1
Return to citation in text: [1] -
Ielo, L.; Touqeer, S.; Roller, A.; Langer, T.; Holzer, W.; Pace, V. Angew. Chem., Int. Ed. 2019, 58, 2479–2484. doi:10.1002/anie.201812525
Return to citation in text: [1] -
Ielo, L.; Pace, V.; Pillari, V.; Miele, M.; Castiglione, D. Synlett 2021. doi:10.1055/s-0040-1706404
Return to citation in text: [1] -
Monticelli, S.; Colella, M.; Pillari, V.; Tota, A.; Langer, T.; Holzer, W.; Degennaro, L.; Luisi, R.; Pace, V. Org. Lett. 2019, 21, 584–588. doi:10.1021/acs.orglett.8b04001
Return to citation in text: [1] -
Degennaro, L.; Trinchera, P.; Luisi, R. Chem. Rev. 2014, 114, 7881–7929. doi:10.1021/cr400553c
Return to citation in text: [1] -
de Ceglie, M. C.; Musio, B.; Affortunato, F.; Moliterni, A.; Altomare, A.; Florio, S.; Luisi, R. Chem. – Eur. J. 2011, 17, 286–296. doi:10.1002/chem.201002172
Return to citation in text: [1] -
Dammacco, M.; Degennaro, L.; Florio, S.; Luisi, R.; Musio, B.; Altomare, A. J. Org. Chem. 2009, 74, 6319–6322. doi:10.1021/jo9011943
Return to citation in text: [1] -
Alves, M. J.; Teixeira Costa, F. 2H-Azirines as electrophiles. In Heterocyclic Targets in Advanced Organic Synthesis, 1st ed.; do Carmo Carreiras, M.; Marco-Contelles, J., Eds.; Research Signpost: Kerala, India, 2010; pp 145–172.
Return to citation in text: [1] -
Davis, F. A.; Liang, C.-H.; Liu, H. J. Org. Chem. 1997, 62, 3796–3797. doi:10.1021/jo9702610
Return to citation in text: [1] -
Carlson, R. M.; Lee, S. Y. Tetrahedron Lett. 1969, 10, 4001–4004. doi:10.1016/s0040-4039(01)88598-1
Return to citation in text: [1] -
Ramkumar, N.; Voskressensky, L. G.; Sharma, U. K.; Van der Eycken, E. V. Chem. Heterocycl. Compd. 2019, 55, 795–801. doi:10.1007/s10593-019-02539-w
Return to citation in text: [1] -
Tiwari, D. K.; Maurya, R. A.; Nanubolu, J. B. Chem. – Eur. J. 2016, 22, 526–530. doi:10.1002/chem.201504292
Return to citation in text: [1] -
O'Brien, A. G.; Lévesque, F.; Seeberger, P. H. Chem. Commun. 2011, 47, 2688–2690. doi:10.1039/c0cc04481d
Return to citation in text: [1] -
Cludius-Brandt, S.; Kupracz, L.; Kirschning, A. Beilstein J. Org. Chem. 2013, 9, 1745–1750. doi:10.3762/bjoc.9.201
Return to citation in text: [1] [2] [3] -
Rossa, T. A.; Suveges, N. S.; Sá, M. M.; Cantillo, D.; Kappe, C. O. Beilstein J. Org. Chem. 2018, 14, 506–514. doi:10.3762/bjoc.14.36
Return to citation in text: [1] [2] -
Koo, H.; Kim, H. Y.; Oh, K. Org. Lett. 2019, 21, 10063–10068. doi:10.1021/acs.orglett.9b04010
Return to citation in text: [1] -
Chandrasekhar, D.; Borra, S.; Nanubolu, J. B.; Maurya, R. A. Org. Lett. 2016, 18, 2974–2977. doi:10.1021/acs.orglett.6b01321
Return to citation in text: [1] [2] -
Azzena, U.; Carraro, M.; Pisano, L.; Monticelli, S.; Bartolotta, R.; Pace, V. ChemSusChem 2019, 12, 40–70. doi:10.1002/cssc.201801768
Return to citation in text: [1] -
Perna, F. M.; Vitale, P.; Capriati, V. Curr. Opin. Green Sustainable Chem. 2020, 21, 27–33. doi:10.1016/j.cogsc.2019.09.004
Return to citation in text: [1] -
Watanabe, K.; Yamagiwa, N.; Torisawa, Y. Org. Process Res. Dev. 2007, 11, 251–258. doi:10.1021/op0680136
Return to citation in text: [1] -
