Abstract
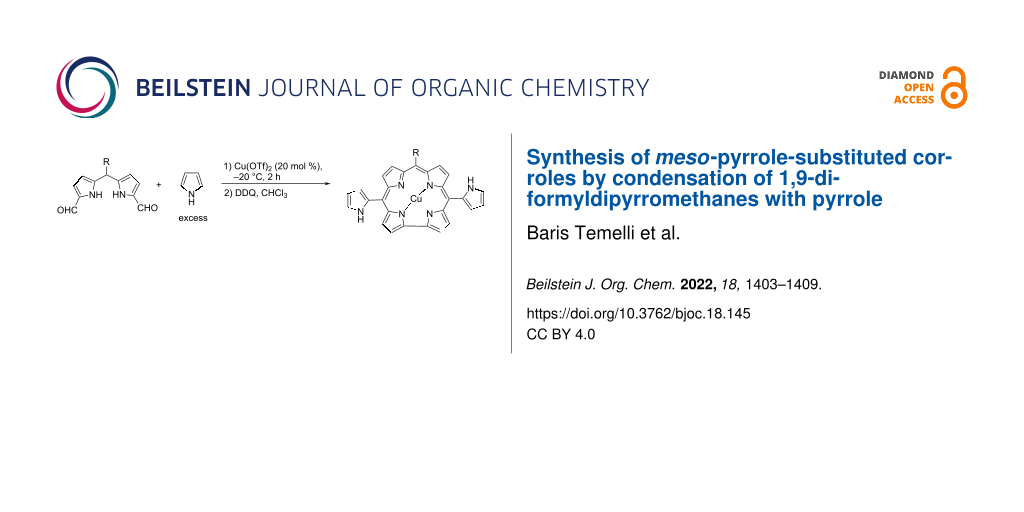
A copper triflate-mediated approach to access copper complexes of pyrrole-substituted corroles from the reaction of 1,9-diformyldipyrromethanes and an excess amount of pyrrole is presented for the first time. This procedure is a simple and efficient way for the preparation of corroles with a polymerizable substituent on meso-positions.
Graphical Abstract
Introduction
Corroles, a member of contracted porphyrins, are tetrapyrrolic aromatic compounds, with the lack of one meso-carbon atom on the macrocycle [1-4]. This feature supplies a smaller ring cavity than in the case of porphyrins, three NH in the core, and coordination ability with high-valence transition metal ions. It is noteworthy that studies on porphyrins, which have many application areas such as photodynamic therapy and photovoltaic systems, have focused on oligomeric and polymeric structures in the last two decades [5-9]. Although such porphyrin structures have been used successfully in the development of molecular devices and functional materials, the synthesis of corrole-based analogues has been rather limited due to very few synthetic methods developed to produce corroles with polymerizable substituents at the meso- or beta positions [10-13].
To date, meso-substituted corroles have been synthesized by several methods including; (i) the condensation of pyrrole or dipyrromethanes with aldehydes [14-16], (ii) the reaction of 2,2’-bipyrrole with dipyrromethane-1,9-dicarbinols [17,18], (iii) the condensation of bipyrrole-5,5’-dicarbinols with dipyrromethanes [19], (iv) the reaction of dipyrromethane-1,9-dicarbinols with pyrrole [20,21], (v) the condensation of dipyrromethane-1-carbinols with dipyrromethanes [22] and (vi) the reaction of tripyrranes with aldehydes [23]. Although many different substituents can be attached to the meso-position of corroles using all these methods, to the best of our knowledge, there is no generally accepted method for the synthesis of pyrrole-substituted corroles. Very recently, we reported the synthesis of porphyrin–corrole [24-26] and porphyrin–porphyrin dyads and triads [27] using formylated porphyrin compounds. In a continuation of research activity with corroles, here we describe the first synthesis of copper complexes of trans-A2B-corroles possessing pyrrol-2-yl substituents at positions 5 and 15 by the condensation reaction of 1,9-diformylated dipyrromethanes with pyrrole in the presence of copper triflate.
