Abstract
Substituted 4-acetyl-3-hydroxy-3-pyrroline-2-ones have been prepared via three-component reactions and the tautomerism of these 3-pyrroline-2-ones is due to the slight difference of energy, and the significantly large rate constant of transformation between two tautomers. 1,4,5-Trisubstituted pyrrolidine-2,3-dione derivatives were prepared from the above mentioned 2-pyrrolidinone derivatives and aliphatic amines, which exist in enamine form and are stabilized by an intramolecular hydrogen bond. A possible reaction mechanism between 3-pyrroline-2-one and aliphatic amine (CH3NH2) was proposed based on computational results and the main product is formed favorably following the PES via the lowest ΔG# pathway in both the gas-phase and an ethanol solvent model. DFT calculations showed that kinetic selectivity is more significant than thermodynamic selectivity for forming main products.
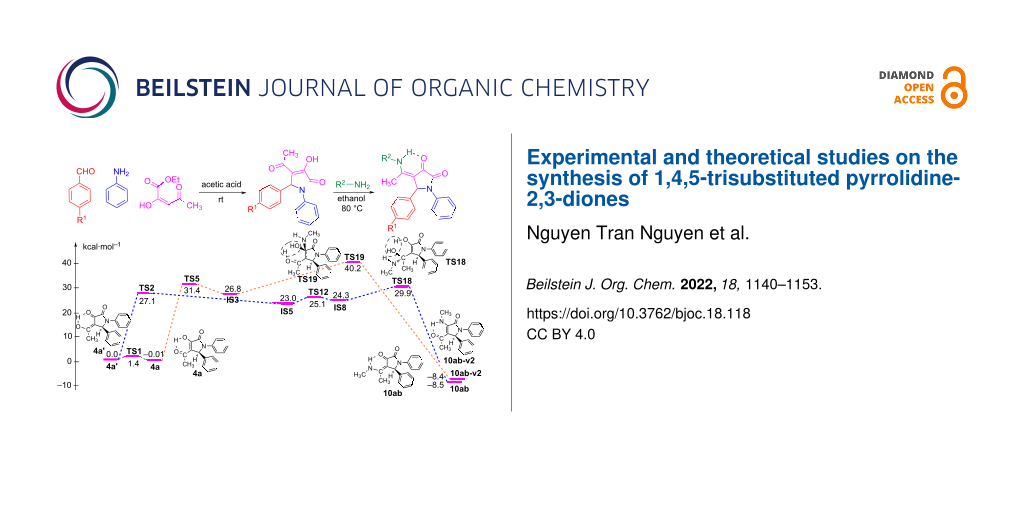
Graphical Abstract
Introduction
2-Pyrrolidone, also known as γ-lactam, is a five-membered heterocyclic ring containing four carbon and one nitrogen atoms [1]. This γ-lactam structure is an important scaffold which can be found in many pharmaceutical active natural products and synthetic medicinal compounds [2]. Pramanicin, for example, is a 2-pyrrolidone-containing natural product isolated from a lactose-containg liquid fermentation of a sterile fungus growing in grass which showed interesting antimicrobial and antibacterial activities [2-4]. (−)-Clausenamide was extracted and isolated from Clausena lansium which exhibited biological activities to enhance learning and memory capacities in amnesia animal models [5,6]. Doxapram, a non-natural compound, has been used to form doxapram hydrochloride which helps to increase the respiratory rate [7] (Figure 1).
Figure 1: Structure of naturally occurring and synthetic 2-pyrrolidone derivatives.
Figure 1: Structure of naturally occurring and synthetic 2-pyrrolidone derivatives.
Among the 2-pyrrolidinone derivatives, 1,5-dihydro-2H-pyrrol-2-ones, also named as 3-pyrrolin-2-ones, are important builiding blocks which can be further modified in organic synthesis and medicinal chemistry [8-10]. In addition, these 2-oxopyrroles are structural subunits of various bioactive natural compounds and synthetic drugs. For example, codinaeopsin has been isolated from a fungal extract of a tree called Vochysia guatemalensis which shows antimalarial activity [11]. Pyrrocidine A was isolated from the fungal endophyte Acremonium zeae and its chemical structure was elucidated in 2002 (Figure 2). This natural 3-pyrrolin-2-one derivative exhibits potent antivity against Gram-positive bacteria [12]. Moreover, 1,5-dihydro-2H-pyrrol-2-ones and especially, 3-hydroxy-1,5-dihydro-2H-pyrrol-2-ones are also important substructures in a variety of non-natural compounds and their pharmacological effects against bacteria [13-16], inflammation [17,18], viruses [19], radical [20], and cancer [21-26] have been proven.
Figure 2: Structure of natural compounds containing a 1,5-dihydro-2H-pyrrol-2-one subunit.
Figure 2: Structure of natural compounds containing a 1,5-dihydro-2H-pyrrol-2-one subunit.
It is undoubtedly true that heterocyclic compounds containing a 3-pyrroline-2-one skeleton could be promising drug candidates. Therefore, these nitrogen-containing heterocycles have attracted attention and they have been investigated most intensively via multicomponent reactions (MCRs). This kind of reaction has been proven to be an efficient synthetic pathway to obtain structurally complicated and biologically active products containing substantial portions of all starting materials [27]. Recently, polysubstituted 3-hydroxy-1,5-dihydro-2H-pyrrol-2-ones have been synthesized via eco-friendly multicomponent reactions of aromatic aldehydes, amines, and dialkyl acetylenedicarboxylate or sodium diethyl oxalacetate [28-34]. The resulting products have the 4-position locked by the alkoxycarbonyl (–COOR) group and these 2-pyrrolidinone derivatives can be functionalized with amines as nucleophiles via the 3-position [10,35-38].
Acyl or aroyl groups can be introduced into the 4-position of 3-hydroxy-1,5-dihydro-2H-pyrrol-2-ones via three-component reactions of esters of acylpyruvic acid or of aroylpyruvic acid, respectively, with aromatic aldehydes, and amines. In addition, the resulting 2-pyrrolidinone derivatives also showed antibacterial and antifungal activities [39-41]. The presence of an acyl group at the 4-position enables these heterocycles to be functionalized via nucleophilic addition reactions between the carbonyl group and nucleophiles like hydroxylamine and semicarbazide [42].
Herein, we report the synthesis of 4-acetyl-3-hydroxy-3-pyrroline-2-ones via multicomponent reactions of ethyl 2,4-dioxovalerate with aromatic aldehydes, and aniline in glacial acetic acid. These 2-pyrrolidinone derivatives were then reacted with aliphatic amines in ethanol to obtain a library of 1,4,5-trisubstituted pyrrolidine-2,3-dione enamine derivatives. As compared to glacial acetic acid [42], ethanol has showed to be the best solvent for the synthesis of these pyrrolidine-2,3-diones and a dramatic increase in the yield of the desired products was also observed. In addition, understanding of the reaction mechanism at the molecular level brings an efficient evaluation and good orientation in experimental work [43]. Density functional theory (DFT) calculations were used to explore the mechanistic aspects of the reaction between 4-acetyl-3-hydroxy-1,5-diphenyl-3-pyrrolin-2-one and methylamine (CH3NH2) to achieve 4-(1-methylamino)ethylene-1,5-diphenylpyrrolidine-2,3-dione in the present work. To the best of our knowledge, it is the first time the reaction mechanism between 3-pyrrolin-2-one derivative and methylamine was explained in detail via computational studies.
Results and Discussion
A model reaction of benzaldehyde (1a), aniline (2), and ethyl 2,4-dioxovalerate (3) in glacial acetic acid was used for optimization (Scheme 1). Reacting equimolar amounts of 1a, 2, and 3, 0.5 M in acetic acid as solvent at room temperature [39], resulted in the formation of 1,5-diphenyl-4-acetyl-3-hydroxy-3-pyrrolin-2-one (4a) in a yield of 70%. However, an increase in the yield of product 4a to 77% could be observed with the ratio of 1:1:1.5 of reactants 1a, 2, and 3, respectively (Table 1).
Scheme 1: Synthesis of substituted 4-acetyl-3-hydroxy-3-pyrroline-2-ones 4 via three-component reaction.
Scheme 1: Synthesis of substituted 4-acetyl-3-hydroxy-3-pyrroline-2-ones 4 via three-component reaction.