Zenzola, M.; Degennaro, L.; Trinchera, P.; Carroccia, L.; Giovine, A.; Romanazzi, G.; Mastrorilli, P.; Rizzi, R.; Pisano, L.; Luisi, R. Chem. – Eur. J. 2014, 20, 12190–12200. doi:10.1002/chem.201403141
Return to citation in text: [1] -
Parisi, G.; Capitanelli, E.; Pierro, A.; Romanazzi, G.; Clarkson, G. J.; Degennaro, L.; Luisi, R. Chem. Commun. 2015, 51, 15588–15591. doi:10.1039/c5cc06323j
Return to citation in text: [1]
| 27. | Cludius-Brandt, S.; Kupracz, L.; Kirschning, A. Beilstein J. Org. Chem. 2013, 9, 1745–1750. doi:10.3762/bjoc.9.201 |
| 30. | Chandrasekhar, D.; Borra, S.; Nanubolu, J. B.; Maurya, R. A. Org. Lett. 2016, 18, 2974–2977. doi:10.1021/acs.orglett.6b01321 |
| 28. | Rossa, T. A.; Suveges, N. S.; Sá, M. M.; Cantillo, D.; Kappe, C. O. Beilstein J. Org. Chem. 2018, 14, 506–514. doi:10.3762/bjoc.14.36 |
| 1. | Chen, T.-L.; Kim, H.; Pan, S.-Y.; Tseng, P.-C.; Lin, Y.-P.; Chiang, P.-C. Sci. Total Environ. 2020, 716, 136998. doi:10.1016/j.scitotenv.2020.136998 |
| 2. | Ratti, R. SN Appl. Sci. 2020, 2, No. 263. doi:10.1007/s42452-020-2019-6 |
| 3. | Loste, N.; Roldán, E.; Giner, B. Environ. Sci. Pollut. Res. 2020, 27, 6215–6227. doi:10.1007/s11356-019-07177-5 |
| 7. | Fanelli, F.; Parisi, G.; Degennaro, L.; Luisi, R. Beilstein J. Org. Chem. 2017, 13, 520–542. doi:10.3762/bjoc.13.51 |
| 8. | Porta, R.; Benaglia, M.; Puglisi, A. Org. Process Res. Dev. 2016, 20, 2–25. doi:10.1021/acs.oprd.5b00325 |
| 9. | Degennaro, L.; Carlucci, C.; De Angelis, S.; Luisi, R. J. Flow Chem. 2016, 6, 136–166. doi:10.1556/1846.2016.00014 |
| 25. | Tiwari, D. K.; Maurya, R. A.; Nanubolu, J. B. Chem. – Eur. J. 2016, 22, 526–530. doi:10.1002/chem.201504292 |
| 26. | O'Brien, A. G.; Lévesque, F.; Seeberger, P. H. Chem. Commun. 2011, 47, 2688–2690. doi:10.1039/c0cc04481d |
| 27. | Cludius-Brandt, S.; Kupracz, L.; Kirschning, A. Beilstein J. Org. Chem. 2013, 9, 1745–1750. doi:10.3762/bjoc.9.201 |
| 28. | Rossa, T. A.; Suveges, N. S.; Sá, M. M.; Cantillo, D.; Kappe, C. O. Beilstein J. Org. Chem. 2018, 14, 506–514. doi:10.3762/bjoc.14.36 |
| 29. | Koo, H.; Kim, H. Y.; Oh, K. Org. Lett. 2019, 21, 10063–10068. doi:10.1021/acs.orglett.9b04010 |
| 30. | Chandrasekhar, D.; Borra, S.; Nanubolu, J. B.; Maurya, R. A. Org. Lett. 2016, 18, 2974–2977. doi:10.1021/acs.orglett.6b01321 |
| 6. | Häckl, K.; Kunz, W. C. R. Chim. 2018, 21, 572–580. doi:10.1016/j.crci.2018.03.010 |
| 27. | Cludius-Brandt, S.; Kupracz, L.; Kirschning, A. Beilstein J. Org. Chem. 2013, 9, 1745–1750. doi:10.3762/bjoc.9.201 |
| 5. | Osei Akoto, C. Industrial Applications of Green Solvents in Organic and Drug Synthesis for Sustainable Development of Chemical Process and Technologies. In Applications of Nanotechnology for Green Synthesis; Inamuddin, A. A., Ed.; Nanotechnology in the Life Sciences; Springer: Cham, Switzerland, 2020; pp 19–40. doi:10.1007/978-3-030-44176-0_2 |
| 22. | Davis, F. A.; Liang, C.-H.; Liu, H. J. Org. Chem. 1997, 62, 3796–3797. doi:10.1021/jo9702610 |
| 23. | Carlson, R. M.; Lee, S. Y. Tetrahedron Lett. 1969, 10, 4001–4004. doi:10.1016/s0040-4039(01)88598-1 |
| 4. | Sharma, S.; Das, J.; Braje, W. M.; Dash, A. K.; Handa, S. ChemSusChem 2020, 13, 2859–2875. doi:10.1002/cssc.202000317 |
