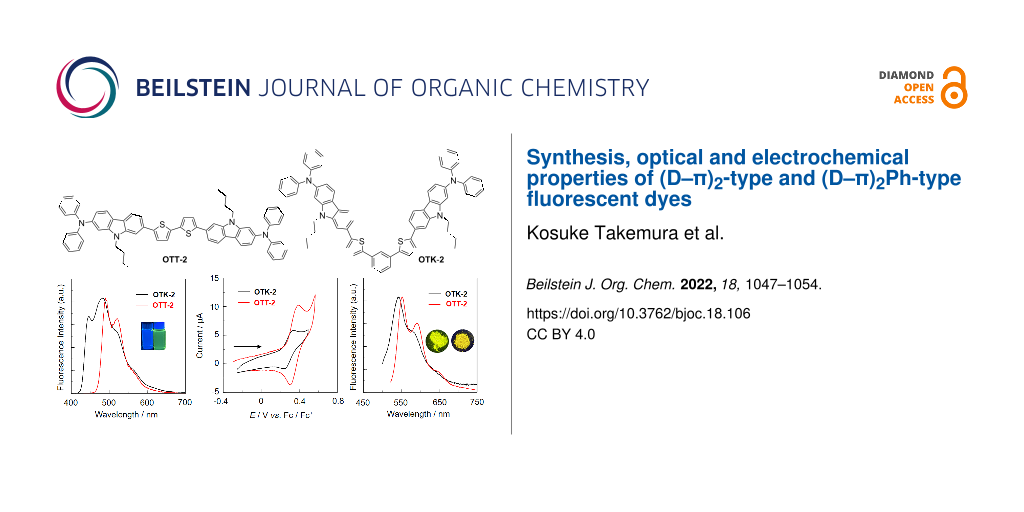
Abstract
The (D–π)2-type fluorescent dye OTT-2 with two (diphenylamino)carbazole-thiophene units as D (electron-donating group)–π (π-conjugated bridge) moiety and the (D–π)2Ph-type fluorescent dye OTK-2 with the two D–π moieties connected through a phenyl ring were derived by oxidative homocoupling of a stannyl D–π unit and Stille coupling of a stannyl D–π unit with 1,3-diiodobenzene, respectively. Their optical and electrochemical properties were investigated by photoabsorption and fluorescence spectroscopy, time-resolved fluorescence spectroscopy, cyclic voltammetry (CV) and molecular orbital (MO) calculations. In toluene the photoabsorption and fluorescence maximum wavelengths (λmax,abs and λmax,fl) of OTT-2 appear in a longer wavelength region than those of OTK-2. The fluorescence quantum yield (Φfl) of OTT-2 is 0.41, which is higher than that (Φfl = 0.36) of OTK-2. In the solid state OTT-2 shows relatively intense fluorescence properties (Φfl-solid = 0.24 nm), compared with OTK-2 (Φfl-solid = 0.15 nm). CV results demonstrated that OTT-2 and OTK-2 exhibit a reversible oxidation wave. Based on photoabsorption, fluorescence spectroscopy and CV for the two dyes, it was found that the lowest unoccupied molecular orbital (LUMO) energy level of OTT-2 is lower than that of OTK-2, but OTT-2 and OTK-2 have comparable highest occupied molecular orbital (HOMO) energy levels. Consequently, this work reveals that compared to the (D–π)2Ph-type structure, the (D–π)2-type structure exhibits not only a bathochromic shift of the photoabsorption band, but also intense fluorescence emission both in solution and the solid state.
Graphical Abstract
Introduction
The design and development of a new type of organic fluorescent dyes have been of considerable scientific and practical concern with the objective of not only fundamental studies [1-13] in synthetic chemistry, electrochemistry and photochemistry, but also their potential applications to emitters for optoelectronic devices, such as organic light-emitting diodes (OLEDs) [14-22], as well as fluorescent probes [23-28] for bioimaging and fluorescent sensors for specific target species [29-32]. Among many kinds of organic fluorescent dyes, much efforts have been made on the development of donor–π–acceptor (D–π–A)-type fluorescent dyes constructed of an electron-donating moiety (D) and an electron-withdrawing moiety (A), linked by a π-conjugated unit thanks to their intense photoabsorption and fluorescence emission characteristics originating from the intramolecular charge transfer (ICT) excitation from the D to the A moiety [4-9,18-20,25,26]. Furthermore, the (D–π–)2A-type fluorescent dyes with two D–π moieties have recently been stimulating intensive research efforts because of their high molar extinction coefficients and fluorescence quantum yields, compared to those of D–π–A-type fluorescent dyes [10-13,21,22,27,28,32].
In our previous work [33], we have reported the synthesis, optical and electrochemical properties of the (D–π)2Ph-type fluorescent dye OTK-2 with two (diphenylamino)carbazole-thiophene units as D–π moiety connected through a phenyl ring (Scheme 1). The ICT-based photoabsorption and fluorescence bands of OTK-2 appear in a shorter wavelength region than those of the corresponding (D–π)2A-type fluorescent dye having an azine ring (pyridine, pyrazine or triazine ring) as a substitute for the phenyl ring. However, the molar extinction coefficient (εmax) and fluorescence quantum yield (Φfl) of OTK-2 are comparable to those of the (D–π)2A-type fluorescent dyes. More recently, we found that the (D–π)2-type fluorescent dye OTT-2 consisting of two D–π moieties is derived by oxidative homocoupling of a stannyl D–π unit. There is an obvious structural difference between the two dyes: OTK-2 has a cross-conjugated system due to the involvement of the 1,3-phenylene unit as an additional linker, but OTT-2 has a conjugated system. Therefore, it is interesting to reveal the optical and electrochemical properties of (D–π)2-type fluorescent dyes, making a comparison with (D–π)2Ph-type fluorescent dyes. Herein, we report the syntheses of (D–π)2-type and (D–π)2Ph-type fluorescent dyes and their optical and electrochemical properties based on photoabsorption and fluorescence spectroscopy, time-resolved fluorescence spectroscopy, cyclic voltammetry (CV) and molecular orbital (MO) calculations.