The product 4a was obtained in 80% yield when the concentration of the aromatic aldehyde 1a in solvent increased to 0.75 M while remaining the amounts of 2 and 3 unchanged. However, increasing the concentration of aniline (2) in acetic acid to 0.75 M while keeping the amounts of 1 and 3 constant at 0.5 M resulted in a slight decrease of the yield (67%) of 2-pyrrolidinone derivative 4a (Table 1). Therefore, the ratio 1.5:1:1 of reactants, the aromatic aldehyde, aniline, and ethyl 2,4-dioxovalerate, respectively, in acetic acid as solvent was used to synthesize other substituted 4-acetyl-3-hydroxy-3-pyrroline-2-ones.
It may be surmised that the first step in the three-component reaction to synthesize substituted 4-acetyl-3-hydroxy-3-pyrroline-2-ones 4a–c occurs via the acid-catalyzed condensation of the aromatic aldehyde 1a–c and aniline (2) to produce imine intermediate 5 which is then protonated to the iminium species 6. In addition, ethyl 2,4-dioxovalerate (3) containing an activated methylene group is in fast equilibrium with enol derivative 7 in acidic media, the latter playing the role of nucleophilic reagent which attacks to iminium salt 6 to give intermediate 8. Subsequently, intramolecular nucleophilic attack of the amino moiety to the carboxylate group in the intermediate 8 leads to the formation of the target products 4a–c (Scheme 2).
Scheme 2: Proposed mechanistic path for the synthesis of substituted 4-acetyl-3-hydroxy-3-pyrroline-2-ones.
Scheme 2: Proposed mechanistic path for the synthesis of substituted 4-acetyl-3-hydroxy-3-pyrroline-2-ones.
The reaction of aromatic aldehyde 1a and aniline (2) in acidic environment is reversible [44] and therefore, according to Le Chatelier’s principle [45], the increase in the concentration of 1a or 2 will shift the equilibrium to the side of the iminium species 6. As the concentration of 2 was increased to 0.75 M as compared to 0.5 M of aldehyde 1a, the equilibrium would favor the side of iminium salt 6. However, the excess amount of the nucleophilic reactant 2 reacted with intermediate 6 and as a consequence, the yield of the product 4a decreased to 67% (Table 1, Scheme 2).
The 1H NMR spectra of compounds 4a–c in DMSO-d6 showed peaks of aromatic protons in the chemical shift region of 6.97–7.59 ppm, a singlet at 6.00–6.06 ppm for the proton at the 5-position of the 3-pyrrroline-2-one moiety. In addition, along with peaks of carbons at the 2,5-positions of the N-containing heterocyclic ring and signals of the aromatic carbons, the 13C NMR spectra of these compounds in DMSO-d6 also exhibited broad peaks at around 30 ppm and 191 ppm. The NMR spectra of the 2-pyrrolidinone derivative 4c were also recorded in CDCl3 and its 1H NMR spectrum is similar to the one observed in DMSO-d6. However, the 13C NMR spectrum of 4c in CDCl3 showed sharp peaks at 29.21 ppm and 194.61 ppm representing the two carbon atoms of the acetyl group attached to the 4-postion of the heterocyclic five-membered ring. Therefore, the broadening of peaks observed in the 13C NMR spectra is due to the tautomerism of compounds 4a–c in DMSO [46], a hydrogen-bond accepting solvent (Scheme 3).
Scheme 3: Tautomerism of compounds 4a–c in DMSO.
Scheme 3: Tautomerism of compounds 4a–c in DMSO.
To clarify further this statement, DFT calculations were performed at the B3LYP/6-311++G(2d,2p)//B3LYP/6-31+G(d,p) level of theory as given in Table 2 and Table 3. The theoretical results show that structure 4a is more stable than 4a’ by 1.3 kcal·mol−1 in the gas phase or by 0.4 kcal·mol−1 in the ethanol solvent model. The transformation of 4a into 4a’ occurs with a small potential barrier of 0.5 kcal·mol−1 (in the gas phase) or 1.0 kcal·mol−1 (in ethanol). This process goes through TS1 by H displacement at the O–H bond of the O–H···O intramolecular hydrogen bond (distance ca. 1.7–1.8 Å) (cf. Figures 4–6). The isomerization of 4a/4a’ is extremely fast due to the high rate constant of about 1012 s−1 at 298 K, calculated by the transitional state theory method and quantum tunneling effect [47]. It is thus impossible to distinguish 4a and 4a’ (Scheme 3). Consequently, isomers 4a and 4a’ coexist during the synthesis of 10ab from the reaction of 4a/4a’ and CH3NH2, being consistent with our experimental work (cf. Table 5).
Table 2: The thermodynamic parameters of possible reaction pathways in the gas phase (kcal·mol−1) at the B3LYP/6-311++G(2d,2p)// B3LYP/6-31+G(d,p) level of theory.
| Entry | Stage | ΔE | ΔG | ΔE# | ΔG# | I-Freq (cm−1) | Note |
| 1 | 4a’→ 4a | −1.3 | −1.0 | 0.5 | 0.6 | −1156.69 | TS1 |
| 2 | 4a’ → IS5 | 10.1 | 22.9 | 21.9 | 34.6 | −212.87 | TS2 |
| 3 | 4a’ → IS3 | 9.0 | 21.3 | 37.5 | 50.0 | −1565.52 | TS3 |
| 4 | 4a’ → IS2 | 14.5 | 27.0 | 44.8 | 57.2 | −1612.98 | TS4 |
| 5 | 4a → IS1 | −3.6 | 5.3 | ||||
| 6 | 4a → IS3 | 10.3 | 22.3 | 22.9 | 34.7 | −186.59 | TS5 |
| 7 | 4a → IS4 | 16.7 | 29.2 | 46.1 | 58.3 | −1630.70 | TS6 |
| 8 | IS2 → IS4 | 0.9 | 1.2 | 1.0 | 1.3 | −1099.78 | TS7 |
| 9 | IS1 → IS5 | 15.0 | 15.9 | 41.7 | 45.2 | −1521.85 | TS8 |
| 10 | IS3 → IS5 | 1.1 | 1.6 | 58.3 | 59.0 | −335.98 | TS9 |
| 11 | IS3 → IS6 | −1.6 | −1.1 | 6.7 | 7.4 | −139.56 | TS10 |
| 12 | IS4 → IS7 | −1.1 | −1.3 | 3.9 | 4.7 | −115.85 | TS11 |
| 13 | IS5 → IS8 | 1.0 | 0.7 | 1.7 | 2.5 | −64.84 | TS12 |
| 14 | IS2 → 10ab-v4 | −6.3 | 15.1 | 26.7 | 26.9 | −216.31 | TS13 |
| 15 | IS5 → IS9 | −8.6 | −19.4 | 41.6 | 31.8 | −1083.98 | TS14 |
| 16 | IS6 → IS10 | −9.3 | −20.7 | 28.8 | 28.4 | −359.26 | TS15 |
| 17 | IS7 → IS11 | −1.0 | −11.8 | 26.5 | 26.9 | −276.33 | TS16 |
| 18 | IS4 → 10ab-v3 | −1.7 | −13.2 | 33.9 | 31.8 | −314.94 | TS17 |
| 19 | IS8 → 10ab | −15.7 | −26.6 | 11.1 | 10.7 | −902.87 | TS18 |
| 20 | IS3 → 10ab-v2 | −20.1 | −30.7 | 17.3 | 17.3 | −1078.98 | TS19 |
| 21 | IS9 → 10ab | −6.1 | −6.5 | ||||
| 22 | IS10 → 10ab-v2 | −9.2 | −8.9 | ||||
| 23 | IS11 → 10ab-v3 | 0.4 | −0.1 | 7.2 | 7.1 | −1366.77 | TS20 |
Table 3: The thermodynamic parameters of selected pathways (1), (2), (3), and (4) in ethanol (kcal·mol−1) at the B3LYP/6-311++G(2d,2p)// B3LYP/6-31+G(d,p) level of theory.