Results and Discussion
At the beginning of our studies, we investigated the synthesis of mono- and dipyrrole-substituted corroles via the condensation reaction of pyrrole-2-carboxaldehyde with 5-phenyldipyrromethane (Scheme 1a) and the reaction between tris(2-pyrrolyl)methane with benzaldehyde (Scheme 1b). Although we tried many reaction conditions and catalysts, unidentified product mixtures were obtained instead of corrole products in both reactions.
Scheme 1: Synthetic studies to obtain mono- and dipyrrole-substituted compounds.
Scheme 1: Synthetic studies to obtain mono- and dipyrrole-substituted compounds.
Then, the reaction of 1,9-diformyl-5-phenyldipyrromethane (1a) in an excess amount of pyrrole was tested to obtain pyrrole-substituted metal-free corrole through the oxidation of the bilane intermediate by using DDQ (Scheme 2). Pyrrole was used as both reagent and solvent in these reactions. The desired product was not observed in the reaction medium when various catalysts (TFA, I2, AlCl3, InCl3, FeCl3, H2SO4, p-TsOH, Mont. KSF, Mont. K-10, and AgOTf) were used at different temperatures (Supporting Information File 1, Table S1). However, the copper complex of the desired product 2a was obtained in 5% yield in the presence of Cu(OTf)2 catalyst at room temperature.
Scheme 2: The reaction of 5-phenyl-1,9-diformyldipyrromethane (1a) with pyrrole.
Scheme 2: The reaction of 5-phenyl-1,9-diformyldipyrromethane (1a) with pyrrole.
When the synthetic methods in the literature are examined to obtain corrole compounds, it is observed that temperature, pyrrole ratio, reaction time, catalyst type, and oxidant are important parameters on the yields of the reactions [28-32]. For this reason, optimization studies were carried out on these parameters. Based on the results of the preliminary studies, optimization studies were carried out in the presence of 10 mol % Cu(OTf)2 catalyst, and the effect of temperature on the synthesis of pyrrole-substituted trans-A2B corrole compounds was investigated in 40 equivalents of pyrrole using a reaction time of 2 hours (Table 1, entries 1–4). No product was formed as a result of increasing the reaction temperature to 40 °C. It was observed that the yield increased gradually when the reaction temperature was decreased. The yield of the product, which was obtained with 5% efficiency at room conditions and 6% at 0 °C, increased to 9% by reducing the temperature to −20 °C. The pyrrole/1a ratio played little role in improving the yield of the product. The yield of 2a decreased to 4% yield when the reaction was carried out in 20 equivalents of pyrrole (Table 1, entry 5). Increasing the amount of pyrrole above 40 equivalents did not affect the reaction yield (Table 1, entries 6 and 7). Then, the effect of the reaction time before the oxidant addition on the yield of product was investigated at −20 °C in 40 equivalents of pyrrole. When the reaction time was 1 hour, the yield decreased to 4% (Table 1, entry 8). If the reaction time exceeded 2 hours, unexpectedly no desired product was found at all (Table 1, entries 9 and 10). This situation can be explained by the instability of the bilane intermediate formed in the reaction medium and its decomposition during long reaction times. It was also investigated whether the product yield would increase with the amount of catalyst since only the copper complex of the expected product could be isolated at the end of the reaction. The reaction was repeated using 20 mol %, 50 mol %, and equimolar amounts of copper triflate under previously optimized conditions. In the case of using 20 mol % copper triflate, the reaction efficiency increased to 12%, while a further increase in the amount of catalyst did not affect the yield (Table 1, entries 11–13). In order to determine the effect of the oxidant type and the oxidant amount, reactions were carried out with 3 and 4 equivalents of DDQ and p-chloranil. While more than 2 equivalents of DDQ did not have a positive effect on the reaction yield (Table 1, entries 14 and 15), p-chloranil formed a product with a lower yield than DDQ (Table 1, entries 16–18). The activities of different copper catalysts were also tested in the model reaction. Only CuCl2 formed the product in 5% yield and the other salts did not catalyze the reaction (Table 1, entries 19–22).