Results and Discussion
Using a toluene solution containing 1,3-diiodobenzene and (diphenylamino)carbazole-thiophenestannane derivative 1 [33] in the presence of Pd(PPh3)4, the (D–π)2-type and (D–π)2Ph-type fluorescent dyes OTK-2 [33] and OTT-2 were obtained by Stille coupling of 1 with 1,3-diiodobenzene and oxidative homocoupling of 1, respectively (Scheme 1).
The photoabsorption and fluorescence spectra of OTK-2 and OTT-2 in toluene are shown in Figure 1a,b, and their optical data are summarized in Table 1. As shown in insets of Figure 1a,b, the toluene solutions of OTK-2 and OTT-2 are nearly-colorless and greenish-yellow, and show blue and green fluorescent colors, respectively. The photoabsorption spectra demonstrate that the photoabsorption maximum wavelength (λmax, abs = 424 nm) of OTT-2 occurs at a by 29 nm longer wavelength than that (λmax, abs = 395 nm) of OTK-2. The εmax value for the λmax, abs of OTT-2 is 89 200 M−1 cm−1, which is comparable to that (εmax = 98 000 M−1 cm−1) of OTK-2. In the corresponding fluorescence spectra, as in the case of OTK-2, OTT-2 exhibited a vibronically-structured fluorescence band. The fluorescence maximum (λmax,fl) of OTT-2 appeared at 490 nm, which is a by 43 nm longer wavelength than that (λmax,fl = 447 nm) of OTK-2. The Stokes shift (SS) value of OTT-2 is estimated to be 3177 cm−1, which is higher than that (2945 cm−1) of OTK-2. In addition, the Φfl of OTT-2 is 0.41, which is higher than that (Φfl = 0.36) of OTK-2. Time-resolved fluorescence spectroscopy of the two dyes revealed that the fluorescence lifetimes (τfl) are 0.62 ns for OTK-2 and 0.66 ns for OTT-2, indicating that there is a little difference in the τfl values of the two dyes. The radiative rate constant (kr = 6.2 × 108 s−1) for OTT-2 is slightly larger than that (5.8 × 108 s−1) for OTK-2. However, the nonradiative rate constant (knr = 8.9 × 108 s−1) of OTT-2 is smaller than that (1.0 × 109 s−1) for OTK-2. As the result, the ratio of nonradiative constant to radiative constant (knr/kr = 1.4) for OTT-2 is smaller than that (1.7) for OTK-2, suggesting that the higher Φfl value of OTT-2 is mainly attributed to the smaller knr value compared with that of OTK-2.
![[1860-5397-18-106-1]](/bjoc/content/figures/1860-5397-18-106-1.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 1: (a) Photoabsorption and (b) fluorescence (λex = λmax,abs) spectra of OTK-2 [33] and OTT-2 in toluene. (c) Solid-state UV–vis diffuse reflection–absorption and (b) and (d) fluorescence spectra (λex = 484 nm for OTK-2 [33] and 512 nm for OTT-2) of OTK-2 and OTT-2 in the solid state. Insets in (a) and (b): color and fluorescence images of OTK-2 (left) and OTT-2 (right) in toluene. Insets in (c) and (d): color and fluorescence images of OTK-2 (left) and OTT-2 (right) in the solid state. The photos depicted as insets in Figure 1a–d were reproduced from [33] (“Mechanofluorochromism of (D–π–)2A-type azine-based fluorescent dyes, © 2022 K. Takemura et al., published by the Royal Society of Chemistry, distributed under the terms of the Creative Commons Attribution 3.0 Unported License, https://creativecommons.org/licenses/by/3.0/).
Figure 1: (a) Photoabsorption and (b) fluorescence (λex = λmax,abs) spectra of OTK-2 [33] and OTT-2 in toluene. (...
Table 1: Optical data of OTK-2 [33] and OTT-2 in toluene.
| Dye |
λmax,abs [nm]
(ε [M−1cm−1]) |
λmax,fl [nm]
(Φf)a |
SS [cm−1]b | τfl [ns]c | kr [s−1]d | knr [s−1]e | knr/kr |
| OTK-2 | 395 (98 000) | 447 (0.36) | 2945 | 0.62 | 5.8 × 108 | 1.0 × 109 | 1.7 |
| OTT-2 | 424 (89 200) | 490 (0.41) | 3177 | 0.66 | 6.2 × 108 | 8.9 × 108 | 1.4 |
aFluorescence quantum yields (Φfl) were determined by using a calibrated integrating sphere system (λex = λmax,abs); bStokes shift; cfluorescence lifetime; dradiative rate constant (kr = Φfl/τfl); enonradiative rate constant (knr = (1 − Φfl)/τfl).
The solid-state optical properties of OTK-2 and OTT-2 were investigated by solid-state UV–vis diffuse reflection–photoabsorption and fluorescence spectral measurements, and time-resolved fluorescence spectroscopy for the solids (Figure 1c,d). As shown in insets of Figure 1c,d, in the solid state, the colors are yellowish orange for OTK-2 and orange for OTT-2, and the fluorescent colors are greenish yellow for OTK-2 and yellow for OTT-2. The photoabsorption bands of OTK-2 and OTT-2 in the solid state are broadened in a longer wavelength region with an onset of ca. 520–550 nm, and the λmax,abs-solid of OTK-2 and OTT-2 appeared at around 480 nm, which showed bathochromic shifts by 85 nm and 56 nm, respectively, compared with those in toluene (Table 2). The corresponding solid-state fluorescence spectra demonstrated that as in the case of toluene solutions, OTK-2 and OTT-2 in the solid state exhibited a vibronically-structured fluorescence band. The λmax,fl-solid of OTK-2 and OTT-2 occur at 543 nm and 552 nm, which exhibited significant bathochromic shifts of 96 nm and 62 nm, respectively, compared with those in toluene. The Φfl-solid (0.24) of OTT-2 is higher than that (Φfl-solid = 0.15) of OTK-2, while the Φfl-solid of OTK-2 and OTT-2 are lower than those in toluene. Although single crystals of OTK-2 and OTT-2 with sufficient size for X-ray structural analysis were not obtained, the intermolecular π–π interactions between the fluorophores leading to delocalization of excitons or excimers in the solid state would be responsible for the bathochromic shifts of λmax,abs and λmax,fl and lowering of Φfl with change of state from solution to solid [34-36]. The τfl-solid values of OTK-2 and OTT-2 are longer than those in toluene, however, the τfl-solid value (1.03 ns) of OTT-2 is comparable to that (τfl-solid = 0.93 ns) of OTK-2. Whereas the kr-solid value (2.3 × 108 s−1) for OTT-2 is larger than that (1.6 × 108 s−1) for OTK-2, the knr-solid value (7.4 × 108 s−1) for OTT-2 is slightly smaller than that (9.4 × 108 s−1) for OTK-2. Consequently, the knr-solid/kr-solid values for OTK-2 and OTT-2 in the solid state are 5.7 and 3.2, respectively, which are larger than those (1.7 and 1.4, respectively) in toluene, indicating that the non-radiative decay in the solid state is accelerated. However, the knr-solid/kr-solid value (3.2) of OTT-2 is smaller than that (5.7) of OTK-2, suggesting that the higher Φfl-solid value of OTT-2 is due to the larger kr value compared with that of OTK-2. Therefore, it was found that the (D–π)2-type structure exhibits not only the bathochromic shift of photoabsorption band but also intense fluorescence emission both in solution and the solid state, compared to the (D–π)2Ph-type structure.