| Entry | Stage | ΔE | ΔG | ΔE# | ΔG# | I-Freq (cm−1) | Note |
| 1 | 4a’ → 4a | −0.4 | −0.01 | 1.0 | 1.4 | −1169.88 | TS1 |
| 2 | 4a’ → IS5 | 10.1 | 23.0 | 14.3 | 27.1 | −240.38 | TS2 |
| 3 | 4a’ → IS3 | 14.5 | 26.8 | 38.4 | 35.7 | −1670.26 | TS3 |
| 4 | 4a → IS1 | −7.3 | 2.2 | ||||
| 5 | 4a → IS3 | 14.9 | 26.8 | 19.4 | 31.4 | −197.98 | TS5 |
| 6 | IS1 → IS5 | 17.8 | 20.8 | 27.0 | 45.4 | −1648.76 | TS8 |
| 7 | IS3 → IS6 | −2.9 | −2.3 | 6.0 | 6.6 | −135.85 | TS10 |
| 8 | IS5 → IS8 | 1.3 | 1.3 | 2.1 | 2.1 | −63.84 | TS12 |
| 9 | IS5 → IS9 | −11.3 | −21.8 | 34.7 | 34.4 | −272.88 | TS14 |
| 10 | IS6 → IS10 | −11.7 | −22.7 | 24.5 | 23.8 | −252.49 | TS15 |
| 11 | IS8 → 10ab | −22.1 | −32.8 | 6.1 | 5.6 | −748.04 | TS18 |
| 12 | IS3 → 10ab-v2 | −24.7 | −35.2 | 13.4 | 13.4 | −831.36 | TS19 |
| 13 | IS9 → 10ab | −9.5 | −9.7 | ||||
| 14 | IS10 → 10ab-v2 | −10.1 | −10.2 | ||||
Three substituted 3-hydroxy-3-pyrroline-2-ones 4a–c were then reacted with aliphatic amines and the structures of the obtained compounds were elucidated by NMR and mass spectrometry. The reaction between 1,5-diphenyl-4-acetyl-3-hydroxy-3-pyrrolin-2-one (4a) and 4-methoxybenzylamine (9a) was chosen to optimize the reaction conditions, such as the reactant ratio and solvent. Heating 2-pyrrolidinone derivative 4a (1 equiv) and aliphatic amine 9a (2.5 equiv) in glacial acetic acid [42] resulted in the formation of product 10aa with a yield of only 36%. It is clear that compound 9a could be protonated in the acidic environment, decreasing its nucleophilicity and therefore, product 10aa was obtained in low yield. The yield of 10aa could be increased to 62% when substrate 4a was reacted with the amine 9a in DMF at 95 °C (Table 4), meaning that the reaction does not require an acidic medium.
Table 4: Optimization of the reaction conditions for the synthesis of compound 10aa.
|
|
||||||
| Entry | Ratio 4a:9a (equiv) | Solvent | Volume (mL) | T (°C) | t (hour) | Yield (%) |
| 1 | 1:2.5 | DMF | 1 | 95 | 6 | 62 |
| 2 | 1:2.5 | AcOH | 1 | 95 | 9.5 | 36 |
| 3 | 1:1 | ethanol | 1 | 80 | 5 | 69 |
| 4 | 1:2.5 | ethanol | 1 | 80 | 3 | 72 |
| 5 | 1:2.5 | ethanol | 1 | 80 | 5 | 73 |
| 6 | 1:4 | ethanol | 1 | 80 | 5 | 82 |
| 7 | 1:4 | ethanol | 0.5 | 80 | 5 | 86 |
| 8 | 1:4 | ethanol | 0.34 | 80 | 5 | 89 |
Ethanol was also used as green solvent to test the above model reaction. As compared to CH3COOH, heating compounds 4a (1 equiv, 0.17 mmol) and 9a (2.5 equiv, 0.43 mmol) in 1 mL of ethanol at 80 °C led to a dramatic increase in the yield of product 10aa, 72%. In addition, 2-pyrrolidinone derivative 10aa could be obtained in 82% yield when the amount of the amine 9a was increased to 4 equiv. Unsurprisingly, the yield of the desired product 10aa reached 89% when the solvent volume was lowered to 0.34 mL (Table 4). The optimized procedure was then applied to the synthesis of other 1,4,5-trisubstituted pyrrolidine-2,3-dione enamine derivatives with high yields (Table 5).
Table 5: Synthesis of a library of 1,4,5-trisubstituted pyrrolidine-2,3-diones.
|
|
|||||
| Entry | Ar1 | Ar2 | R | Product | Yield (%) |
| 1 | C6H5 | C6H5 | 4-MeOC6H4CH2 | 10aa | 89 |
| 2 | C6H5 | C6H5 | CH3 | 10ab | 88 |
| 3 | C6H5 | C6H5 | C6H5CH2 | 10ac | 90 |
| 4 | C6H5 | C6H5 | CH3CH2OCOCH2 | 10ad | 82 |
| 5 | 4-MeC6H4 | C6H5 | 4-MeOC6H4CH2 | 10ba | 76 |
| 6 | 4-MeC6H4 | C6H5 | CH3 | 10bb | 84 |
| 7 | 4-MeC6H4 | C6H5 | C6H5CH2 | 10bc | 81 |
| 8 | 4-MeC6H4 | C6H5 | CH3CH2OCOCH2 | 10bd | 87 |
| 9 | 4-ClC6H4 | C6H5 | 4-MeOC6H4CH2 | 10ca | 81 |
| 10 | 4-ClC6H4 | C6H5 | CH3 | 10cb | 77 |
| 11 | 4-ClC6H4 | C6H5 | C6H5CH2 | 10cc | 79 |
| 12 | 4-ClC6H4 | C6H5 | CH3CH2OCOCH2 | 10cd | 80 |
The 1H NMR spectrum of product 10aa showed a broad peak corresponding to the proton of the secondary amino group (NH) at a chemical shift of 11.74 ppm. The intramolecular hydrogen bond led to the appearance of a resonance signal of the amino group at high chemical shift. Also, the coupling between the amino proton and methylene protons was observed. In addition, the methylene protons are diastereotopic and they show geminal coupling. As a consequence, the two methylene protons were observed as two doublets of doublets at 4.40 ppm and 4.49 ppm, respectively. Moreover, the HMBC 2D NMR spectrum of compound 10aa also exhibited the correlation between the amino proton and the carbon of the methyl group separated by three σ-bonds. Thus, the reaction between 2-pyrrolidinone derivatives 4a–c and nucleophilic amines 9a–d is confirmed to occur at the carbonyl group of the acetyl moiety to achieve 1,4,5-trisubstituted pyrrolidine-2,3-dione derivatives present in the enamine form and stabilized by intramolecular hydrogen bonds (Table 5).
Single-crystal X-ray diffraction confirmed the structure of 10aa (Figure 3) as well as the presence of the intramolecular N15–H15···O13 hydrogen bond [N15–H15: 0.92(3) Å, H15···O13: 1.90(3) Å, N15···O13: 2.699(3) Å, N15–H15···O13: 145(3)˚]. The pyrrolidine-2,3-dione ring is planar (rms deviation: 0.003 Å) and makes an angle of 40.39(14)˚ with the phenyl ring C6–C11 and of 82.23(15)˚ with the phenyl ring C26–C31. The dihedral angle between these phenyl rings is 74.87(14)˚. The crystal packing of product 10aa (Figure S1 in Supporting Information File 1) is characterized by C7–H7···O12i hydrogen bonds resulting in chain formation along the a direction [C7–H7: 0.93 Å, H7···O12: 2.42 Å, C7···O12: 3.325(3) Å, C7–H7···O12: 164˚; symmetry code: (i) 1 + x, y, z]. Neighboring chains connect by C–H···π interactions between C29–H29 and phenyl ring C6–C11 [H29···Cgii: 2.77 Å, Cg is the centroid of ring C6–C11, symmetry code: (ii) 1 − x, 2 − y, 1 − z].
![[1860-5397-18-118-3]](/bjoc/content/figures/1860-5397-18-118-3.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 3: View of the molecular structure of compound 10aa with atom labeling. Displacement ellipsoids are drawn at the 30% probability level. The intramolecular hydrogen bond N15–H15···O13 is shown as dashed line.
Figure 3: View of the molecular structure of compound 10aa with atom labeling. Displacement ellipsoids are dr...
