Abstract
During the continued isolation of different bacteria from highly diverse, low human activity environments in Ghana and the subsequent characterization and biological activity studies of their secondary metabolites, we found both Gram-positive and Gram-negative Bacillus strains to be ubiquitous and widespread. One of such strains, the Ghanaian novel Bacillus sp. strain DE2B was isolated from rhizosphere soils collected from the Digya National Park in Ghana. Chromatographic purifications of the fermented culture extract of the strain DE2B, led to the isolation of a cyclic lipopeptide, digyalipopeptide A (1). Using 1D and 2D NMR data, mass spectrometry sequence tagging, advanced Marfey’s analysis, and the GNPS molecular networking we solved the full structure of digyalipopeptide A (1). We found that compound 1 is a member of a somewhat homologous series of peptides produced as a mixture by the strain containing the same amino acid sequence in the cyclic peptide backbone but differing only by the length of aliphatic fatty acid side chains. When tested against Trypanosoma brucei subsp. brucei strain GUTat 3.1 and Leishmania donovani (Laveran and Mesnil) Ross (D10), digyalipopeptide A (1) gave IC50 values of 12.89 µM (suramin IC50 0.96 µM) and 4.85 µM (amphotericin B IC50 4.87 µM), respectively. Furthermore, digyalipopeptide A (1) produced IC50 values of 10.07 µM (ampicillin IC50 0.18 µM) and 10.01 µM (ampicillin IC50 1.53 µM) for Staphylococcus aureus and Shigella sonnei, respectively. The selectivity and toxicity profile of compound 1 was investigated using normal cell lines, macrophages RAW 264.7. When tested against normal macrophages, compound 1 gave an IC50 value of 71.32 μM. Selectivity indices (SI) were obtained by calculating the ratio of the IC50 in RAW 264.7 to the IC50 in the respective microbe and neglected parasite. In the presence of RAW 264.7 cell lines, compound 1 was particularly selective towards Leishmania donovani (Laveran and Mesnil) Ross (D10) with an SI value of 14.71. The bioactivity studies conducted confirm the role of these cyclic lipopeptides as defense chemicals in their natural environment and their ability to be biologically active across different species.
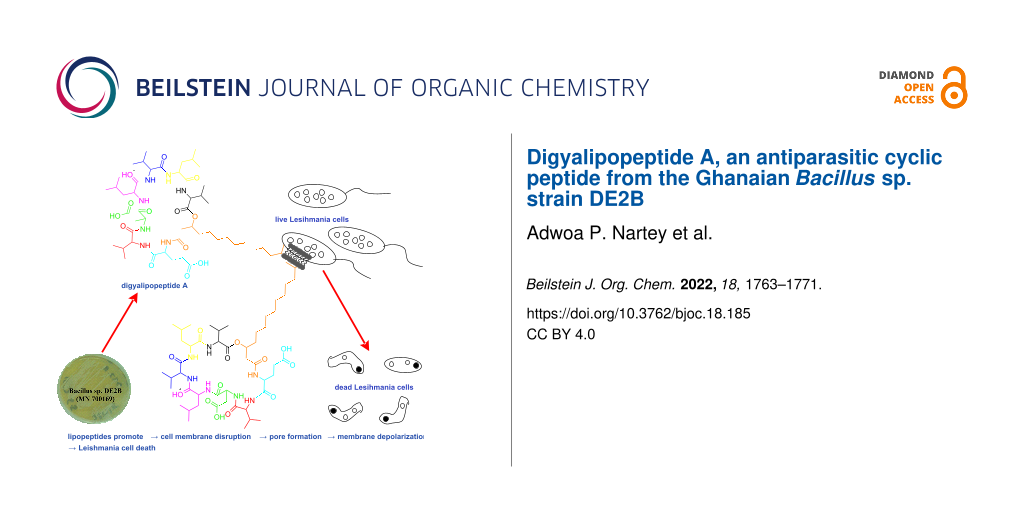
Graphical Abstract
Introduction
Microbes belonging to the genus Bacillus are mainly Gram-positive with only a few examples characterized as Gram-negative [1]. Bacillus demonstrates a variety of colony morphologies and is ubiquitous in nature, being omnipresent in aquatic, benthic, halophilic, soil, and pathogenic environments [2]. Together, this group constitutes one of the most genetically and environmentally diverse genera in the microbial world [3]. The major secondary metabolic pathways, defense mechanisms, and intracellular transport processes vary widely among Bacillus depending on their natural habitats [4-6]. Although the majority of Bacillus species are non-pathogenic, the few well-studied and genome-sequenced pathogenic strains among the group have instilled fear in the scientists that may wish to investigate their potential. This has rendered the genera much less studied when compared to their Streptomyces counterparts in terms of bioprospecting for novel drug scaffolds. However, a substantial number of Bacillus species have proven to be of clinical, health, industrial research and agrochemical importance [7-12].