Table 1: Optimization of reaction conditions.a
|
|
|||||||
| Entry | Catalyst | Catalyst amount (%) | Temp (°C) | Pyrrole/1a | Time (h) |
Oxidant
(oxidant/1a) |
Yield (%)b |
| 1 | Cu(OTf)2 | 10 | 40 | 40 | 2 | DDQ (2) | – |
| 2 | Cu(OTf)2 | 10 | rt | 40 | 2 | DDQ (2) | 5 |
| 3 | Cu(OTf)2 | 10 | 0 | 40 | 2 | DDQ (2) | 6 |
| 4 | Cu(OTf)2 | 10 | −20 | 40 | 2 | DDQ (2) | 9 |
| 5 | Cu(OTf)2 | 10 | −20 | 20 | 2 | DDQ (2) | 4 |
| 6 | Cu(OTf)2 | 10 | −20 | 60 | 2 | DDQ (2) | 9 |
| 7 | Cu(OTf)2 | 10 | −20 | 80 | 2 | DDQ (2) | 9 |
| 8 | Cu(OTf)2 | 10 | −20 | 40 | 1 | DDQ (2) | 4 |
| 9 | Cu(OTf)2 | 10 | −20 | 40 | 4 | DDQ (2) | – |
| 10 | Cu(OTf)2 | 10 | −20 | 40 | 6 | DDQ (2) | – |
| 11 | Cu(OTf)2 | 20 | −20 | 40 | 2 | DDQ (2) | 12 |
| 12 | Cu(OTf)2 | 50 | −20 | 40 | 2 | DDQ (2) | 12 |
| 13 | Cu(OTf)2 | 100 | −20 | 40 | 2 | DDQ (2) | 12 |
| 14 | Cu(OTf)2 | 20 | −20 | 40 | 2 | DDQ (3) | 12 |
| 15 | Cu(OTf)2 | 20 | −20 | 40 | 2 | DDQ (4) | 12 |
| 16 | Cu(OTf)2 | 20 | −20 | 40 | 2 | p-chloranil (2) | 10 |
| 17 | Cu(OTf)2 | 20 | −20 | 40 | 2 | p-chloranil (3) | 10 |
| 18 | Cu(OTf)2 | 20 | −20 | 40 | 2 | p-chloranil (4) | 10 |
| 19 | CuCl2 | 100 | −20 | 40 | 2 | DDQ (2) | 5 |
| 20 | CuCl | 100 | −20 | 40 | 2 | DDQ (2) | – |
| 21 | Cu(OAc)2 | 100 | −20 | 40 | 2 | DDQ (2) | – |
| 22 | Cu(NO3)2 | 100 | −20 | 40 | 2 | DDQ (2) | – |
aReaction conditions: 1a (0.36 mmol, 0.10 g), pyrrole (14.4 mmol, 0.97 g, 1 mL), CHCl3 (2 mL). bIsolated yield.
With the best conditions in our hands (Table 1, entry 11), different diformylated dipyrromethanes were subjected to condensation reactions. Electron-withdrawing 4-chlorophenyl, pentafluorophenyl, and 4-nitrophenyl-substituted corrole compounds were isolated in 13% yields (Table 2, entries 2–4). While electron-donating 4-methoxyphenyl (2e) and p-tolyl-substituted corrole (2f) were isolated in 8% yield and 12% yields respectively, a p-bromophenyl substituent resulted in a mixture of undefined products after the reaction. This might be due to scrambling, which is an acid-catalyzed rearrangement of the substituent in intermediates of the condensation reaction.
Table 2: Synthesis of pyrrole-substituted corroles.a
|
|
|||
| Entry | R | 2 | Yield (%)b |
| 1 | C6H5 | 2a | 12 |
| 2 | 4-ClC6H4 | 2b | 13 |
| 3 | C6F5 | 2c | 13 |
| 4 | 4-NO2C6H4 | 2d | 13 |
| 5 | 4-CH3OC6H4 | 2e | 8 |
| 6 | 4-CH3C6H4 | 2f | 12 |
| 7 | 4-BrC6H4 | 2g | – |
aReaction conditions: 1a–g (0.36 mmol), pyrrole (14.4 mmol, 0.97 g, 1 mL), Cu(OTf)2 (0.072 mmol, 0.026 g), DDQ (0.72 mmol, 0.16 g ), CHCl3 (2 mL). bIsolated yield.