As a model example, the reaction between substrate 4a and methylamine (9b) was used to elucidate the mechanism to obtain product 10ab via DFT calculations at the B3LYP/6-311++G(2d,2p)// B3LYP/6-31+G(d,p) level of theory. To consider the reaction pathways of 4a, 4a’ to form product 10ab synthesized from experimental work, we optimized all possible geometries of 4a, 4a’, and 10ab. The structures and their corresponding energy values are listed in Table S2 and shown in Figures S2, S3, and S4 in Supporting Information File 1. Accordingly, the most stable shapes of 4a, 4a’, and 10ab are considered in the potential energy surface (PES) and displayed in Figure 4 and Figure 6. Fortunately, these structures have similar stereochemical configurations. Further, the structures of IS5 and 10ab-v2 are also considered with all possible isomers and are listed in Figures S5 and S6, and Table S2 (Supporting Information File 1). It is noticeable that the IS5 and 10ab-v2 are formed via the reaction between 4a/4a’ and 9b (CH3NH2) without any changes in stereochemistry. The reactants, intermediates, transition states, and products were optimized and are illustrated in Figure S7 in Supporting Information File 1. The relative free energy (ΔG, ΔG#) values for all species are presented in Figure 4, the reaction potential energy surface. This figure is a detailed reaction mechanism to form product 10ab and isomers from 4a/4a’ in the gas phase. The ΔG, ΔG# values for each stage in the PES at the gas phase are collected in Table 2.
![[1860-5397-18-118-4]](/bjoc/content/figures/1860-5397-18-118-4.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 4: The PES of reaction for the synthesis of 1,4,5-trisubstituted pyrrolidine-2,3-dione 10ab enamine derivative in the gas phase.
Figure 4: The PES of reaction for the synthesis of 1,4,5-trisubstituted pyrrolidine-2,3-dione 10ab enamine de...
The 4a and 4a’ tautomers react with CH3NH2 to yield products, as detailed in Figure 4. All three C (C−O) sites in 4a, 4a’ are possible for interaction with CH3NH2 to obtain 2-pyrrolidinone derivative 10ab and its isomers (10ab-v2, 10ab-v3, 10ab-v4) (Figure S7 in Supporting Information File 1). It is clear that the attack of CH3NH2 on the C (C−O) sites to form intermediates (ISs) takes place on the opposite side of the benzene ring (C−C6H5). This is due to the steric hindrance of the C6H5-groups attached to the 1- and 5-position of the heterocyclic ring. The 3D-optimized structures of substances on the PES are illustrated in Figures S2, S3, S4, S5, S6 and S7 in Supporting Information File 1.
For the reaction pathway starting from 4a, firstly, it interacts with CH3NH2 to form a stable complex (IS1), which is then transfered to the IS5 intermediate via the TS8 transition state with a high potential barrier of 49.5 kcal·mol−1. From the IS5 intermediate, there are two possible ways to yield product 10ab by removing one H2O molecule from either two O–H groups or O–H and N–H groups. Before a H2O molecule is released from two O–H groups, IS5 firstly converts to its isomer IS8 through the TS12 transition state with a relatively small potential barrier (ca. 2.6 kcal·mol−1). IS8 then removes H2O from two close O–H bonds to form the product 10ab via TS18 with an activation energy of about 10.7 kcal·mol−1. On the other hand, if the removal of one H2O molecule occurs from the O–H and N–H groups, IS5 transforms into IS9 through TS14 with a high barrier of 31.8 kcal·mol−1. IS9 is then converted to product 10ab based on the shifting of H atom from the O–H bond to N atom (Scheme 4). This process is thermodynamically favorable by a Gibbs free energy (ΔG) of −6.5 kcal·mol−1.
Scheme 4: Reaction pathways from 4a, 4a’ to 10ab via IS5 in the gase phase.
Scheme 4: Reaction pathways from 4a, 4a’ to 10ab via IS5 in the gase phase.
In addition, a second way from 4a, through the TS5 transition state, to form the IS3 intermediate requires an activation energy of ca. 33.7 kcal·mol−1. IS3 could release one H2O molecule from two O–H groups to obtain 10ab-v2 through the TS19 transition state with a barrier of 17.3 kcal·mol−1. Alternatively, IS3 will firstly transfer to its isomer IS6, and then IS10 by removing one H2O molecule from O–H and N–H groups and finally produce 10ab-v2 via isomerization with H shift (Scheme 5). The first two stages occur through the TS10, TS15 transition states with Ea values ca. 7.4 and 28.4 kcal·mol−1, respectively. The last stage is a thermodynamically favorable H-shift process with ΔG of −8.9 kcal·mol−1 which is similar to the transformation from IS9 to 10ab. In addition, we found a more difficult way to yield IS5 from IS3 via TS9 with a high barrier of 59.0 kcal·mol−1.
Scheme 5: Reaction pathways from 4a to 10ab-v2 via IS3 in the gase phase.
Scheme 5: Reaction pathways from 4a to 10ab-v2 via IS3 in the gase phase.
The most unlikely direction from 4a is to create an IS4 intermediate via the TS6 transition state with a high potential barrier (ca. 58.3 kcal·mol−1). From IS4, one H2O molecule is cleaved from two O–H bonds to produce 10ab-v3. This process passes through TS17 with an Ea of 33.8 kcal·mol−1. By performing an isomerization of IS4 it is easy to obtain IS7, then removal of one H2O molecule from O–H and N–H groups occurs to yield IS11. The isomerization of IS11 via the shift of a H atom in the O–H group to form an N–H bond leads to 10ab-v3 (Scheme S1, Supporting Information File 1). These processes are carried out through the TS11, TS16, and TS20 transition states with ΔG# values of 4.7, 26.9, and 7.1 kcal·mol−1, respectively.
For the reaction pathway starting from 4a’, the first steps lead to intermediates IS5 and IS3 via transitions states TS2 and TS3 with ΔG# values of 34.6 and 50.0 kcal·mol−1, respectively. Then, as compared to the pathways of 4a, IS5 and IS3 follow the same directions to obtain 10ab, 10ab-v2. Particularly, the reaction occurs through TS4 to give IS2 with a significant potential barrier of about 57.2 kcal·mol−1. The 10ab-v4 product is formed later by the removal of a H2O molecule from IS2 via TS13 with ΔG# about 26.9 kcal·mol−1. Furthermore, IS2 could convert efficiently to the IS4 isomer by a rapid isomerization with a low barrier of 1.3 kcal·mol−1.
In summary, in the gas phase, the reaction pathway starting from 4a through IS3 and finally yielding 10ab-v2 is a more favorable one in both thermodynamic (ΔG) and kinetic (ΔG#) aspects (red and black lines) in comparision with going through IS5 to achieve the 10ab product. For the reaction originating from 4a’, passing through IS5 and finally forming product 10ab is the most preferred pathway, and even more favorable than both directions from 4a to yield 10ab-v2. Hence, the reaction routes to obtain 10ab-v2 are thermodynamically favorable, while yielding 10ab is under kinetic control.
In order to better evaluate the reaction mechanism of 10ab synthesis, we continued to investigate four possible reaction pathways (1–4) corresponding to the green, blue, red, and black lines on the PES in different solvents, including ethanol, chloroform and dimethyl sulfoxide (DMSO) at the same level of theory. Especially, ethanol is the solvent used experimentally for the synthesis of 10ab. The calculated results in the solvent model are shown in Figure 5, Table 3, and Table S3 in Supporting Information File 1, and the stable structures are further illustrated in Figure 6 and Figure S7 (see Supporting Information File 1).
![[1860-5397-18-118-5]](/bjoc/content/figures/1860-5397-18-118-5.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 5: The PES for the possible pathways (1), (2), (3), and (4) in ethanol solvent.
Figure 5: The PES for the possible pathways (1), (2), (3), and (4) in ethanol solvent.
Figure 6: The optimized structures of some reactants, intermediates, transition states, and products in the possible pathways.
Figure 6: The optimized structures of some reactants, intermediates, transition states, and products in the p...