Interestingly, the resistance of Bacillus spores to heat, radiation, and disinfection coupled with an inherent biosynthetic potential that enables them to grow even in the presence of antibiotics such as nystatin and nalidixic acid makes it easier to isolate them from a number of soil and sediment types using the spread plate method (SPM) in our laboratory. Therefore, in our continued research on the discovery of new bioactive compounds from Ghanaian microbes, we have started to study interesting strains belonging to Bacillus genus. Previously, we reported a Ghanaian Paenibacillus sp. strain DE2SH which produced the novel antiparasitics paenidigyamycins A–J and digyaindoleacid A [13-15].
Herein, we report a novel Ghanaian Bacillus sp. strain DE2B with GenBank Accession Number: MN700169 (Figures S1 and S2 in Supporting Information File 1), isolated from the Digya National Park in the Brong Ahafo Region of Ghana. We found this strain to produce a cyclic lipopeptide which we have named digyalipopeptide A (1).
Results and Discussion
Structure elucidation
Compound 1 (digyalipopeptide A) was isolated by repeated semi-preparative reversed-phase HPLC with diode array detection (0–400 nm) at retention time of 27.5 min (see Supporting Information File 1, Figures S3–S7). It was obtained as a white amorphous powder which was bright yellow when in solution. The molecular formula of 1 was determined to be C51H89N7O13 based on the high resolution electrospray ionization mass spectrometry (HRESIMSMS) at m/z 1008.6610 for [M + H]+ similar to the calculated value of m/z 1008.6591 with Δ = −1.9 ppm. The molecular formula indicated 11 degrees of unsaturation and the analyses of the 13C NMR spectrum acquired in DMSO-d6, together with the distortionless enhancement by polarization transfer (DEPT-135) and the 1H, 13C, multiplicity edited pulsed field gradient heteronuclear single quantum coherence (HSQC) spectra showed the presence of ten sp2-hybridized carbon resonances all of which were assigned to carbonyl carbons of seven amides, two carboxylic acids, and one ester. Also present were forty-one sp3-hybridized carbon signals which were assigned to fourteen methines, fifteen methylenes and twelve methyl groups
The 1H NMR spectrum exhibited signals at δH 4.19 (ov., 1H, H-2), 4.08 (dd, J = 8.8, 6.0 Hz, 1H, H-7), 4.53 (ov., 1H, H-12), 4.52 (ov., 1H, H-16), 4.18 (ov., 1H, H-22), 4.08 (dd, J = 8.8, 6.0 Hz, 1H, H-33), and 4.44 (ov., 1H, H-27) which were indicative of the presence of α-hydrogens belonging to amino acid residues typical for peptides. Using 1H,1H homonuclear correlation spectroscopy (COSY) and 1H,13C total correlation spectroscopy (HSQC-TOCSY), the individual spin systems within each amino acid residue were fully established.
Subsequently, seven amino acid residues were identified as glutamic acid, valine-1, aspartic acid, leucine-1, valine-2, leucine-2 and valine-3, see Table 1 and Tables S1 and S2 in Supporting Information File 1. The addition of carbonyl carbon HMBC data gave the complete amino acids which were further sequenced using LC–HRESIMSn sequence tag data.
Table 1: 1H NMR and 13C NMR spectroscopic data for compound 1 in DMSO-d6.
| Residue | # | δc, mult |
δH, mult
(J in Hz) |
Residue | # | δc, mult |
δH, mult
(J in Hz) |
|
| glutamic acid | 1 | 171.8, C | leucine-2 | 26 | 171.6, C | |||
| 2 | 51.9, CH | 4.19, ov. | 27 | 50.4, CH | 4.44, ov. | |||
| 3 | 27.3, CH2 |
1.91, ov.