The structures of the meso-pyrrole substituted corroles were identified by using 1H NMR, 1H,1H-COSY NMR and HRMS techniques (see Supporting Information File 1). The 1H NMR spectrum of 2a is shown in Figure 1. As expected, pyrrole C4, C3, C5 and NH protons appeared at 6.51, 7.05, 7.27 and 8.90 ppm, respectively. Coupling of all pyrrole protons can be seen in the 1H,1H-COSY NMR spectrum (Supporting Information File 1, Figure S3). The β-protons of the corrole macrocycle and the phenyl group gave signals between 7.40–8.20 ppm.
![[1860-5397-18-145-1]](/bjoc/content/figures/1860-5397-18-145-1.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 1: 1H NMR spectrum of 2a in THF-d8.
Figure 1: 1H NMR spectrum of 2a in THF-d8.
Electronic absorption spectra of corroles 2a–g were recorded in CHCl3 at 2.0 × 10−5 M. The Soret bands of all compounds are located between 410–420 nm (see Supporting Information File 1). The Q bands of the compounds are seen as broad absorptions in the 500–700 nm region. Figure 2 shows the absorption spectrum of 2a with a strong Soret band at 412 nm and weak Q-bands at 542 and 611 nm.
![[1860-5397-18-145-2]](/bjoc/content/figures/1860-5397-18-145-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: Electronic absorption spectrum of 2a in CHCl3.
Figure 2: Electronic absorption spectrum of 2a in CHCl3.
After the synthesis of corrole compounds, we tried to extent our studies to obtain meso-pyrrole-substituted porphyrin compounds. For this purpose, the MacDonald [2 + 2] porphyrin macrocyclization reaction of 1,9-diformyl-5-phenyl dipyrromethane (1a) with tris(2-pyrrolyl)methane was investigated by changing the reaction conditions in the presence of various acids such as acetic acid, hydrochloric acid and p-toluenesulfonic acid (Scheme 3). Among these reactions, the reaction in acetic acid resulted in 5,15-diphenylporphyrin (3) and 5-phenylporphyrin (4) in 4% and 1% yields, respectively. No pyrrole-substituted porphyrin product was detected. The structures of compounds 3 [33] and 4 [34] are in agreement with the literature data.
Scheme 3: [2 + 2] Mac Donald type condensation reaction.
Scheme 3: [2 + 2] Mac Donald type condensation reaction.
Conclusion
In summary, we report the first example of copper complexes of A2B-type pyrrole substituted corroles. We believe that the placement of the polymerizable pyrrole as a conjugated substituent to the macrocycle is an important contribution to the polymerization of corroles and the expansion of the usage areas of these compounds. Further studies on the production of mono pyrrole metal-free corrole compounds and the polymerization reactions of the obtained compounds are ongoing in our laboratory.
Experimental
General information
All reactions were performed under N2 atmosphere. All reagents and solvents were of reagent grade. The NMR spectra were recorded in CDCl3 and THF-d8 on a Bruker AV Ultra Shield 400 MHz instrument. Absorption spectra were obtained with PG T80. NMR data are represented as follows: chemical shift (ppm), multiplicity (s = singlet, brs = broad singlet, d = doublet, t = triplet, q = quartet, m = multiplet), coupling constants in hertz (Hz). IR spectra were recorded on FTIR spectrometer (Thermo Scientific, Nicolet IS10). HRMS were measured in ESI mode and the mass analyzer of the HRMS was TOF (Agilent 6224 TOF LC–MS). Flash column chromatography was performed on silica gel (230–400 mesh). 5-Substituted dipyrromethanes [35] and 1,9-diformyldipyrromethanes [36] were prepared according to literature methods and their spectral data matched literature values.