| 24. | Ramkumar, N.; Voskressensky, L. G.; Sharma, U. K.; Van der Eycken, E. V. Chem. Heterocycl. Compd. 2019, 55, 795–801. doi:10.1007/s10593-019-02539-w |
| 13. | Jat, J. L.; Paudyal, M. P.; Gao, H.; Xu, Q.-L.; Yousufuddin, M.; Devarajan, D.; Ess, D. H.; Kürti, L.; Falck, J. R. Science 2014, 343, 61–65. doi:10.1126/science.1245727 |
| 14. | Oseka, M.; Laudadio, G.; van Leest, N. P.; Dyga, M.; de Andrade Bartolomeu, A.; Goossen, L.; de Bruin, B.; Thiago de Oliveira, K.; Noel, T. ChemRxiv 2020. doi:10.26434/chemrxiv.12824135.v1 |
| 18. | Degennaro, L.; Trinchera, P.; Luisi, R. Chem. Rev. 2014, 114, 7881–7929. doi:10.1021/cr400553c |
| 19. | de Ceglie, M. C.; Musio, B.; Affortunato, F.; Moliterni, A.; Altomare, A.; Florio, S.; Luisi, R. Chem. – Eur. J. 2011, 17, 286–296. doi:10.1002/chem.201002172 |
| 20. | Dammacco, M.; Degennaro, L.; Florio, S.; Luisi, R.; Musio, B.; Altomare, A. J. Org. Chem. 2009, 74, 6319–6322. doi:10.1021/jo9011943 |
| 12. | Dembitsky, V. M.; Terent’ev, A. O.; Levitsky, D. O. Aziridine Alkaloids: Origin, Chemistry and Activity. Natural Products; Springer: Berlin, Heidelberg, Germany, 2013; pp 977–1006. doi:10.1007/978-3-642-22144-6_93 |
| 21. | Alves, M. J.; Teixeira Costa, F. 2H-Azirines as electrophiles. In Heterocyclic Targets in Advanced Organic Synthesis, 1st ed.; do Carmo Carreiras, M.; Marco-Contelles, J., Eds.; Research Signpost: Kerala, India, 2010; pp 145–172. |
| 11. | Bogdan, A. R.; Dombrowski, A. W. J. Med. Chem. 2019, 62, 6422–6468. doi:10.1021/acs.jmedchem.8b01760 |
| 31. | Azzena, U.; Carraro, M.; Pisano, L.; Monticelli, S.; Bartolotta, R.; Pace, V. ChemSusChem 2019, 12, 40–70. doi:10.1002/cssc.201801768 |
| 32. | Perna, F. M.; Vitale, P.; Capriati, V. Curr. Opin. Green Sustainable Chem. 2020, 21, 27–33. doi:10.1016/j.cogsc.2019.09.004 |
| 33. | Watanabe, K.; Yamagiwa, N.; Torisawa, Y. Org. Process Res. Dev. 2007, 11, 251–258. doi:10.1021/op0680136 |
| 10. | Plutschack, M. B.; Pieber, B.; Gilmore, K.; Seeberger, P. H. Chem. Rev. 2017, 117, 11796–11893. doi:10.1021/acs.chemrev.7b00183 |
| 15. | Ielo, L.; Touqeer, S.; Roller, A.; Langer, T.; Holzer, W.; Pace, V. Angew. Chem., Int. Ed. 2019, 58, 2479–2484. doi:10.1002/anie.201812525 |
| 16. | Ielo, L.; Pace, V.; Pillari, V.; Miele, M.; Castiglione, D. Synlett 2021. doi:10.1055/s-0040-1706404 |
| 17. | Monticelli, S.; Colella, M.; Pillari, V.; Tota, A.; Langer, T.; Holzer, W.; Degennaro, L.; Luisi, R.; Pace, V. Org. Lett. 2019, 21, 584–588. doi:10.1021/acs.orglett.8b04001 |
| 34. | Zenzola, M.; Degennaro, L.; Trinchera, P.; Carroccia, L.; Giovine, A.; Romanazzi, G.; Mastrorilli, P.; Rizzi, R.; Pisano, L.; Luisi, R. Chem. – Eur. J. 2014, 20, 12190–12200. doi:10.1002/chem.201403141 |
| 35. | Parisi, G.; Capitanelli, E.; Pierro, A.; Romanazzi, G.; Clarkson, G. J.; Degennaro, L.; Luisi, R. Chem. Commun. 2015, 51, 15588–15591. doi:10.1039/c5cc06323j |
© 2021 Andresini et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0). Please note that the reuse, redistribution and reproduction in particular requires that the author(s) and source are credited and that individual graphics may be subject to special legal provisions.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (https://www.beilstein-journals.org/bjoc/terms)