1.77, ov. |
28 | 41.6, CH2 | 1.42, ov. | |||
| 4 | 29.9, CH2 | 2.22, t (7.9) | 29 | 24.1, CH | 1.53, ov. | |||
| 5 | 174.0, C | 30, 31 | 23.0, CH3 | 0.86, ov. | ||||
| 1NH | 7.82, d (7.2) | 6NH | 8.30, ov. | |||||
| OH | 12.24, s | |||||||
| valine-1 | 6 | 170.6, C | valine-3 | 32 | 170.6, C | |||
| 7 | 58.2, CH | 4.08, dd (8.8, 6.0) | 33 | 58.2, CH | 4.08, dd (8.8, 6.0) | |||
| 8 | 30.5, CH | 1.96, ov. | 34 | 30.5, CH | 1.96, ov. | |||
| 9,10 | 17.9, CH3 | 0.75, d (6.8) | 35, 36 | 17.9, CH3 | 0.75, d (6.8) | |||
| 2NH | 7.77, ov. | 7NH | 8.26, ov. | |||||
| aspartic acid | 11 | 169.8, C | fatty acid | 37 | 169.4, C | |||
| 12 | 49.6, CH | 4.53, ov. | 38 | 40.4, CH2 |
2.41, ov.
2.38, ov. |
|||
| 13 | 35.9, CH2 |
2.70, dd (7.2, 18)
2.59, dd (9.5, 18) |
39 | 71.6, CH | 5.00, m | |||
| 14 | 171.3, C | 40 | 33.2, CH2 | 1.55, ov. | ||||
| 3NH | 8.17, m | 41 | 28.5, CH2 | 1.21, ov. | ||||
| OH | 12.24, s | 42 | 28.8, CH2 | 1.21, ov. | ||||
| 43 | 28.9, CH2 | 1.21, ov. | ||||||
| leucine-1 | 15 | 171.8, C | 44 | 28.9, CH2 | 1.21, ov. | |||
| 16 | 50.6, CH | 4.52, ov. | 45 | 28.9, CH2 | 1.21, ov. | |||
| 17 | 41.6, CH2 | 1.42, m | 46 | 28.9, CH2 | 1.21, ov. | |||
| 18 | 24.1, CH | 1.53, ov. | 47 | 26.7, CH2 | 1.22, ov. | |||
| 19,20 | 22.9, CH3 | 0.86, ov. | 48 | 39.7, CH2 | 1.53, ov. | |||
| 4NH | 7.68, d (8.6) | 49 | 27.3, CH | 1.49, ov. | ||||
| 50, 51 | 23.0, CH3 | 0.86, ov. | ||||||
| valine-2 | 21 | 172.2, C | ||||||
| 22 | 57.2, CH | 4.18, ov. | ||||||
| 23 | 29.5, CH | 2.07, h (6.0) | ||||||
| 24 | 19.0, CH3 | 0.84, ov. | ||||||
| 25 | 18.9, CH3 | 0.84, ov. | ||||||
| 5NH | 8.26, ov. | |||||||
The 1H NMR data also showed the presence of diastereotopic methylene protons at δH 2.41, 2.38 (ov., 2H, H-38) which were characteristic for a –CH2– sandwiched between the carbonyl carbon of an amide and a hydroxylated methine proton δH 5.00 (m, 1H, H-39). Interestingly, this spin system expands to include protons at δH 1.55 (ov., 2H, H-40), 1.21 (ov., 2H, H-41), 1.21 (ov., 2H, H-42), 1.21 (ov., 2H, H-43), 1.21 (ov., 2H, H-44), 1.21 (ov., 2H, H-45), and 1.21 (ov., 2H, H-46) suggesting the presence of a long aliphatic chain. Careful study of the protons at δH 1.21 (ov., 2H, H-47), 1.53 (ov., 2H, H-48), 1.49 (ov., 1H, H-49), and 0.86 (ov., 6H, H-50, H-51) also suggested that the long aliphatic chain terminated as an isopropyl.