Notably, comparing the reaction pathways and the energy values illustrated in Figure 5 (ethanol solvent model) with that in Figure 4 (gas phase), most of the directions take place with similar trends. In fact, the most preferred way is from 4a’ to create IS5 and finally to form 10ab by removal of one H2O molecule from two –OH groups close together (blue line). Another competitive direction is from 4a to IS3 and finally to 10ab-v2 by the release of one H2O molecule from O–H and N–H groups. Noticeably, in the ethanol solvent model, the formation of 10ab and 10ab-v2 is thermodynamically similar (the ΔG difference is minimal, about 0.1 kcal·mol−1). In this case, the kinetic factor mainly determines the predominant product. With a difference of potential barrier of ca. 4.4 kcal·mol−1 in the first stage (to form intermediate), or 7.8 kcal·mol−1 in the final stage (for H2O separation) (IS5 isomerization to yield IS8 can be omitted because it is so fast with a small activation energy of 2.1 kcal·mol−1), the formation of product 10ab is superior as compared to its isomer. In terms of the value of the rate constant k, the direction of 10ab formation is in the range of 103–106 times faster than 10ab-v2. The remaining two reaction routes are to form the intermediates IS9, IS10 with the separation of a H2O molecule from the O–H and N–H bonds resulting in 10ab and 10ab-v2, respectively, which are evaluated lower than the above two pathways in releasing H2O molecule from two –OH groups. Hence, from the calculated results in the solvent model, it could be confirmed that, in terms of thermodynamic and kinetic aspects, compound 10ab is the most preferred product as compared to others for the reaction between 4a/4a’ with CH3NH2. In addition, removing one H2O molecule from two –OH groups in the intermediates is more favorable than from –OH and –NH groups. Moreover, the reaction mechanism in the chloroform and DMSO solvent model is also considered to confirm the main reaction pathway (Table S3 in Supporting Information File 1). The results indicate that the same trends and slight differences of free energy values are shown in solvent models used in this work.
Conclusion
4-Acetyl-3-hydroxy-3-pyrrolin-2-ones were synthesized successfully via the three-component reaction of an aromatic aldehyde, aniline, and ethyl 2,4-dioxovalerate with acceptable yields. The broadening of peaks in the 13C NMR spectra for the two carbon atoms of the acetyl group is due to the tautomerism of 4-acetyl-3-hydroxy-3-pyrrolin-2-ones in DMSO. The isomers 4a and 4a’ exist together upon synthesis with the significantly large rate constant of transformation of 4a into 4a’ of ca. 1012 s−1 in the gas phase and solvents mode (ethanol, chloroform, DMSO).
The reaction between 4-acetyl-3-hydroxy-3-pyrrolin-2-ones and an aliphatic amine in ethanol occurs at the carbonyl group attached to the 4-position of the heterocyclic ring to obtain 1,4,5-trisubstituted pyrrolidine-2,3-dione enamine derivatives. Remarkably, the theoretical results demonstrate that the possible pathway to form product 10ab from 4a/4a’ is the direction (1) following the PES in the gas phase and the ethanol/CHCl3/DMSO solvents model. It can be suggested that 10ab is the most favorable product as a result of the reaction process via transition state TS2 and intermediate IS5, in good agreement with the experimental results. Besides, the kinetic selectivity is more significant than the thermodynamic one for forming the main product 10ab with a rate constant ≈ 103–106 times higher (from the first step to the final step) in ethanol solvent (also in chloroform, DMSO) as compared to 10ab-v2. Moreover, CH3NH2 approaches 4a/4a’ at the opposite side of the C–C6H5 sites to form IS3/IS5 and finally 10ab-v2/10ab . The reaction mechanisms in the gas phase and in the solvents model are only slightly different.
Experimental
General experimental methods
NMR spectra were acquired on commercial instruments (Bruker Avance 300 MHz, Bruker Avance II+ 500 MHz, or Bruker Avance II+ 600 MHz) and chemical shifts (δ) are reported in parts per million (ppm) referenced to tetramethylsilane (TMS) or the internal (NMR) solvent signals. For column chromatography, 70–230 mesh silica 60 (E. M. Merck) was used as the stationary phase. Chemicals received from commercial sources were used without further purification. Melting points (not corrected) were determined using a Büchi Melting Point B-545. Exact mass measurements were acquired on a quadrupole orthogonal acceleration time-of-flight mass spectrometer (Synapt G2 HDMS, Waters, Milford, MA). Samples were infused at 3 μL/min and spectra were obtained in positive (or negative) ionization mode with a resolution of 15000 (FWHM) using leucine enkephalin as lock mass. Besides, exact mass measurements were also recorded on a SCIEX X500 QTOF with electrospray ionization (ESI) source in a positive mode. The temperatures of the source were set at 300 °C. Curtain gas (25 psi) chambers were filled with high-purity nitrogen. The capillary voltage was constantly kept at 5500 V. Collision energies was set at 10 V and zero collision energy spread. IDA mode was used to find mass in range (100 to 1000).
Computational methods
In this work, DFT calculations were used to investigate the synthesis of 1,4,5-trisubstituted pyrrolidine-2,3-dione (10ab) from 2-pyrrolidinone (4a/4a’) and methylamine (9b, CH3NH2). The stable structures of reactants, intermediates, transition states, and products were optimized at the B3LYP/6-31+G(d,p) level of theory by Gaussian 09 package [48]. The vibrational frequencies of these structures were also computed at the same level to determine the minima and transition states on the potential energy surface. Further, some highly possible pathways of forming 10ab were considered in ethanol, chloroform, and dimethyl sulfoxide (DMSO) solvents, in which ethanol was used in the experimental work, based on the Polarizable Continuum Model (PCM) method [49]. Similar to the calculations in the gas phase, the geometrical structures were optimized, and vibrational frequencies were computed at the same level in the solvent model. Especially, the single-point energy values of structures wee examined at the higher level B3LYP/6-311++G(2df,2pd) using the optimized geometries at B3LYP/6-31+G(d,p) in the gas phase and solvents. The intrinsic reaction coordinates (IRCs) were performed at the same level of theory to determine two local minima through transition states in each stepwise [50]. Besides, the rate constant of reaction based on the transitional state theory method and quantum tunneling effect was calculated by the following expression [47]:
with Qtun, ΔG#, ωi are tunneling transmission coefficient, Gibbs free energy values, and imaginary frequencies of transition states, respectively.
Supporting Information
| Supporting Information File 1: Experimental part, additional DFT data and NMR spectra. | ||
| Format: PDF | Size: 6.0 MB | Download |
References
-
Harreus, A. L.; Backes, R.; Eichloer, J.-O.; Feuerhake, R.; Jäkel, C.; Mahn, U.; Pinkos, R.; Vogelsang, R. 2-Pyrrolidone. In Ullmann's Encyclopedia of Industrial Chemistry; Elvers, B., Ed.; Wiley-VCH: Weinheim, Germany, 2011; pp 1–7. doi:10.1002/14356007.a22_457.pub2
Return to citation in text: [1] -
Caruano, J.; Muccioli, G. G.; Robiette, R. Org. Biomol. Chem. 2016, 14, 10134–10156. doi:10.1039/c6ob01349j
Return to citation in text: [1] [2] -
Barrett, A. G. M.; Head, J.; Smith, M. L.; Stock, N. S.; White, A. J. P.; Williams, D. J. J. Org. Chem. 1999, 64, 6005–6018. doi:10.1021/jo9905672
Return to citation in text: [1] -
Schwartz, R. E.; Helms, G. L.; Bolessa, E. A.; Wilson, K. E.; Giacobbe, R. A.; Tkacz, J. S.; Bills, G. F.; Liesch, J. M.; Zink, D. L.; Curotto, J. E.; Pramanik, B.; Onishi, J. C. Tetrahedron 1994, 50, 1675–1686. doi:10.1016/s0040-4020(01)80843-7
Return to citation in text: [1] -
Zhu, X. Z.; Li, X.-Y.; Liu, J. Eur. J. Pharmacol. 2004, 500, 221–230. doi:10.1016/j.ejphar.2004.07.027
Return to citation in text: [1] -
Ning, N.; Hu, J.-F.; Sun, J.-D.; Han, N.; Zhang, J.-T.; Chen, N.-H. Eur. J. Pharmacol. 2012, 682, 50–55. doi:10.1016/j.ejphar.2012.02.004
Return to citation in text: [1] -
Singh, P.; Dimitriou, V.; Mahajan, R. P.; Crossley, A. W. A. Br. J. Anaesth. 1993, 71, 685–688. doi:10.1093/bja/71.5.685
Return to citation in text: [1] -
Albrecht, D.; Basler, B.; Bach, T. J. Org. Chem. 2008, 73, 2345–2356. doi:10.1021/jo7027129
Return to citation in text: [1] -
Metten, B.; Kostermans, M.; Van Baelen, G.; Smet, M.; Dehaen, W. Tetrahedron 2006, 62, 6018–6028. doi:10.1016/j.tet.2006.04.005
Return to citation in text: [1] -
Manta, S.; Gkaragkouni, D.-N.; Kaffesaki, E.; Gkizis, P.; Hadjipavlou-Litina, D.; Pontiki, E.; Balzarini, J.; Dehaen, W.; Komiotis, D. Tetrahedron Lett. 2014, 55, 1873–1876. doi:10.1016/j.tetlet.2014.01.106
Return to citation in text: [1] [2] -
Kontnik, R.; Clardy, J. Org. Lett. 2008, 10, 4149–4151. doi:10.1021/ol801726k
Return to citation in text: [1] -
He, H.; Yang, H. Y.; Bigelis, R.; Solum, E. H.; Greenstein, M.; Carter, G. T. Tetrahedron Lett. 2002, 43, 1633–1636. doi:10.1016/s0040-4039(02)00099-0
Return to citation in text: [1] -
Gein, V. L.; Mihalev, V. A.; Kasimova, N. N.; Voronina, E. V.; Vakhrin, M. I.; Babushkina, E. B. Khim.-Farm. Zh. 2007, 41, 30–32.