Table 2: Optical data of OTK-2 [33] and OTT-2 in the solid-state.
| Dye | λmax,abs-solid [nm] |
λmax,fl-solid [nm]
(Φfl-solid)a |
τfl-solid [ns]b | kr-solid [s−1]c | knr-solid [s−1]d | knr-solid/kr-solid |
| OTK-2 | 480shoulder | 543 (0.15) | 0.93 | 1.6 × 108 | 9.1 × 108 | 5.7 |
| OTT-2 | 480shoulder | 552 (0.24) | 1.03 | 2.3 × 108 | 7.4 × 108 | 3.2 |
aFluorescence quantum yields (Φfl-solid) were determined by using a calibrated integrating sphere system (484 nm for OTK-2 and λex = 512 nm for OTT-2, respectively); bfluorescence lifetime; cradiative rate constant (kr-solid = Φfl-solid/τfl-solid); dnonradiative rate constant (knr-solid = (1 − Φfl-solid)/τfl-solid).
The electrochemical properties of OTK-2 and OTT-2 (0.1 mM) were evaluated using CV in DMF containing 0.1 M tetrabutylammonium perchlorate (Bu4NClO4), in which the potentials were internally referenced to ferrocene/ferrocenium (Fc/Fc+). The electrochemical data are summarized in Table 3. The cyclic voltammograms of the two dyes show a reversible oxidation wave with the anodic peak potential (Epaox) at 0.32 V for OTK-2 and 0.40 V for OTT-2 (Figure 2), while any obvious reduction waves and another oxidation waves did not appear within the potential window (Figure 3a and Figure S2a, Supporting Information File 1). The corresponding cathodic peak potential (Epcox) appeared at 0.24 V for OTK-2 and 0.30 V for OTT-2, and thus the peak separations between the Epaox and Epcox waves are ca. 80–100 mV. This result may indicate that the two dyes undergo an electrochemically stable one-electron oxidation–reduction process, but further studies are necessary to exactly determine the number of electrons in the oxidation–reduction process. The half-wave potential (E1/2ox) was evaluated to be 0.28 V for OTK-2 and 0.35 V for OTT-2. Therefore, the E1/2ox for OTK-2 with the (D–π)2Ph-type structure is cathodically shifted by 0.07 V, compared with that for OTT-2 with the (D–π)2-type structure. Furthermore, we investigated the diffusion-controlled process form CV at different scan rates (50, 100, 200, 400, 600 and 1000 mV s−1) and reversibility of the oxidation process by repeated potential cycling (20 cycles). For both OTK-2 and OTT-2, the Epaox remained steady at different scan rates while the anodic peak current (Ipa) increased with the increase in scan rate. The Ipa showed a negligible change during 20 cycles at a scan rate of 100 mV s−1, indicating diffusion control and good reversibility of the oxidation process (Figures S2b,c and S3b,c, Supporting Information File 1). The highest occupied molecular orbital (HOMO) energy level versus vacuum level was estimated from the E1/2ox, that is, −[E1/2ox + 4.8] eV. On the other hand, the lowest unoccupied molecular orbital (LUMO) energy level versus the vacuum level was estimated by using [HOMO + E0–0] eV from the E1/2ox and intersections (optical energy gap: E0-0 = 2.79 eV for OTK-2 and 2.61 eV for OTT-2) of the photoabsorption and fluorescence spectra in toluene. It was found that the HOMO energy level (−5.15 eV) of OTT-2 is slightly lower than that (−5.08 eV) of OTK-2, indicating that the two dyes have comparable HOMO energy levels. On the other hand, the LUMO energy level (−2.54 eV) of OTT-2 is significantly lower than that (−2.29 eV) of OTK-2. Semi-empirical MO calculations (PM5, INDO/S method) revealed that for OTK-2 both the HOMO and LUMO were mostly localized on the two (diphenylamino)carbazole-thiophene moieties. On the other hand, for OTT-2 both the HOMO and LUMO are delocalized over the whole molecule through the thiophene units (Figure 3). Consequently, the fact reveals that compared to the (D–π)2Ph-type structure, the (D–π)2-type structure can cause not only the stabilization of the LUMO energy level but also the delocalization of the HOMO and LUMO over the whole molecule, leading to a narrower HOMO–LUMO band gap of OTT-2 than OTK-2, that is, the bathochromic shift of the photoabsorption band from OTT-2 to OTK-2.
Table 3: Electrochemical data, and HOMO and LUMO energy levels of OTK-2 and OTT-2.
| Dye | Epaox [V]a | Epcox [V]a | E1/2ox [V]a | HOMO [eV]b | LUMO [eV]c | E0–0 [eV]d |
| OTK-2 | 0.32 | 0.24 | 0.28 | −5.08 | −2.29 | 2.79 eV |
| OTT-2 | 0.40 | 0.30 | 0.35 | −5.15 | −2.54 | 2.61 eV |
aThe anodic peak (Epaox), the cathodic peak (Epcox) and the half-wave (E1/2ox) potentials for oxidation vs Fc/Fc+ were recorded in DMF/Bu4NClO4 (0.1 M) solution; b−[Eox1/2 + 4.8] eV; c[HOMO + E0–0] eV; d444 nm for OTK-2 and 475 nm for OTT-2.