The COSY correlation H-38 (δH 2.41, 2.38)/H-39 (δH 5.00) which for this spin system is largely extended by the gHSQC-TOCSY correlations C-38 (δC 40.4)/H-39 (δH 5.00), C-39 (δC 71.6)/H-38 (δH 2.41, 2.38), H-40 (δH 1.55), H-41 (δH 1.21), H-42 (δH 1.21), H-43 (δH 1.21), H-44 (δH 1.21), H-45 (δH 1.21), H-46 (δH 1.21), H-48 (δH 1.21), C-40 (δC 33.2)/H-38 (δH 2.41, 2.38), H-39 (δH 5.00), H-41 (δH 1.21), H-42 (δH 1.21), H-43 (δH 1.21), H-44 (δH 1.21), H-45 (δH 1.21), H-46 (δH 1.21), C-42 (δC 28.8)/H-39 (δH 5.00), H-40 (δH 1.55), H-48 (δH 1.21), C-47 (δC 26.7)/H-41 (δH 1.21), H-42 (δH 1.21), H-43 (δH 1.21), H-44 (δH 1.21), H-45 (δH 1.21), H-46 (δH 1.21), and C-48 (δC 39.7)/H-50 (δH 0.86) fully confirms the aliphatic side chain terminating by an isopropyl moiety. Some of the HMBC correlations used to support this substructure are, H-38 (δH 2.41, 2.38)/C-39 (δC 71.6), H-39 (δH 5.00)/C-37 (δC 169.4), H-45 (δH 1.21)/C-47 (δC 26.7), H-46 (δH 1.21)/C-47 (δC 26.7), and H-47 (δH 1.22)/C-45 (δC 28.9).
The structure of compound 1 was further confirmed by the analysis of NOESY data, which along with the other 2D NMR data, are summarized in Figure 1, Figure 2 and Figure 3 and Table 1. The full details of all the NMR data with correlations can be found in Table S1 and Figures S22–S29 in Supporting Information File 1 but the summary for the data acquired in DMSO-d6 solvent is shown in Table 1. Subsequently, we dissolved compound 1 in deuterated chloroform, measured all 1D- and 2D NMR data and repeated the structure elucidation (see Supporting Information File 1, Table S2, and Figures S30–S37). The resultant structure confirmed the structural information initially obtained from the NMR data in DMSO-d6 solvent.
Figure 1: Primary structure of digyalipopeptide A (1).
Figure 1: Primary structure of digyalipopeptide A (1).
![[1860-5397-18-185-2]](/bjoc/content/figures/1860-5397-18-185-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: Key COSY and HMBC correlations for compound 1.
Figure 2: Key COSY and HMBC correlations for compound 1.
![[1860-5397-18-185-3]](/bjoc/content/figures/1860-5397-18-185-3.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 3: Key TOCSY and NOESY correlations for compound 1.
Figure 3: Key TOCSY and NOESY correlations for compound 1.
High-resolution ESI mass spectrometry (HRESIMS) sequence tags
Furthermore, analysis of the LC–HRESIMSn showed the presence of several b and y sequence tag ions indicating fragmentations from the middle of the cyclic peptide and most importantly the specific positions of each amino acid residue (Figure 4). Due to the presence of the free hydroxy groups of aspartic and glutamic acid and the elaborate system of aliphatic side chains, the sequence tag ions generated by systematic loss of amino acids were each subjected to several facile losses of H2O, –CH3, –C2H2O and CO resulting in the generation of several peaks in the LC–HRESIMSn spectrum. The b and y ion fragmentation derived sequence tags corresponding to m/z 909 (− 99Val), 796 (− 99Val − 113Leu), 794 (− 99Val − 115Asp), 780 (− 99Val − 129Glu), 697 (− 99Val − 113Leu − 99Val), 582 (− 99Val − 113Leu − 99Val − 113Leu), 469 (− 99Val − 113Leu − 99Val − 113Leu − 115Asp) and 370 (− 99Val − 113Leu − 99Val − 113Leu − 115Asp − 99Val) provided the needed sequence information for the structure of compound 1.
Figure 4: Liquid chromatography high resolution electrospray ionization mass spectrometry (LC–HRESIMS) sequence tags for compound 1.
Figure 4: Liquid chromatography high resolution electrospray ionization mass spectrometry (LC–HRESIMS) sequen...
Stereochemistry determination
Historically, the determination of the regiochemistry of enantiomeric amino acid residues within natural product peptides has proved challenging sometimes requiring the strenuous efforts of de novo total synthesis or degradation. This is because in natural product peptides both the ᴅ- and ʟ-forms of the different amino acids may be incorporated into the peptide structure. In order to determine the regiochemistry of the Val, Leu, Asp, and Glu amino acid residues in compound 1, we deployed the advanced Marfey’s method [16-18] involving the complete hydrolysis of compound 1 followed by derivatization of the resultant amino acid residues with N-α-(2,4-dinitro-5-fluorophenyl)-ʟ-alanine amide (ʟ-FDAA).