Pharm. Chem. J. 2007, 41, 208–210. doi:10.1007/s11094-007-0047-9
Return to citation in text: [1] -
Gein, V. L.; Rubtsova, D. D.; Bobyleva, A. A.; Ryabova, O. V.; Novikova, V. V.; Kasimova, N. N.; Yankin, A. N. Russ. J. Gen. Chem. 2020, 90, 1222–1228. doi:10.1134/s1070363220070087
Return to citation in text: [1] -
Cusumano, A. Q.; Pierce, J. G. Bioorg. Med. Chem. Lett. 2018, 28, 2732–2735. doi:10.1016/j.bmcl.2018.02.047
Return to citation in text: [1] -
López-Pérez, A.; Freischem, S.; Grimm, I.; Weiergräber, O.; Dingley, A. J.; López-Alberca, M. P.; Waldmann, H.; Vollmer, W.; Kumar, K.; Vuong, C. Antibiotics (Basel, Switz.) 2021, 10, 529. doi:10.3390/antibiotics10050529
Return to citation in text: [1] -
Gein, V. L.; Yushkov, V. V.; Kasimova, N. N.; Shuklina, N. S.; Vasil'eva, M. Yu.; Gubanova, M. V. Khim.-Farm. Zh. 2005, 39, 33–36.
Pharm. Chem. J. 2005, 39, 484–487. doi:10.1007/s11094-006-0006-x
Return to citation in text: [1] -
Ortiz Zacarías, N. V.; van Veldhoven, J. P. D.; Portner, L.; van Spronsen, E.; Ullo, S.; Veenhuizen, M.; van der Velden, W. J. C.; Zweemer, A. J. M.; Kreekel, R. M.; Oenema, K.; Lenselink, E. B.; Heitman, L. H.; IJzerman, A. P. J. Med. Chem. 2018, 61, 9146–9161. doi:10.1021/acs.jmedchem.8b00605
Return to citation in text: [1] -
Barreca, M. L.; Rao, A.; De Luca, L.; Zappalà, M.; Gurnari, C.; Monforte, P.; De Clercq, E.; Van Maele, B.; Debyser, Z.; Witvrouw, M.; Briggs, J. M.; Chimirri, A. J. Chem. Inf. Comput. Sci. 2004, 44, 1450–1455. doi:10.1021/ci034296e
Return to citation in text: [1] -
Zykova, S. S.; Shurov, S. N.; Talismanov, V. S.; Karmanova, O. G.; Shavrina, T. V.; Ponosov, S. V.; Savinkov, S. A.; Zykov, N. A. J. Pharm. Sci. Res. 2018, 10, 947–949.
Return to citation in text: [1] -
Surmiak, E.; Twarda-Clapa, A.; Zak, K. M.; Musielak, B.; Tomala, M. D.; Kubica, K.; Grudnik, P.; Madej, M.; Jablonski, M.; Potempa, J.; Kalinowska-Tluscik, J.; Dömling, A.; Dubin, G.; Holak, T. A. ACS Chem. Biol. 2016, 11, 3310–3318. doi:10.1021/acschembio.6b00596
Return to citation in text: [1] -
Lauro, G.; Cantone, V.; Potenza, M.; Fischer, K.; Koeberle, A.; Werz, O.; Riccio, R.; Bifulco, G. Med. Chem. Commun. 2018, 9, 2028–2036. doi:10.1039/c8md00497h
Return to citation in text: [1] -
Pelkey, E. T.; Pelkey, S. J.; Greger, J. G. De Novo Synthesis of 3-Pyrrolin-2-Ones. In Advances in Heterocyclic Chemistry; Scriven, E. F. V.; Ramsden, C. A., Eds.; Elsevier: New York, NY, USA, 2015; Vol. 115, pp 151–285. doi:10.1016/bs.aihch.2015.04.001
Return to citation in text: [1] -
Joksimović, N.; Petronijević, J.; Janković, N.; Baskić, D.; Popović, S.; Todorović, D.; Matić, S.; Bogdanović, G. A.; Vraneš, M.; Tot, A.; Bugarčić, Z. Bioorg. Chem. 2019, 88, 102954. doi:10.1016/j.bioorg.2019.102954
Return to citation in text: [1] -
Kuznecovs, J.; Vorona, M.; Domraceva, I.; Kanepe-Lapsa, I.; Petrova, M.; Liepins, E.; Belyakov, S.; Leonchiks, A.; Veinberg, G. Chem. Heterocycl. Compd. 2018, 54, 514–519. doi:10.1007/s10593-018-2298-7
Return to citation in text: [1] -
del Corte, X.; López-Francés, A.; Maestro, A.; Villate-Beitia, I.; Sainz-Ramos, M.; Martínez de Marigorta, E.; Pedraz, J. L.; Palacios, F.; Vicario, J. Pharmaceuticals 2021, 14, 782. doi:10.3390/ph14080782
Return to citation in text: [1] -
Ameta, K. L.; Dandia, A., Eds. Multicomponent reactions: Synthesis of bioactive heterocycles; CRC Press: Boca Raton, FL, USA, 2017. doi:10.1201/9781315369754
Return to citation in text: [1] -
Ghorbani-Vaghei, R.; Sarmast, N.; Mahmoodi, J. Appl. Organomet. Chem. 2017, 31, e3681. doi:10.1002/aoc.3681
Return to citation in text: [1] -
Esmaeilzadeh, S.; Setamdideh, D. J. Serb. Chem. Soc. 2021, 86, 1039–1052. doi:10.2298/jsc210521059e
Return to citation in text: [1] -
Saha, A.; Payra, S.; Banerjee, S. RSC Adv. 2016, 6, 101953–101959. doi:10.1039/c6ra24367c
Return to citation in text: [1] -
Moshtaghi Zonouz, A.; Eskandari, I.; Notash, B. Synth. Commun. 2015, 45, 2115–2121. doi:10.1080/00397911.2015.1065506
Return to citation in text: [1] -
Khaligh, N. G.; Mihankhah, T.; Johan, M. R. Synth. Commun. 2019, 49, 1334–1342. doi:10.1080/00397911.2019.1601225
Return to citation in text: [1] -
Ahankar, H.; Ramazani, A.; Ślepokura, K.; Lis, T.; Joo, S. W. Green Chem. 2016, 18, 3582–3593. doi:10.1039/c6gc00157b
Return to citation in text: [1] -
Dutta, A.; Rohman, M. A.; Nongrum, R.; Thongni, A.; Mitra, S.; Nongkhlaw, R. New J. Chem. 2021, 45, 8136–8148. doi:10.1039/d1nj00343g
Return to citation in text: [1] -
Saha, M.; Das, A. R. ChemistrySelect 2017, 2, 10249–10260. doi:10.1002/slct.201701989
Return to citation in text: [1] -
Zhu, Q.; Jiang, H.; Li, J.; Liu, S.; Xia, C.; Zhang, M. J. Comb. Chem. 2009, 11, 685–696. doi:10.1021/cc900046f
Return to citation in text: [1] -
del Corte, X.; Martinez de Marigorta, E.; Palacios, F.; Vicario, J. Molecules 2019, 24, 2951. doi:10.3390/molecules24162951
Return to citation in text: [1] -
Ghasemzadeh, M. A.; Abdollahi-Basir, M. H.; Elyasi, Z. Curr. Organocatal. 2019, 6, 61–68. doi:10.2174/2213337206666181211125226
Return to citation in text: [1] -
Gein, V. L.; Armisheva, M. N.; Rassudikhina, N. A.; Vakhrin, M. I.; Voronina, E. V. Khim.-Farm. Zh. 2011, 45, 33–35.