General synthetic procedure for pyrrole-substituted corroles
A solution of 1,9-diformyldipyrromethanes 1a–g (0.36 mmol) and pyrrole (14.4 mmol, 0.97 g, 1 mL) was cooled under N2 at −20 °C for 1 h. Cu(OTf)2 (0,072 mmol, 0.026 g) was added to the mixture at the same temperature and stirred for 2 h. A solution of DDQ (0.72 mmol, 0.16 g) in 2 mL CHCl3 was added to the reaction. The reaction was removed from the cold bath and left to stir overnight. The mixture passed from the silica column to remove Cu(OTf)2. The solvent was removed under reduced pressure and the crude product was purified by flash column chromatography over silica gel with CH2Cl2/hexane (1:1).
Synthesis of 3 and 4 by [2 + 2] MacDonald coupling reaction
A solution of 5-phenyl-1,9-diformyldipyrromethane (1a, 0.40 mmol, 0.11 g) and tris(2-pyrrolyl)methane (0.40 mmol, 0.085 g) in 60 mL HOAc was stirred for 2 hours in the dark and 4 g NaOAc was added. The reaction was stirred for 12 h. in the dark. Solvent was removed by distillation in vacuum and 120 mL MeOH and 2 mL concd H2SO4 was added to the residue. The mixture was refluxed for 12 h. The solvent was removed under reduced pressure and the crude product was purified by flash column chromatography over silica gel with CHCl3/hexane (1:1).
Supporting Information
| Supporting Information File 1: Table S1 and experimental part. | ||
| Format: PDF | Size: 1.7 MB | Download |
References
-
Johnson, A. W.; Kay, I. T. Proc. Chem. Soc., London 1964, 89–90.
Return to citation in text: [1] -
Johnson, A. W.; Kay, I. T. J. Chem. Soc. 1965, 1620–1629. doi:10.1039/jr9650001620
Return to citation in text: [1] -
Paolesse, R. Synlett 2008, 2215–2230. doi:10.1055/s-2008-1078687
Return to citation in text: [1] -
Aviv-Harel, I.; Gross, Z. Chem. – Eur. J. 2009, 15, 8382–8394. doi:10.1002/chem.200900920
Return to citation in text: [1] -
Ji, W.; Wang, T.-X.; Ding, X.; Lei, S.; Han, B.-H. Coord. Chem. Rev. 2021, 439, 213875. doi:10.1016/j.ccr.2021.213875
Return to citation in text: [1] -
Jin, L.; Lv, S.; Miao, Y.; Liu, D.; Song, F. ChemCatChem 2021, 13, 140–152. doi:10.1002/cctc.202001179
Return to citation in text: [1] -
Fuhrhop, J.-H. Langmuir 2014, 30, 1–12. doi:10.1021/la402228g
Return to citation in text: [1] -
Day, N. U.; Wamser, C. C.; Walter, M. G. Polym. Int. 2015, 64, 833–857. doi:10.1002/pi.4908
Return to citation in text: [1] -
Di Natale, C.; Gros, C. P.; Paolesse, R. Chem. Soc. Rev. 2022, 51, 1277–1335. doi:10.1039/d1cs00662b
Return to citation in text: [1] -
Friedman, A.; Landau, L.; Gonen, S.; Gross, Z.; Elbaz, L. ACS Catal. 2018, 8, 5024–5031. doi:10.1021/acscatal.8b00876
Return to citation in text: [1] -
Zhao, Y.; Dai, W.; Peng, Y.; Niu, Z.; Sun, Q.; Shan, C.; Yang, H.; Verma, G.; Wojtas, L.; Yuan, D.; Zhang, Z.; Dong, H.; Zhang, X.; Zhang, B.; Feng, Y.; Ma, S. Angew. Chem., Int. Ed. 2020, 59, 4354–4359. doi:10.1002/anie.201915569