Similar derivatization reactions between ʟ-FDAA and authentic amino acid standards, followed by their injection on analytical reversed-phase HPLC-DAD in comparison to the naturally derived amino acid residue derivatives of 1 provided information on the absolute stereochemistry of the component amino acids in compound 1 (see Figure 5 and Figures S9–S13 and Table S3 in Supporting Information File 1).
Figure 5: The absolute stereochemistry of compound 1.
Figure 5: The absolute stereochemistry of compound 1.
However, it was difficult to determine the absolute stereochemistry around the esterified methine carbon C-39 (δC 71.6) of the fatty acid chain. In the absence of an authentic laboratory standard for the fatty acid chain coupled with the inability to obtain well-defined multiplicity patterns in the 1H NMR spectrum amidst huge overlaps of signals for δH 2.41, 2.38 (ov., 2H, H-38), δH 5.00 (m, 1H, H-39), δH 1.55 (ov., 2H, H-40) made it difficult to also obtain coupling constants (3JHH) for information on the relative spatial orientation of residues in this part of the structure.
In order to confirm the presence of the cyclic ester and the possible absolute stereochemistry around C-39 (δC 71.6) of the fatty acid chain we first of all consulted the global natural products social molecular networking (GNPS) [19-21]. The GNPS network data (Figure 6) provided the opportunity to examine similar lipopeptide structures previously characterized in the literature that possessed both the fatty acid chain terminating as an isopropyl and adjacent to Glu residue connected by an amide bond. This detailed examination revealed that in the structure of these cyclic lipopeptides, the configuration of the methine carbon C-39 (δC 71.6) of the fatty acid chain is always opposite to that of the adjacent Glu residue connected by an amide bond with typical examples being the pumilacidin B and surfactin C [22]. Therefore, since the stereochemistry of the Glu residue was S we tentatively proposed that the absolute stereochemistry of the methine carbon C-39 (δC 71.6) of the fatty acid chain is most likely to be R. In order to verify this hypothesis, we used the many accumulated NOESY correlations to make relative deductions that established the stereochemistry of the C-39 (δC 71.6). Firstly, H-2 has an S configuration pointing into the plane of the screen and oriented away from us. In this configuration, H-2 shows NOESY correlations H-2/1NH and H-2/2NH suggesting that protons 1NH and 2-NH also orient similarly to H-2. Therefore, the NOESY correlation H-39/1NH shows that the H-39 proton must point away from us resulting in an S configuration at C-39 as opposed to and R configuration. Subsequently, we propose the 3D structure of compound 1 as shown in Figure 5.
![[1860-5397-18-185-6]](/bjoc/content/figures/1860-5397-18-185-6.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 6: Global natural products social molecular networking (GNPS).
Figure 6: Global natural products social molecular networking (GNPS).
Structurally, compound 1 belongs to the surfactin group of lipopeptides that exhibit huge chemical diversity through the alternate insertion of different amino acids, homologous extension and linear, isopropyl or isobutyl termination of fatty acid chains. Therefore, the biosynthesis of compound 1 is speculated to occur via a similar pathway like the surfactins, which is known to be modulated by four enzyme subunits SrfAA, SrfAB, SrfAC, and SrfAD on the surfactin synthetase complex [23,24]. We hope to confirm this similarity in the near future as we proceed to further study the whole genome sequence of strain DE2B.
Subsequently, using the fully elucidated structure of compound 1, we noticed that this compound was part of a homologous series that included the reversed phase HPLC co-eluting compounds shown in Figure S5 (Supporting Information File 1).
Biological activity
Generally, cyclic lipopeptides have an amphipathic physicochemical property that directly promotes their ability to be compatible with both hydrophobic and hydrophilic biological environments. Repeated studies on the bioactivity of lipopeptides have shown that the primary mechanism of action of these peptides involves interactions with the double layer of lipids and proteins that constitute the cell membrane [24-26]. This mechanism of action is important, since it promotes the ability of these ubiquitous cyclic lipopeptides to be effective and biologically active across different organism types. Hence, cyclic lipopeptides have been previously reported to possess antimicrobial, antifungal, antiviral, anticancer, and cytotoxic biological activities, but their ability to produce antiparasitic activity against neglected tropical parasites such as trypanosomes and leishmanias is largely underinvestigated.