Pharm. Chem. J. 2011, 45, 162. doi:10.1007/s11094-011-0584-0
Return to citation in text: [1] [2] -
Gein, V. L.; Pastukhova, E. V. Russ. J. Gen. Chem. 2021, 91, 1261–1264. doi:10.1134/s107036322107001x
Return to citation in text: [1] -
Gein, V. L.; Rubtsova, D. D.; Bobyleva, A. A.; Yankin, A. N. Russ. J. Gen. Chem. 2020, 90, 804–808. doi:10.1134/s1070363220050072
Return to citation in text: [1] -
Gein, V. L.; Armisheva, M. N.; Kornienko, N. A.; Gein, L. F. Russ. J. Gen. Chem. 2014, 84, 2270–2272. doi:10.1134/s1070363214110395
Return to citation in text: [1] [2] [3] -
Yao, Y.; Zhang, X.; Ma, S. Org. Chem. Front. 2020, 7, 2047–2054. doi:10.1039/d0qo00564a
Return to citation in text: [1] -
Clayden, J.; Greeves, N.; Warren, S. Nucleophilic substitution at C=O with loss of carbonyl oxygen. Organic Chemistry, 2nd ed.; Oxford University Press: New York, NY, USA, 2012; pp 339–360.
Return to citation in text: [1] -
Silberberg, M. Equilibrium: The Extent of Chemical Reactions. Principles of general chemistry; McGraw Hill: New York, NY, USA, 2012; pp 540–570.
Return to citation in text: [1] -
Atta-ur-Rahman. Experimental Procedures in NMR Spectroscopy. Nuclear Magnetic Resonance: Basic Principles; Springer US: New York, NY, USA, 1986; pp 87–139. doi:10.1007/978-1-4612-4894-1_3
Return to citation in text: [1] -
Bao, J. L.; Truhlar, D. G. Chem. Soc. Rev. 2017, 46, 7548–7596. doi:10.1039/c7cs00602k
Return to citation in text: [1] [2] -
Gaussian 09, Revision A.02; Gaussian, Inc.: Wallingford, CT, 2016.
Return to citation in text: [1] -
Cramer, C. J. Essentials of computational chemistry: theories and models, 2nd ed.; John Wiley & Sons: Chichester, UK, 2004.
Return to citation in text: [1] -
Lipparini, F.; Scalmani, G.; Mennucci, B.; Cancès, E.; Caricato, M.; Frisch, M. J. J. Chem. Phys. 2010, 133, 014106. doi:10.1063/1.3454683
Return to citation in text: [1]
| 49. | Cramer, C. J. Essentials of computational chemistry: theories and models, 2nd ed.; John Wiley & Sons: Chichester, UK, 2004. |
| 50. | Lipparini, F.; Scalmani, G.; Mennucci, B.; Cancès, E.; Caricato, M.; Frisch, M. J. J. Chem. Phys. 2010, 133, 014106. doi:10.1063/1.3454683 |
| 47. | Bao, J. L.; Truhlar, D. G. Chem. Soc. Rev. 2017, 46, 7548–7596. doi:10.1039/c7cs00602k |
| 1. | Harreus, A. L.; Backes, R.; Eichloer, J.-O.; Feuerhake, R.; Jäkel, C.; Mahn, U.; Pinkos, R.; Vogelsang, R. 2-Pyrrolidone. In Ullmann's Encyclopedia of Industrial Chemistry; Elvers, B., Ed.; Wiley-VCH: Weinheim, Germany, 2011; pp 1–7. doi:10.1002/14356007.a22_457.pub2 |
| 7. | Singh, P.; Dimitriou, V.; Mahajan, R. P.; Crossley, A. W. A. Br. J. Anaesth. 1993, 71, 685–688. doi:10.1093/bja/71.5.685 |
| 28. | Ghorbani-Vaghei, R.; Sarmast, N.; Mahmoodi, J. Appl. Organomet. Chem. 2017, 31, e3681. doi:10.1002/aoc.3681 |
| 29. | Esmaeilzadeh, S.; Setamdideh, D. J. Serb. Chem. Soc. 2021, 86, 1039–1052. doi:10.2298/jsc210521059e |
| 30. | Saha, A.; Payra, S.; Banerjee, S. RSC Adv. 2016, 6, 101953–101959. doi:10.1039/c6ra24367c |
| 31. | Moshtaghi Zonouz, A.; Eskandari, I.; Notash, B. Synth. Commun. 2015, 45, 2115–2121. doi:10.1080/00397911.2015.1065506 |
| 32. | Khaligh, N. G.; Mihankhah, T.; Johan, M. R. Synth. Commun. 2019, 49, 1334–1342. doi:10.1080/00397911.2019.1601225 |
| 33. | Ahankar, H.; Ramazani, A.; Ślepokura, K.; Lis, T.; Joo, S. W. Green Chem. 2016, 18, 3582–3593. doi:10.1039/c6gc00157b |
| 34. | Dutta, A.; Rohman, M. A.; Nongrum, R.; Thongni, A.; Mitra, S.; Nongkhlaw, R. New J. Chem. 2021, 45, 8136–8148. doi:10.1039/d1nj00343g |
| 5. | Zhu, X. Z.; Li, X.-Y.; Liu, J. Eur. J. Pharmacol. 2004, 500, 221–230. doi:10.1016/j.ejphar.2004.07.027 |
| 6. | Ning, N.; Hu, J.-F.; Sun, J.-D.; Han, N.; Zhang, J.-T.; Chen, N.-H. Eur. J. Pharmacol. 2012, 682, 50–55. doi:10.1016/j.ejphar.2012.02.004 |
| 10. | Manta, S.; Gkaragkouni, D.-N.; Kaffesaki, E.; Gkizis, P.; Hadjipavlou-Litina, D.; Pontiki, E.; Balzarini, J.; Dehaen, W.; Komiotis, D. Tetrahedron Lett. 2014, 55, 1873–1876. doi:10.1016/j.tetlet.2014.01.106 |
| 35. | Saha, M.; Das, A. R. ChemistrySelect 2017, 2, 10249–10260. doi:10.1002/slct.201701989 |
| 36. | Zhu, Q.; Jiang, H.; Li, J.; Liu, S.; Xia, C.; Zhang, M. J. Comb. Chem. 2009, 11, 685–696. doi:10.1021/cc900046f |
| 37. | del Corte, X.; Martinez de Marigorta, E.; Palacios, F.; Vicario, J. Molecules 2019, 24, 2951. doi:10.3390/molecules24162951 |
| 38. | Ghasemzadeh, M. A.; Abdollahi-Basir, M. H.; Elyasi, Z. Curr. Organocatal. 2019, 6, 61–68. doi:10.2174/2213337206666181211125226 |
| 2. | Caruano, J.; Muccioli, G. G.; Robiette, R. Org. Biomol. Chem. 2016, 14, 10134–10156. doi:10.1039/c6ob01349j |
| 3. | Barrett, A. G. M.; Head, J.; Smith, M. L.; Stock, N. S.; White, A. J. P.; Williams, D. J. J. Org. Chem. 1999, 64, 6005–6018. doi:10.1021/jo9905672 |
| 4. | Schwartz, R. E.; Helms, G. L.; Bolessa, E. A.; Wilson, K. E.; Giacobbe, R. A.; Tkacz, J. S.; Bills, G. F.; Liesch, J. M.; Zink, D. L.; Curotto, J. E.; Pramanik, B.; Onishi, J. C. Tetrahedron 1994, 50, 1675–1686. doi:10.1016/s0040-4020(01)80843-7 |
| 21. | Surmiak, E.; Twarda-Clapa, A.; Zak, K. M.; Musielak, B.; Tomala, M. D.; Kubica, K.; Grudnik, P.; Madej, M.; Jablonski, M.; Potempa, J.; Kalinowska-Tluscik, J.; Dömling, A.; Dubin, G.; Holak, T. A. ACS Chem. Biol. 2016, 11, 3310–3318. doi:10.1021/acschembio.6b00596 |
| 22. | Lauro, G.; Cantone, V.; Potenza, M.; Fischer, K.; Koeberle, A.; Werz, O.; Riccio, R.; Bifulco, G. Med. Chem. Commun. 2018, 9, 2028–2036. doi:10.1039/c8md00497h |
| 23. | Pelkey, E. T.; Pelkey, S. J.; Greger, J. G. De Novo Synthesis of 3-Pyrrolin-2-Ones. In Advances in Heterocyclic Chemistry; Scriven, E. F. V.; Ramsden, C. A., Eds.; Elsevier: New York, NY, USA, 2015; Vol. 115, pp 151–285. doi:10.1016/bs.aihch.2015.04.001 |
| 24. | Joksimović, N.; Petronijević, J.; Janković, N.; Baskić, D.; Popović, S.; Todorović, D.; Matić, S.; Bogdanović, G. A.; Vraneš, M.; Tot, A.; Bugarčić, Z. Bioorg. Chem. 2019, 88, 102954. doi:10.1016/j.bioorg.2019.102954 |
| 25. | Kuznecovs, J.; Vorona, M.; Domraceva, I.; Kanepe-Lapsa, I.; Petrova, M.; Liepins, E.; Belyakov, S.; Leonchiks, A.; Veinberg, G. Chem. Heterocycl. Compd. 2018, 54, 514–519. doi:10.1007/s10593-018-2298-7 |
| 26. | del Corte, X.; López-Francés, A.; Maestro, A.; Villate-Beitia, I.; Sainz-Ramos, M.; Martínez de Marigorta, E.; Pedraz, J. L.; Palacios, F.; Vicario, J. Pharmaceuticals 2021, 14, 782. doi:10.3390/ph14080782 |
| 2. | Caruano, J.; Muccioli, G. G.; Robiette, R. Org. Biomol. Chem. 2016, 14, 10134–10156. doi:10.1039/c6ob01349j |
| 27. | Ameta, K. L.; Dandia, A., Eds. Multicomponent reactions: Synthesis of bioactive heterocycles; CRC Press: Boca Raton, FL, USA, 2017. doi:10.1201/9781315369754 |
| 13. |
Gein, V. L.; Mihalev, V. A.; Kasimova, N. N.; Voronina, E. V.; Vakhrin, M. I.; Babushkina, E. B. Khim.-Farm. Zh. 2007, 41, 30–32.