Return to citation in text: [1] -
Brandès, S.; Quesneau, V.; Fonquernie, O.; Desbois, N.; Blondeau-Patissier, V.; Gros, C. P. Dalton Trans. 2019, 48, 11651–11662. doi:10.1039/c9dt01599j
Return to citation in text: [1] -
Zhao, Y.; Peng, Y.; Shan, C.; Lu, Z.; Wojtas, L.; Zhang, Z.; Zhang, B.; Feng, Y.; Ma, S. Nano Res. 2022, 15, 1145–1152. doi:10.1007/s12274-021-3617-3
Return to citation in text: [1] -
Gryko, D. T.; Koszarna, B. Org. Biomol. Chem. 2003, 1, 350–357. doi:10.1039/b208950e
Return to citation in text: [1] -
Paolesse, R.; Nardis, S.; Sagone, F.; Khoury, R. G. J. Org. Chem. 2001, 66, 550–556. doi:10.1021/jo005661t
Return to citation in text: [1] -
Gryko, D. T.; Jadach, K. J. Org. Chem. 2001, 66, 4267–4275. doi:10.1021/jo010146w
Return to citation in text: [1] -
Decréau, R. A.; Collman, J. P. Tetrahedron Lett. 2003, 44, 3323–3327. doi:10.1016/s0040-4039(03)00617-8
Return to citation in text: [1] -
Geier, G. R., III; Grindrod, S. C. J. Org. Chem. 2004, 69, 6404–6412. doi:10.1021/jo049131z
Return to citation in text: [1] -
Braaten, K. C.; Gordon, D. G.; Aphibal, M. M.; Geier, G. R., III. Tetrahedron 2008, 64, 9828–9836. doi:10.1016/j.tet.2008.08.025
Return to citation in text: [1] -
Geier, G. R., III; Chick, J. F. B.; Callinan, J. B.; Reid, C. G.; Auguscinski, W. P. J. Org. Chem. 2004, 69, 4159–4169. doi:10.1021/jo0496493
Return to citation in text: [1] -
Guilard, R.; Gryko, D. T.; Canard, G.; Barbe, J.-M.; Koszarna, B.; Brandès, S.; Tasior, M. Org. Lett. 2002, 4, 4491–4494. doi:10.1021/ol027003w
Return to citation in text: [1] -
Ooi, S.; Tanaka, T.; Osuka, A. Eur. J. Org. Chem. 2015, 130–134. doi:10.1002/ejoc.201403217
Return to citation in text: [1] -
Sankar, J.; Anand, V. G.; Venkatraman, S.; Rath, H.; Chandrashekar, T. K. Org. Lett. 2002, 4, 4233–4235. doi:10.1021/ol026728x
Return to citation in text: [1] -
Temelli, B.; Gündüz, M.; Yüksel, D. Tetrahedron 2018, 74, 4476–4488. doi:10.1016/j.tet.2018.07.007
Return to citation in text: [1] -
Temelli, B.; Kalkan, H. Beilstein J. Org. Chem. 2018, 14, 187–193. doi:10.3762/bjoc.14.13
Return to citation in text: [1] -
Temelli, B.; Ozasik, O.; Yüksel, D. Eur. J. Org. Chem. 2017, 4905–4915. doi:10.1002/ejoc.201700896
Return to citation in text: [1] -
Temelli, B.; Kalkan, H. Synth. Commun. 2018, 48, 2112–2117. doi:10.1080/00397911.2018.1486426
Return to citation in text: [1] -
Gryko, D. T. Eur. J. Org. Chem. 2002, 1735–1743. doi:10.1002/1099-0690(200206)2002:11<1735::aid-ejoc1735>3.0.co;2-k
Return to citation in text: [1] -
Gryko, D. T.; Fox, J. P.; Goldberg, D. P. J. Porphyrins Phthalocyanines 2004, 8, 1091–1105. doi:10.1142/s1088424604000465
Return to citation in text: [1] -
Nardis, S.; Monti, D.; Paolesse, R. Mini-Rev. Org. Chem. 2005, 2, 355–374. doi:10.2174/157019305774322716