Therefore, we studied the biological activity profile of compound 1 against Trypanosoma brucei subsp. brucei strain GUTat 3.1 and Leishmania donovani (Laveran and Mesnil) Ross (D10). Compound 1 showed promising antiparasitic activity against Leishmania donovani with IC50 of 4.85 µM compared to the laboratory standard amphotericin B with IC50 value of 4.87 µM (Table 2 and Figure S13 in Supporting Information File 1). When tested against Trypanosoma brucei subsp. brucei strain GUTat 3.1, compound 1 gave an IC50 value of 12.89 µM compared to the laboratory standard, suramin with IC50 of 0.96 µM (Table 2 and Figure S14 in Supporting Information File 1).
Furthermore, we investigated the biological activity profile of compound 1 against a number of Gram-negative and Gram-positive standard laboratory bacteria including Escherichia coli, Staphylococcus aureus, Bacillus cereus, Shigella flexneri, Shigella sonnei, Salmonella paratyphi B, Salmonella enteritidis, Salmonella typhimurium, and Shigella dysenteriae (Table 3). Compound 1 was found to possess promising antimicrobial properties in comparison to the laboratory standards ampicillin and amphotericin B. Compound 1 produced interesting biological activity against the Gram-negative bacteria Shigella sonnei, Shigella flexneri and the multidrug resistant Gram-positive Staphylococcus aureus with IC50 of 10.01, 18.71, and 10.01 µM, respectively. The laboratory standard itself produced IC50 of 1.53 µM for Shigella sonnei, Shigella flexneri 1.76 µM, and Staphylococcus aureus 0.18 µM, respectively (Table 3).
Table 3: Antibacterial activity data for compound 1.
| Microbe | IC50 (µM) ± SEM | Positive control | IC50 (µM) ± SEM | |
| compound 1 | E. coli | >100 ± 0.31 | ampicillin | 10.44 ±0.18 |
| S. typhimurium | 34.27 ± 0.25 | ampicillin | 1.27 ± 0.15 | |
| B. cereus | 38.35 ± 0.21 | ampicillin | 1.70 ± 0.19 | |
| S. flexneri | 18.71 ± 0.18 | ampicillin | 1.76 ± 0.21 | |
| S. paratyphi B | 27.90 ± 0.19 | amphotericin B | 1.53 ± 0.20 | |
| S. enteritidis | >100 ± 0.34 | ampicillin | 0.76 ± 0.30 | |
| S. aureus | 10.07 ± 0.29 | ampicillin | 0.18 ± 0.16 | |
| S. sonnei | 10.01 ± 0.15 | ampicillin | 1.53 ± 0.19 | |
| S. dysenteriae | 19.72 ± 0.20 | ampicillin | 1.07 ± 0.22 | |
The selectivity and toxicity profile of compound 1 was also investigated using normal cell lines, macrophages RAW 264.7. When tested against normal macrophages, compound 1 produced an IC50 value of 71.32 μM. Selectivity indices (SI) were obtained by calculating the ratio of the IC50 in RAW 264.7 to the IC50 in the respective microbe or neglected parasite. In comparison to RAW 264.7 cell line, compound 1 was particularly selective towards Leishmania donovani (Laveran and Mesnil) Ross (D10) with an SI value of 14.71. Other promising SI values of 2.08, 1.86, 3.81, and 2.26 were also recorded for Salmonella typhimurium, Bacillus cereus, Shigella flexneri, and Salmonella paratyphi B, respectively, as shown in Table 4 and Figure S15 in Supporting Information File 1.
Table 4: Cytotoxicity and selectivity profiles of compound 1 using normal macrophages.
| Microbe | Activity in microbe or parasite [IC50 (µM)]) ± SEM | Selectivity Index (SI) |
| E. coli | >100 ± 0.29 | <0.71 |
| S. typhimurium | 34.27 ± 0.21 | 2.08 |
| B. cereus | 38.35 ± 0.18 | 1.86 |
| S. flexneri | 18.71 ± 0.24 | 3.81 |
| S. paratyphi B | 27.90 ± 0.28 | 2.26 |
| S. enteritidis | >100 ± 0.20 | <0.71 |
| Parasite | ||
| T. brucei brucei | 12.89 ± 0.19 | 5.53 |
| L. donovani | 4.85 ± 0.22 | 14.71 |
Conclusion
In conclusion, a new cyclic lipopeptide has been isolated from the novel Ghanaian Bacillus sp. Strain DE2B. The structure of this peptide was established through detailed analysis of 1D and 2D NMR spectroscopic data acquired in DMSO-d6, HRESIMSMS sequence tagging data with GNPS molecular networking and the advanced Marfey’s test. We proposed that the biosynthesis of compound 1 is similar to that of other surfactin-like lipopeptides previously reported in the literature. We found compound 1 to possess remarkable antiparasitic activity against Trypanosoma brucei subsp. brucei strain GUTat 3.1 and Leishmania donovani (Laveran and Mesnil) Ross (D10).