![[1860-5397-18-106-2]](/bjoc/content/figures/1860-5397-18-106-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: Cyclic voltammograms of OTK-2 and OTT-2 (0.1 mM) in DMF containing 0.1 M Bu4NClO4 at a scan rate of 100 mV s−1. The arrow denotes the direction of the potential scan.
Figure 2: Cyclic voltammograms of OTK-2 and OTT-2 (0.1 mM) in DMF containing 0.1 M Bu4NClO4 at a scan rate of...
![[1860-5397-18-106-3]](/bjoc/content/figures/1860-5397-18-106-3.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 3: (a) HOMO and (b) LUMO of OTK-2 [33] and OTT-2 derived from MO calculations (PM5, INDO/S method). The red and blue lobes denote the positive and negative signs of the coefficients of the molecular orbitals. The size of each lobe is proportional to the MO coefficient.
Figure 3: (a) HOMO and (b) LUMO of OTK-2 [33] and OTT-2 derived from MO calculations (PM5, INDO/S method). The re...
Conclusion
We have developed the (D–π)2-type fluorescent dye OTT-2 and the (D–π)2Ph-type fluorescent dye OTK-2 and evaluated their optical and electrochemical properties. Both in solution and the solid state, the photoabsorption and fluorescence maximum wavelengths of OTT-2 appear in a longer wavelength region than those of OTK-2, and the fluorescence quantum yields of OTT-2 are higher than those of OTK-2. The cyclic voltammograms demonstrated that OTT-2 and OTK-2 exhibit a reversible oxidation wave, indicating that the two dyes undergo an electrochemically stable oxidation–reduction process. It was found that the LUMO energy level of OTT-2 is lower than that of OTK-2, while OTT-2 and OTK-2 have comparable HOMO energy levels. Semi-empirical MO calculations showed that for OTK-2 both the HOMO and LUMO were mostly localized on the two D–π moieties, whereas for OTK-2 both the HOMO and LUMO are delocalized over the whole molecule through the thiophene units. Consequently, this work reveals that compared to the (D–π)2Ph-type structure, the (D–π)2-type structure not only has intense fluorescence emission properties both in solution and the solid state, but also can cause delocalization of the HOMO and the LUMO over the whole molecule as well as the stabilization of the LUMO energy level, leading to a narrower HOMO–LUMO band gap of OTT-2 than OTK-2, that is, the bathochromic shift of photoabsorption band from OTT-2 to OTK-2.
Experimental
General methods
Melting points were measured with an AS ONE ATM-02 apparatus. IR spectra were recorded on a SHIMADZU IRTracer-100 spectrometer by ATR method. 1H NMR and 13C NMR spectra were recorded on a Varian-500 FT NMR spectrometer. High-resolution mass spectral data by APCI were acquired on a Thermo Fisher Scientific LTQ Orbitrap XL apparatus. Photoabsorption spectra of solutions were observed with a Shimadzu UV-3600 plus spectrophotometer. Photoabsorption spectra of solids were recorded by a Shimadzu UV-3600 plus spectrophotometer with a calibrated integrating sphere system. Fluorescence spectra of solutions and solids were measured with a HORIBA FluoroMax-4 spectrofluorometer. Fluorescence quantum yields in solution and in the solid state were determined using a HORIBA FluoroMax-4 spectrofluorometer with a calibrated integrating sphere system. Fluorescence decay measurements were performed on a HORIBA DeltaFlex modular fluorescence lifetime system using a Nano LED pulsed diode excitation source (451 nm). Cyclic voltammetry (CV) curves were recorded in DMF/Bu4NClO4 (0.1 M) solution with a three-electrode system consisting of Ag/Ag+ as the reference electrode, a Pt plate as the working electrode and a Pt wire as the counter electrode using an Electrochemical Measurement System HZ-7000 (HOKUTO DENKO). Semi-empirical molecular orbital calculations were carried out with the WinMOPAC Ver. 3.9 package (Fujitsu, Chiba, Japan), where geometry calculations of the compounds in the ground state were made using the PM5 method. Dipole moments and HOMO and LUMO energy levels of the compounds were also evaluated from INDO/S calculations.
Synthesis
7,7'-(1,3-Phenylenebis(thiophene-5,2-diyl))bis(9-butyl-N,N-diphenyl-9H-carbazol-2-amine) (OTK-2) and 7,7'-([2,2'-bithiophene]-5,5'-diyl)bis(9-butyl-N,N-diphenyl-9H-carbazol-2-amine) (OTT-2): A solution of 1 (87 mg, 0.137 mmol), 1,3-diiodobenzene (14 mg, 0.041 mmol), and Pd(PPh3)4 (2 mg, 0.001 mmol) in toluene (1 mL) was stirred for 29 h at 110 ºC under an argon atmosphere. The reaction mixture was diluted with water, and then, the solution was extracted with dichloromethane. The dichloromethane extract was dried over anhydrous MgSO4, filtrated, and concentrated. The residue was chromatographed on silica gel (ethyl acetate/hexane 1:4 ) to give OTK-2 (19 mg, yield 27%) and OTT-2 (9 mg, yield 14%) as a light yellow solid and an orange solid, respectively; the characterization data for OTK-2 are in agreement with those reported in the literature [33]; OTT-2: mp >300 °C; FTIR (ATR) ν̄: 1591, 1491, 1460 cm−1; 1H NMR (500 MHz, CD2Cl2) δ 0.76–1.02 (m, 6H), 1.23–1.38 (m, 4H), 1.71–1.81 (m, 4H), 4.12–4.21 (m, 4H), 6.95 (dd, J = 1.8 and 8.4 Hz, 2H), 7.01–7.05 (m, 4H), 7.10–7.16 (m, 10H), 7.24–7.31 (m, 10H), 7.39 (d, J = 3.8 Hz, 2H), 7.50 (dd, J = 1.4 and 8.0 Hz, 2H), 7.59 (d, J = 1.2 Hz, 2H), 7.93 (d, J = 8.3 Hz, 2H), 7.99 (d, J = 8.0 Hz, 2H) ppm; 13C NMR (125 MHz, CD2Cl2) δ 14.04, 20.85, 31.44, 43.04, 105.37, 105.84, 117.54, 117.56, 118.73, 120.46, 121.16, 122.94, 122.96, 124.02, 124.36, 124.84, 129.55, 131.14, 136.68, 141.67, 142.73, 144.74, 146.92, 148.61 ppm; HRMS (APCI) m/z (%): [M + H+] calcd. for C64H55N4S2, 943.38627; found, 943.38635.