Pharm. Chem. J. 2007, 41, 208–210. doi:10.1007/s11094-007-0047-9 |
| 14. | Gein, V. L.; Rubtsova, D. D.; Bobyleva, A. A.; Ryabova, O. V.; Novikova, V. V.; Kasimova, N. N.; Yankin, A. N. Russ. J. Gen. Chem. 2020, 90, 1222–1228. doi:10.1134/s1070363220070087 |
| 15. | Cusumano, A. Q.; Pierce, J. G. Bioorg. Med. Chem. Lett. 2018, 28, 2732–2735. doi:10.1016/j.bmcl.2018.02.047 |
| 16. | López-Pérez, A.; Freischem, S.; Grimm, I.; Weiergräber, O.; Dingley, A. J.; López-Alberca, M. P.; Waldmann, H.; Vollmer, W.; Kumar, K.; Vuong, C. Antibiotics (Basel, Switz.) 2021, 10, 529. doi:10.3390/antibiotics10050529 |
| 19. | Barreca, M. L.; Rao, A.; De Luca, L.; Zappalà, M.; Gurnari, C.; Monforte, P.; De Clercq, E.; Van Maele, B.; Debyser, Z.; Witvrouw, M.; Briggs, J. M.; Chimirri, A. J. Chem. Inf. Comput. Sci. 2004, 44, 1450–1455. doi:10.1021/ci034296e |
| 12. | He, H.; Yang, H. Y.; Bigelis, R.; Solum, E. H.; Greenstein, M.; Carter, G. T. Tetrahedron Lett. 2002, 43, 1633–1636. doi:10.1016/s0040-4039(02)00099-0 |
| 20. | Zykova, S. S.; Shurov, S. N.; Talismanov, V. S.; Karmanova, O. G.; Shavrina, T. V.; Ponosov, S. V.; Savinkov, S. A.; Zykov, N. A. J. Pharm. Sci. Res. 2018, 10, 947–949. |
| 11. | Kontnik, R.; Clardy, J. Org. Lett. 2008, 10, 4149–4151. doi:10.1021/ol801726k |
| 8. | Albrecht, D.; Basler, B.; Bach, T. J. Org. Chem. 2008, 73, 2345–2356. doi:10.1021/jo7027129 |
| 9. | Metten, B.; Kostermans, M.; Van Baelen, G.; Smet, M.; Dehaen, W. Tetrahedron 2006, 62, 6018–6028. doi:10.1016/j.tet.2006.04.005 |
| 10. | Manta, S.; Gkaragkouni, D.-N.; Kaffesaki, E.; Gkizis, P.; Hadjipavlou-Litina, D.; Pontiki, E.; Balzarini, J.; Dehaen, W.; Komiotis, D. Tetrahedron Lett. 2014, 55, 1873–1876. doi:10.1016/j.tetlet.2014.01.106 |
| 17. |
Gein, V. L.; Yushkov, V. V.; Kasimova, N. N.; Shuklina, N. S.; Vasil'eva, M. Yu.; Gubanova, M. V. Khim.-Farm. Zh. 2005, 39, 33–36.
Pharm. Chem. J. 2005, 39, 484–487. doi:10.1007/s11094-006-0006-x |
| 18. | Ortiz Zacarías, N. V.; van Veldhoven, J. P. D.; Portner, L.; van Spronsen, E.; Ullo, S.; Veenhuizen, M.; van der Velden, W. J. C.; Zweemer, A. J. M.; Kreekel, R. M.; Oenema, K.; Lenselink, E. B.; Heitman, L. H.; IJzerman, A. P. J. Med. Chem. 2018, 61, 9146–9161. doi:10.1021/acs.jmedchem.8b00605 |
| 42. | Gein, V. L.; Armisheva, M. N.; Kornienko, N. A.; Gein, L. F. Russ. J. Gen. Chem. 2014, 84, 2270–2272. doi:10.1134/s1070363214110395 |
| 39. |
Gein, V. L.; Armisheva, M. N.; Rassudikhina, N. A.; Vakhrin, M. I.; Voronina, E. V. Khim.-Farm. Zh. 2011, 45, 33–35.
Pharm. Chem. J. 2011, 45, 162. doi:10.1007/s11094-011-0584-0 |
| 40. | Gein, V. L.; Pastukhova, E. V. Russ. J. Gen. Chem. 2021, 91, 1261–1264. doi:10.1134/s107036322107001x |
| 41. | Gein, V. L.; Rubtsova, D. D.; Bobyleva, A. A.; Yankin, A. N. Russ. J. Gen. Chem. 2020, 90, 804–808. doi:10.1134/s1070363220050072 |
| 42. | Gein, V. L.; Armisheva, M. N.; Kornienko, N. A.; Gein, L. F. Russ. J. Gen. Chem. 2014, 84, 2270–2272. doi:10.1134/s1070363214110395 |
| 42. | Gein, V. L.; Armisheva, M. N.; Kornienko, N. A.; Gein, L. F. Russ. J. Gen. Chem. 2014, 84, 2270–2272. doi:10.1134/s1070363214110395 |
| 46. | Atta-ur-Rahman. Experimental Procedures in NMR Spectroscopy. Nuclear Magnetic Resonance: Basic Principles; Springer US: New York, NY, USA, 1986; pp 87–139. doi:10.1007/978-1-4612-4894-1_3 |
| 47. | Bao, J. L.; Truhlar, D. G. Chem. Soc. Rev. 2017, 46, 7548–7596. doi:10.1039/c7cs00602k |
| 44. | Clayden, J.; Greeves, N.; Warren, S. Nucleophilic substitution at C=O with loss of carbonyl oxygen. Organic Chemistry, 2nd ed.; Oxford University Press: New York, NY, USA, 2012; pp 339–360. |
| 45. | Silberberg, M. Equilibrium: The Extent of Chemical Reactions. Principles of general chemistry; McGraw Hill: New York, NY, USA, 2012; pp 540–570. |
| 43. | Yao, Y.; Zhang, X.; Ma, S. Org. Chem. Front. 2020, 7, 2047–2054. doi:10.1039/d0qo00564a |
| 39. |
Gein, V. L.; Armisheva, M. N.; Rassudikhina, N. A.; Vakhrin, M. I.; Voronina, E. V. Khim.-Farm. Zh. 2011, 45, 33–35.
Pharm. Chem. J. 2011, 45, 162. doi:10.1007/s11094-011-0584-0 |
© 2022 Nguyen et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.