Return to citation in text: [1] -
Gryko, D. T. J. Porphyrins Phthalocyanines 2008, 12, 906–917. doi:10.1142/s1088424608000297
Return to citation in text: [1] -
König, M.; Faschinger, F.; Reith, L. M.; Schöfberger, W. J. Porphyrins Phthalocyanines 2016, 20, 96–107. doi:10.1142/s1088424616300056
Return to citation in text: [1] -
Brückner, C.; Posakony, J. J.; Johnson, C. K.; Boyle, R. W.; James, B. R.; Dolphin, D. J. Porphyrins Phthalocyanines 1998, 2, 455–465. doi:10.1002/(sici)1099-1409(199811/12)2:6<455::aid-jpp67>3.0.co;2-c
Return to citation in text: [1] -
Ryppa, C.; Senge, M. O.; Hatscher, S. S.; Kleinpeter, E.; Wacker, P.; Schilde, U.; Wiehe, A. Chem. – Eur. J. 2005, 11, 3427–3442. doi:10.1002/chem.200500001
Return to citation in text: [1] -
Rohand, T.; Dolusic, E.; Ngo, T. H.; Maes, W.; Dehaen, W. ARKIVOC 2007, No. x, 307–324. doi:10.3998/ark.5550190.0008.a20
Return to citation in text: [1] -
Taniguchi, M.; Balakumar, A.; Fan, D.; McDowell, B. E.; Lindsey, J. S. J. Porphyrins Phthalocyanines 2005, 9, 554–574. doi:10.1142/s1088424605000678
Return to citation in text: [1]
| 1. | Johnson, A. W.; Kay, I. T. Proc. Chem. Soc., London 1964, 89–90. |
| 2. | Johnson, A. W.; Kay, I. T. J. Chem. Soc. 1965, 1620–1629. doi:10.1039/jr9650001620 |
| 3. | Paolesse, R. Synlett 2008, 2215–2230. doi:10.1055/s-2008-1078687 |
| 4. | Aviv-Harel, I.; Gross, Z. Chem. – Eur. J. 2009, 15, 8382–8394. doi:10.1002/chem.200900920 |
| 17. | Decréau, R. A.; Collman, J. P. Tetrahedron Lett. 2003, 44, 3323–3327. doi:10.1016/s0040-4039(03)00617-8 |
| 18. | Geier, G. R., III; Grindrod, S. C. J. Org. Chem. 2004, 69, 6404–6412. doi:10.1021/jo049131z |
| 35. | Rohand, T.; Dolusic, E.; Ngo, T. H.; Maes, W.; Dehaen, W. ARKIVOC 2007, No. x, 307–324. doi:10.3998/ark.5550190.0008.a20 |
| 14. | Gryko, D. T.; Koszarna, B. Org. Biomol. Chem. 2003, 1, 350–357. doi:10.1039/b208950e |
| 15. | Paolesse, R.; Nardis, S.; Sagone, F.; Khoury, R. G. J. Org. Chem. 2001, 66, 550–556. doi:10.1021/jo005661t |
| 16. | Gryko, D. T.; Jadach, K. J. Org. Chem. 2001, 66, 4267–4275. doi:10.1021/jo010146w |
| 36. | Taniguchi, M.; Balakumar, A.; Fan, D.; McDowell, B. E.; Lindsey, J. S. J. Porphyrins Phthalocyanines 2005, 9, 554–574. doi:10.1142/s1088424605000678 |
| 10. | Friedman, A.; Landau, L.; Gonen, S.; Gross, Z.; Elbaz, L. ACS Catal. 2018, 8, 5024–5031. doi:10.1021/acscatal.8b00876 |
| 11. | Zhao, Y.; Dai, W.; Peng, Y.; Niu, Z.; Sun, Q.; Shan, C.; Yang, H.; Verma, G.; Wojtas, L.; Yuan, D.; Zhang, Z.; Dong, H.; Zhang, X.; Zhang, B.; Feng, Y.; Ma, S. Angew. Chem., Int. Ed. 2020, 59, 4354–4359. doi:10.1002/anie.201915569 |
| 12. | Brandès, S.; Quesneau, V.; Fonquernie, O.; Desbois, N.; Blondeau-Patissier, V.; Gros, C. P. Dalton Trans. 2019, 48, 11651–11662. doi:10.1039/c9dt01599j |