Supporting Information
| Supporting Information File 1: 1H and 13C NMR spectra of OTT-2. | ||
| Format: PDF | Size: 410.6 KB | Download |
References
-
Kubo, Y.; Nozawa, T.; Maeda, K.; Hashimoto, Y. Mater. Adv. 2021, 2, 1059–1071. doi:10.1039/d0ma00910e
Return to citation in text: [1] -
Dai, J.; Yao, L.; Wang, C.; Wang, Y.; Liu, F.; Yan, X.; Sun, P.; Zhang, H.; Wang, Y.; Zhou, J.; Lu, G. J. Phys. Chem. Lett. 2022, 13, 4754–4761. doi:10.1021/acs.jpclett.2c01226
Return to citation in text: [1] -
Wałęsa-Chorab, M.; Muras, K.; Filiatrault, H. L.; Skene, W. G. J. Mater. Chem. C 2022, 10, 3691–3703. doi:10.1039/d1tc03388c
Return to citation in text: [1] -
Ooyama, Y.; Ito, G.; Kushimoto, K.; Komaguchi, K.; Imae, I.; Harima, Y. Org. Biomol. Chem. 2010, 8, 2756–2770. doi:10.1039/c003526b
Return to citation in text: [1] [2] -
Ooyama, Y.; Harima, Y. J. Mater. Chem. 2011, 21, 8372–8380. doi:10.1039/c0jm03601c
Return to citation in text: [1] [2] -
Josse, P.; Allain, M.; Calupitan, J. P.; Jiang, Y.; Cabanetos, C.; Roncali, J. Adv. Opt. Mater. 2020, 8, 2000420. doi:10.1002/adom.202000420
Return to citation in text: [1] [2] -
Feng, X.; Chen, Y.; Lei, Y.; Zhou, Y.; Gao, W.; Liu, M.; Huang, X.; Wu, H. Chem. Commun. 2020, 56, 13638–13641. doi:10.1039/d0cc05538g
Return to citation in text: [1] [2] -
Zhang, X.; Ma, Z.; Yang, Y.; Zhang, X.; Jia, X.; Wei, Y. J. Mater. Chem. C 2014, 2, 8932–8938. doi:10.1039/c4tc01457j
Return to citation in text: [1] [2] -
Liu, J.; Cui, Y.; Pan, Y.; Chen, Z.; Jia, T.; Li, C.; Wang, Y. Angew. Chem., Int. Ed. 2022, 61, e202117087. doi:10.1002/anie.202117087
Return to citation in text: [1] [2] -
Narayanaswamy, K.; Venkateswararao, A.; Gupta, V.; Chand, S.; Singh, S. P. Chem. Commun. 2016, 52, 210–213. doi:10.1039/c5cc07435e
Return to citation in text: [1] [2] -
Roy, S.; Nandi, S. K.; Haldar, D.; Pal, B. J. Mater. Chem. C 2022, 10, 8767–8775. doi:10.1039/d2tc00951j
Return to citation in text: [1] [2] -
Yu, L.; Xi, J.; Chan, H. T.; Su, T.; Antrobus, L. J.; Tong, B.; Dong, Y.; Chan, W. K.; Phillips, D. L. J. Phys. Chem. C 2013, 117, 2041–2052. doi:10.1021/jp3113182
Return to citation in text: [1] [2] -
Enoki, T.; Ohshita, J.; Ooyama, Y. Bull. Chem. Soc. Jpn. 2018, 91, 1704–1709. doi:10.1246/bcsj.20180210
Return to citation in text: [1] [2] -
Yagi, S. Luminescent Materials for Organic Light-Emitting Diodes. In Progress in the Science of Functional Dyes; Ooyama, Y.; Yagi, S., Eds.; Springer Nature: Singapore, 2021; pp 561–601. doi:10.1007/978-981-33-4392-4_16
Return to citation in text: [1] -
Gregory, P. Functional Dyes. In Industrial Dyes; Hunger, K., Ed.; Wiley-VCH: Weinheim, Germany, 2003; pp 543–584. doi:10.1002/3527602011.ch6
Return to citation in text: [1] -
Zollinger, H. Photo-, Thermo- , and Electrochemical Reactions of Colorants. Wiley-VCH: Weinheim, Germany, 2003; pp 429–504.
Return to citation in text: [1] -
Rao, J.; Yang, L.; Li, X.; Zhao, L.; Wang, S.; Tian, H.; Ding, J.; Wang, L. Angew. Chem., Int. Ed. 2021, 60, 9635–9641. doi:10.1002/anie.202016428
Return to citation in text: [1] -
Tagare, J.; Vaidyanathan, S. J. Mater. Chem. C 2018, 6, 10138–10173. doi:10.1039/c8tc03689f
Return to citation in text: [1] [2] -
Shi, J.; Ding, Q.; Xu, L.; Lv, X.; Liu, Z.; Sun, Q.; Pan, Y.; Xue, S.; Yang, W. J. Mater. Chem. C 2018, 6, 11063–11070. doi:10.1039/c8tc03777a
Return to citation in text: [1] [2] -
Qiu, X.; Ying, S.; Wang, C.; Hanif, M.; Xu, Y.; Li, Y.; Zhao, R.; Hu, D.; Ma, D.; Ma, Y. J. Mater. Chem. C 2019, 7, 592–600. doi:10.1039/c8tc05469j
Return to citation in text: [1] [2] -
Cai, X.; Li, X.; Xie, G.; He, Z.; Gao, K.; Liu, K.; Chen, D.; Cao, Y.; Su, S.-J. Chem. Sci. 2016, 7, 4264–4275. doi:10.1039/c6sc00542j
Return to citation in text: [1] [2] -
Ji, S.-C.; Jiang, S.; Zhao, T.; Meng, L.; Chen, X.-L.; Lu, C.-Z. New J. Chem. 2022, 46, 8991–8998. doi:10.1039/d2nj01072k
Return to citation in text: [1] [2] -
Kundu, S.; Chowdhury, A.; Nandi, S.; Bhattacharyya, K.; Patra, A. Chem. Sci. 2021, 12, 5874–5882. doi:10.1039/d0sc07050e
Return to citation in text: [1] -
Liu, X.-Y.; Wang, X.-J.; Shi, L.; Liu, Y.-H.; Wang, L.; Li, K.; Bu, Q.; Cen, X.-B.; Yu, X.-Q. Anal. Chem. (Washington, DC, U. S.) 2022, 94, 7665–7673. doi:10.1021/acs.analchem.2c01046
Return to citation in text: [1] -
Xu, C.; Li, Y.; Wu, X.; Li, X.; Li, L.; Kong, F.; Tang, B. Chem. Commun. 2022, 58, 5976–5979. doi:10.1039/d2cc01607a
Return to citation in text: [1] [2] -
Liu, Z.; Wang, Q.; Qiu, W.; Lyu, Y.; Zhu, Z.; Zhao, X.; Zhu, W.-H. Chem. Sci. 2022, 13, 3599–3608. doi:10.1039/d2sc00067a
Return to citation in text: [1] [2] -
Dai, X.; Dong, B.; Ren, M.; Lin, W. J. Mater. Chem. B 2018, 6, 381–385. doi:10.1039/c7tb02414b
Return to citation in text: [1] [2] -
Li, Y.; Wang, K.; Zhou, K.; Guo, W.; Dai, B.; Liang, Y.; Dai, J.; Cui, M. Chem. Commun. 2018, 54, 8717–8720. doi:10.1039/c8cc05259j
Return to citation in text: [1] [2] -
Jacob, F. Fluorescent Molecular Sensors of Ions and Molecules. In Molecular Fluorescence; Valeur, B., Ed.; Wiley-VCH: Weinheim, Germany, 2002; pp 273–350. doi:10.1002/3527600248.ch10
Return to citation in text: [1] -
Ooyama, Y. Fluorescent Sensors for Water. In Sustainable and Functional Redox Chemistry; Inagi, S., Ed.; Royal Society of Chemistry: Cambridge, UK, 2022; pp 300–330. doi:10.1039/9781839164828-00300
Return to citation in text: [1] -
Sarkar, B.; Prasad, E.; Gardas, R. L. Mater. Adv. 2022, 3, 2871–2883. doi:10.1039/d1ma01162f
Return to citation in text: [1] -
Tsumura, S.; Enoki, T.; Ooyama, Y. Chem. Commun. 2018, 54, 10144–10147. doi:10.1039/c8cc06257a
Return to citation in text: [1] [2] -
Takemura, K.; Imato, K.; Ooyama, Y. RSC Adv. 2022, 12, 13797–13809. doi:10.1039/d2ra02431d
Return to citation in text: [1] [2] [3] [4] [5] [6] [7] [8] [9] [10] -
Ooyama, Y.; Okamoto, T.; Yamaguchi, T.; Suzuki, T.; Hayashi, A.; Yoshida, K. Chem. – Eur. J. 2006, 12, 7827–7838. doi:10.1002/chem.200600094
Return to citation in text: [1] -
Langhals, H.; Potrawa, T.; Nöth, H.; Linti, G. Angew. Chem., Int. Ed. Engl. 1989, 28, 478–480. doi:10.1002/anie.198904781
Return to citation in text: [1] -
Yeh, H.-C.; Wu, W.-C.; Wen, Y.-S.; Dai, D.-C.; Wang, J.-K.; Chen, C.-T. J. Org. Chem. 2004, 69, 6455–6462. doi:10.1021/jo049512c
Return to citation in text: [1]
| 33. | Takemura, K.; Imato, K.; Ooyama, Y. RSC Adv. 2022, 12, 13797–13809. doi:10.1039/d2ra02431d |
| 1. | Kubo, Y.; Nozawa, T.; Maeda, K.; Hashimoto, Y. Mater. Adv. 2021, 2, 1059–1071. doi:10.1039/d0ma00910e |
| 2. | Dai, J.; Yao, L.; Wang, C.; Wang, Y.; Liu, F.; Yan, X.; Sun, P.; Zhang, H.; Wang, Y.; Zhou, J.; Lu, G. J. Phys. Chem. Lett. 2022, 13, 4754–4761. doi:10.1021/acs.jpclett.2c01226 |
| 3. | Wałęsa-Chorab, M.; Muras, K.; Filiatrault, H. L.; Skene, W. G. J. Mater. Chem. C 2022, 10, 3691–3703. doi:10.1039/d1tc03388c |
| 4. | Ooyama, Y.; Ito, G.; Kushimoto, K.; Komaguchi, K.; Imae, I.; Harima, Y. Org. Biomol. Chem. 2010, 8, 2756–2770. doi:10.1039/c003526b |