| 13. | Zhao, Y.; Peng, Y.; Shan, C.; Lu, Z.; Wojtas, L.; Zhang, Z.; Zhang, B.; Feng, Y.; Ma, S. Nano Res. 2022, 15, 1145–1152. doi:10.1007/s12274-021-3617-3 |
| 33. | Brückner, C.; Posakony, J. J.; Johnson, C. K.; Boyle, R. W.; James, B. R.; Dolphin, D. J. Porphyrins Phthalocyanines 1998, 2, 455–465. doi:10.1002/(sici)1099-1409(199811/12)2:6<455::aid-jpp67>3.0.co;2-c |
| 5. | Ji, W.; Wang, T.-X.; Ding, X.; Lei, S.; Han, B.-H. Coord. Chem. Rev. 2021, 439, 213875. doi:10.1016/j.ccr.2021.213875 |
| 6. | Jin, L.; Lv, S.; Miao, Y.; Liu, D.; Song, F. ChemCatChem 2021, 13, 140–152. doi:10.1002/cctc.202001179 |
| 7. | Fuhrhop, J.-H. Langmuir 2014, 30, 1–12. doi:10.1021/la402228g |
| 8. | Day, N. U.; Wamser, C. C.; Walter, M. G. Polym. Int. 2015, 64, 833–857. doi:10.1002/pi.4908 |
| 9. | Di Natale, C.; Gros, C. P.; Paolesse, R. Chem. Soc. Rev. 2022, 51, 1277–1335. doi:10.1039/d1cs00662b |
| 34. | Ryppa, C.; Senge, M. O.; Hatscher, S. S.; Kleinpeter, E.; Wacker, P.; Schilde, U.; Wiehe, A. Chem. – Eur. J. 2005, 11, 3427–3442. doi:10.1002/chem.200500001 |
| 23. | Sankar, J.; Anand, V. G.; Venkatraman, S.; Rath, H.; Chandrashekar, T. K. Org. Lett. 2002, 4, 4233–4235. doi:10.1021/ol026728x |
| 27. | Temelli, B.; Kalkan, H. Synth. Commun. 2018, 48, 2112–2117. doi:10.1080/00397911.2018.1486426 |
| 22. | Ooi, S.; Tanaka, T.; Osuka, A. Eur. J. Org. Chem. 2015, 130–134. doi:10.1002/ejoc.201403217 |
| 28. | Gryko, D. T. Eur. J. Org. Chem. 2002, 1735–1743. doi:10.1002/1099-0690(200206)2002:11<1735::aid-ejoc1735>3.0.co;2-k |
| 29. | Gryko, D. T.; Fox, J. P.; Goldberg, D. P. J. Porphyrins Phthalocyanines 2004, 8, 1091–1105. doi:10.1142/s1088424604000465 |
| 30. | Nardis, S.; Monti, D.; Paolesse, R. Mini-Rev. Org. Chem. 2005, 2, 355–374. doi:10.2174/157019305774322716 |
| 31. | Gryko, D. T. J. Porphyrins Phthalocyanines 2008, 12, 906–917. doi:10.1142/s1088424608000297 |
| 32. | König, M.; Faschinger, F.; Reith, L. M.; Schöfberger, W. J. Porphyrins Phthalocyanines 2016, 20, 96–107. doi:10.1142/s1088424616300056 |
| 20. | Geier, G. R., III; Chick, J. F. B.; Callinan, J. B.; Reid, C. G.; Auguscinski, W. P. J. Org. Chem. 2004, 69, 4159–4169. doi:10.1021/jo0496493 |
| 21. | Guilard, R.; Gryko, D. T.; Canard, G.; Barbe, J.-M.; Koszarna, B.; Brandès, S.; Tasior, M. Org. Lett. 2002, 4, 4491–4494. doi:10.1021/ol027003w |
| 19. | Braaten, K. C.; Gordon, D. G.; Aphibal, M. M.; Geier, G. R., III. Tetrahedron 2008, 64, 9828–9836. doi:10.1016/j.tet.2008.08.025 |
| 24. | Temelli, B.; Gündüz, M.; Yüksel, D. Tetrahedron 2018, 74, 4476–4488. doi:10.1016/j.tet.2018.07.007 |
| 25. | Temelli, B.; Kalkan, H. Beilstein J. Org. Chem. 2018, 14, 187–193. doi:10.3762/bjoc.14.13 |
| 26. | Temelli, B.; Ozasik, O.; Yüksel, D. Eur. J. Org. Chem. 2017, 4905–4915. doi:10.1002/ejoc.201700896 |
© 2022 Temelli and Kapci; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.