| 5. | Ooyama, Y.; Harima, Y. J. Mater. Chem. 2011, 21, 8372–8380. doi:10.1039/c0jm03601c |
| 6. | Josse, P.; Allain, M.; Calupitan, J. P.; Jiang, Y.; Cabanetos, C.; Roncali, J. Adv. Opt. Mater. 2020, 8, 2000420. doi:10.1002/adom.202000420 |
| 7. | Feng, X.; Chen, Y.; Lei, Y.; Zhou, Y.; Gao, W.; Liu, M.; Huang, X.; Wu, H. Chem. Commun. 2020, 56, 13638–13641. doi:10.1039/d0cc05538g |
| 8. | Zhang, X.; Ma, Z.; Yang, Y.; Zhang, X.; Jia, X.; Wei, Y. J. Mater. Chem. C 2014, 2, 8932–8938. doi:10.1039/c4tc01457j |
| 9. | Liu, J.; Cui, Y.; Pan, Y.; Chen, Z.; Jia, T.; Li, C.; Wang, Y. Angew. Chem., Int. Ed. 2022, 61, e202117087. doi:10.1002/anie.202117087 |
| 10. | Narayanaswamy, K.; Venkateswararao, A.; Gupta, V.; Chand, S.; Singh, S. P. Chem. Commun. 2016, 52, 210–213. doi:10.1039/c5cc07435e |
| 11. | Roy, S.; Nandi, S. K.; Haldar, D.; Pal, B. J. Mater. Chem. C 2022, 10, 8767–8775. doi:10.1039/d2tc00951j |
| 12. | Yu, L.; Xi, J.; Chan, H. T.; Su, T.; Antrobus, L. J.; Tong, B.; Dong, Y.; Chan, W. K.; Phillips, D. L. J. Phys. Chem. C 2013, 117, 2041–2052. doi:10.1021/jp3113182 |
| 13. | Enoki, T.; Ohshita, J.; Ooyama, Y. Bull. Chem. Soc. Jpn. 2018, 91, 1704–1709. doi:10.1246/bcsj.20180210 |
| 4. | Ooyama, Y.; Ito, G.; Kushimoto, K.; Komaguchi, K.; Imae, I.; Harima, Y. Org. Biomol. Chem. 2010, 8, 2756–2770. doi:10.1039/c003526b |
| 5. | Ooyama, Y.; Harima, Y. J. Mater. Chem. 2011, 21, 8372–8380. doi:10.1039/c0jm03601c |
| 6. | Josse, P.; Allain, M.; Calupitan, J. P.; Jiang, Y.; Cabanetos, C.; Roncali, J. Adv. Opt. Mater. 2020, 8, 2000420. doi:10.1002/adom.202000420 |
| 7. | Feng, X.; Chen, Y.; Lei, Y.; Zhou, Y.; Gao, W.; Liu, M.; Huang, X.; Wu, H. Chem. Commun. 2020, 56, 13638–13641. doi:10.1039/d0cc05538g |
| 8. | Zhang, X.; Ma, Z.; Yang, Y.; Zhang, X.; Jia, X.; Wei, Y. J. Mater. Chem. C 2014, 2, 8932–8938. doi:10.1039/c4tc01457j |
| 9. | Liu, J.; Cui, Y.; Pan, Y.; Chen, Z.; Jia, T.; Li, C.; Wang, Y. Angew. Chem., Int. Ed. 2022, 61, e202117087. doi:10.1002/anie.202117087 |
| 18. | Tagare, J.; Vaidyanathan, S. J. Mater. Chem. C 2018, 6, 10138–10173. doi:10.1039/c8tc03689f |
| 19. | Shi, J.; Ding, Q.; Xu, L.; Lv, X.; Liu, Z.; Sun, Q.; Pan, Y.; Xue, S.; Yang, W. J. Mater. Chem. C 2018, 6, 11063–11070. doi:10.1039/c8tc03777a |
| 20. | Qiu, X.; Ying, S.; Wang, C.; Hanif, M.; Xu, Y.; Li, Y.; Zhao, R.; Hu, D.; Ma, D.; Ma, Y. J. Mater. Chem. C 2019, 7, 592–600. doi:10.1039/c8tc05469j |
| 25. | Xu, C.; Li, Y.; Wu, X.; Li, X.; Li, L.; Kong, F.; Tang, B. Chem. Commun. 2022, 58, 5976–5979. doi:10.1039/d2cc01607a |
| 26. | Liu, Z.; Wang, Q.; Qiu, W.; Lyu, Y.; Zhu, Z.; Zhao, X.; Zhu, W.-H. Chem. Sci. 2022, 13, 3599–3608. doi:10.1039/d2sc00067a |
| 33. | Takemura, K.; Imato, K.; Ooyama, Y. RSC Adv. 2022, 12, 13797–13809. doi:10.1039/d2ra02431d |
| 29. | Jacob, F. Fluorescent Molecular Sensors of Ions and Molecules. In Molecular Fluorescence; Valeur, B., Ed.; Wiley-VCH: Weinheim, Germany, 2002; pp 273–350. doi:10.1002/3527600248.ch10 |
| 30. | Ooyama, Y. Fluorescent Sensors for Water. In Sustainable and Functional Redox Chemistry; Inagi, S., Ed.; Royal Society of Chemistry: Cambridge, UK, 2022; pp 300–330. doi:10.1039/9781839164828-00300 |
| 31. | Sarkar, B.; Prasad, E.; Gardas, R. L. Mater. Adv. 2022, 3, 2871–2883. doi:10.1039/d1ma01162f |
| 32. | Tsumura, S.; Enoki, T.; Ooyama, Y. Chem. Commun. 2018, 54, 10144–10147. doi:10.1039/c8cc06257a |
| 33. | Takemura, K.; Imato, K.; Ooyama, Y. RSC Adv. 2022, 12, 13797–13809. doi:10.1039/d2ra02431d |
| 23. | Kundu, S.; Chowdhury, A.; Nandi, S.; Bhattacharyya, K.; Patra, A. Chem. Sci. 2021, 12, 5874–5882. doi:10.1039/d0sc07050e |
| 24. | Liu, X.-Y.; Wang, X.-J.; Shi, L.; Liu, Y.-H.; Wang, L.; Li, K.; Bu, Q.; Cen, X.-B.; Yu, X.-Q. Anal. Chem. (Washington, DC, U. S.) 2022, 94, 7665–7673. doi:10.1021/acs.analchem.2c01046 |
| 25. | Xu, C.; Li, Y.; Wu, X.; Li, X.; Li, L.; Kong, F.; Tang, B. Chem. Commun. 2022, 58, 5976–5979. doi:10.1039/d2cc01607a |
| 26. | Liu, Z.; Wang, Q.; Qiu, W.; Lyu, Y.; Zhu, Z.; Zhao, X.; Zhu, W.-H. Chem. Sci. 2022, 13, 3599–3608. doi:10.1039/d2sc00067a |
| 27. | Dai, X.; Dong, B.; Ren, M.; Lin, W. J. Mater. Chem. B 2018, 6, 381–385. doi:10.1039/c7tb02414b |
| 28. | Li, Y.; Wang, K.; Zhou, K.; Guo, W.; Dai, B.; Liang, Y.; Dai, J.; Cui, M. Chem. Commun. 2018, 54, 8717–8720. doi:10.1039/c8cc05259j |
| 33. | Takemura, K.; Imato, K.; Ooyama, Y. RSC Adv. 2022, 12, 13797–13809. doi:10.1039/d2ra02431d |
| 14. | Yagi, S. Luminescent Materials for Organic Light-Emitting Diodes. In Progress in the Science of Functional Dyes; Ooyama, Y.; Yagi, S., Eds.; Springer Nature: Singapore, 2021; pp 561–601. doi:10.1007/978-981-33-4392-4_16 |
| 15. | Gregory, P. Functional Dyes. In Industrial Dyes; Hunger, K., Ed.; Wiley-VCH: Weinheim, Germany, 2003; pp 543–584. doi:10.1002/3527602011.ch6 |
| 16. | Zollinger, H. Photo-, Thermo- , and Electrochemical Reactions of Colorants. Wiley-VCH: Weinheim, Germany, 2003; pp 429–504. |
| 17. | Rao, J.; Yang, L.; Li, X.; Zhao, L.; Wang, S.; Tian, H.; Ding, J.; Wang, L. Angew. Chem., Int. Ed. 2021, 60, 9635–9641. doi:10.1002/anie.202016428 |
| 18. | Tagare, J.; Vaidyanathan, S. J. Mater. Chem. C 2018, 6, 10138–10173. doi:10.1039/c8tc03689f |
| 19. | Shi, J.; Ding, Q.; Xu, L.; Lv, X.; Liu, Z.; Sun, Q.; Pan, Y.; Xue, S.; Yang, W. J. Mater. Chem. C 2018, 6, 11063–11070. doi:10.1039/c8tc03777a |
| 20. | Qiu, X.; Ying, S.; Wang, C.; Hanif, M.; Xu, Y.; Li, Y.; Zhao, R.; Hu, D.; Ma, D.; Ma, Y. J. Mater. Chem. C 2019, 7, 592–600. doi:10.1039/c8tc05469j |
| 21. | Cai, X.; Li, X.; Xie, G.; He, Z.; Gao, K.; Liu, K.; Chen, D.; Cao, Y.; Su, S.-J. Chem. Sci. 2016, 7, 4264–4275. doi:10.1039/c6sc00542j |
| 22. | Ji, S.-C.; Jiang, S.; Zhao, T.; Meng, L.; Chen, X.-L.; Lu, C.-Z. New J. Chem. 2022, 46, 8991–8998. doi:10.1039/d2nj01072k |
| 34. | Ooyama, Y.; Okamoto, T.; Yamaguchi, T.; Suzuki, T.; Hayashi, A.; Yoshida, K. Chem. – Eur. J. 2006, 12, 7827–7838. doi:10.1002/chem.200600094 |
| 35. | Langhals, H.; Potrawa, T.; Nöth, H.; Linti, G. Angew. Chem., Int. Ed. Engl. 1989, 28, 478–480. doi:10.1002/anie.198904781 |
| 36. | Yeh, H.-C.; Wu, W.-C.; Wen, Y.-S.; Dai, D.-C.; Wang, J.-K.; Chen, C.-T. J. Org. Chem. 2004, 69, 6455–6462. doi:10.1021/jo049512c |
| 33. | Takemura, K.; Imato, K.; Ooyama, Y. RSC Adv. 2022, 12, 13797–13809. doi:10.1039/d2ra02431d |
| 33. | Takemura, K.; Imato, K.; Ooyama, Y. RSC Adv. 2022, 12, 13797–13809. doi:10.1039/d2ra02431d |
| 33. | Takemura, K.; Imato, K.; Ooyama, Y. RSC Adv. 2022, 12, 13797–13809. doi:10.1039/d2ra02431d |
| 33. | Takemura, K.; Imato, K.; Ooyama, Y. RSC Adv. 2022, 12, 13797–13809. doi:10.1039/d2ra02431d |
| 33. | Takemura, K.; Imato, K.; Ooyama, Y. RSC Adv. 2022, 12, 13797–13809. doi:10.1039/d2ra02431d |
| 10. | Narayanaswamy, K.; Venkateswararao, A.; Gupta, V.; Chand, S.; Singh, S. P. Chem. Commun. 2016, 52, 210–213. doi:10.1039/c5cc07435e |
| 11. | Roy, S.; Nandi, S. K.; Haldar, D.; Pal, B. J. Mater. Chem. C 2022, 10, 8767–8775. doi:10.1039/d2tc00951j |
| 12. | Yu, L.; Xi, J.; Chan, H. T.; Su, T.; Antrobus, L. J.; Tong, B.; Dong, Y.; Chan, W. K.; Phillips, D. L. J. Phys. Chem. C 2013, 117, 2041–2052. doi:10.1021/jp3113182 |
| 13. | Enoki, T.; Ohshita, J.; Ooyama, Y. Bull. Chem. Soc. Jpn. 2018, 91, 1704–1709. doi:10.1246/bcsj.20180210 |
| 21. | Cai, X.; Li, X.; Xie, G.; He, Z.; Gao, K.; Liu, K.; Chen, D.; Cao, Y.; Su, S.-J. Chem. Sci. 2016, 7, 4264–4275. doi:10.1039/c6sc00542j |
| 22. | Ji, S.-C.; Jiang, S.; Zhao, T.; Meng, L.; Chen, X.-L.; Lu, C.-Z. New J. Chem. 2022, 46, 8991–8998. doi:10.1039/d2nj01072k |
| 27. | Dai, X.; Dong, B.; Ren, M.; Lin, W. J. Mater. Chem. B 2018, 6, 381–385. doi:10.1039/c7tb02414b |
| 28. | Li, Y.; Wang, K.; Zhou, K.; Guo, W.; Dai, B.; Liang, Y.; Dai, J.; Cui, M. Chem. Commun. 2018, 54, 8717–8720. doi:10.1039/c8cc05259j |
| 32. | Tsumura, S.; Enoki, T.; Ooyama, Y. Chem. Commun. 2018, 54, 10144–10147. doi:10.1039/c8cc06257a |
| 33. | Takemura, K.; Imato, K.; Ooyama, Y. RSC Adv. 2022, 12, 13797–13809. doi:10.1039/d2ra02431d |
© 2022 Takemura et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.