Furthermore, the antimicrobial bioactivity of compound 1 was also evaluated against Escherichia coli, Staphylococcus aureus, Bacillus cereus, Shigella flexneri, Shigella sonnei, Salmonella paratyphi B, Salmonella enteritidis, Salmonella typhimurium, and Shigella dysenteriae. Compound 1 produced the best antimicrobial activity results against Shigella sonnei, Shigella flexneri, and the multidrug resistant Gram-positive Staphylococcus aureus.
Subsequently, we reiterate the fact that it is important not to overlook Bacillus and their natural products especially in this current era of spreading antibiotic resistance and demands for ecologically friendly pest control strategies.
Supporting Information
| Supporting Information File 1: Experimental protocols, phylogenetic data, compound characterization data (1D, 2D NMR), stereochemistry determination, tables, and figures. | ||
| Format: PDF | Size: 7.1 MB | Download |
Acknowledgements
We acknowledge the mass spectrometry data received from the laboratory of Professor Pieter C. Dorrestein and Andrés Mauricio Caraballo Rodrígueze.
Funding
K.K., H.D and M.J. are grateful for the financial support of Leverhulme Trust-Royal Society Africa award (AA090088) and the jointly funded UK Medical Research Council–UK Department for International Development (MRC/DFID) Concordat Agreement African Research Leaders Award (MR/S00520X/1). A.P.N. is thankful for the award of a Ph.D. scholarship by the Organization for Women in Science for the Developing World (OWSD), the Swedish International Development Cooperation Agency (SIDA) and the Carnegie BANGA-Africa Project Award for a Ph.D. scholarship.
References
-
Cote, C. K.; Heffron, J. D.; Bozue, J. A.; Welkos, S. L.; Liu, D. Bacillus anthracis and other Bacillus species. In Molecular Medical Microbiology, 2nd ed.; Schwartzman, J.; Tang, Y.-W.; Sussman, M.; Poxton, I., Eds.; Academic Press: Boston, USA, 2015; pp 1789–1844. doi:10.1016/b978-0-12-397169-2.00102-5
Return to citation in text: [1] -
Checinska, A.; Paszczynski, A.; Burbank, M. Annu. Rev. Food Sci. Technol. 2015, 6, 351–369. doi:10.1146/annurev-food-030713-092332
Return to citation in text: [1] -
Cihan, A. C.; Tekin, N.; Ozcan, B.; Cokmus, C. Braz. J. Microbiol. 2012, 43, 309–324. doi:10.1590/s1517-83822012000100037
Return to citation in text: [1] -
Sansinenea, E.; Ortiz, A. Biotechnol. Lett. 2011, 33, 1523–1538. doi:10.1007/s10529-011-0617-5
Return to citation in text: [1] -
Harwood, C. R.; Mouillon, J.-M.; Pohl, S.; Arnau, J. FEMS Microbiol. Rev. 2018, 42, 721–738. doi:10.1093/femsre/fuy028
Return to citation in text: [1] -
Boottanun, P.; Potisap, C.; Hurdle, J. G.; Sermswan, R. W. AMB Express 2017, 7, No. 16. doi:10.1186/s13568-016-0302-0
Return to citation in text: [1] -
Raddadi, N.; Crotti, E.; Rolli, E.; Marasco, R.; Fava, F.; Daffonchio, D. The Most Important Bacillus Species in Biotechnology. In Bacillus thuringiensis Biotechnology; Sansinenea, E., Ed.; Springer: Dordrecht, 2012; pp 347–366. doi:10.1007/978-94-007-3021-2_17
Return to citation in text: [1] -
Kandi, V. J. Med. Microb. Diagn. 2016, 5, No. 2. doi:10.4172/2161-0703.1000e130
Return to citation in text: [1] -
Nemutanzhela, M. E.; Roets, Y.; Gardiner, N.; Lalloo, R. The use and benefits of Bacillus based biological agents in aquaculture. In Sustainable Aquaculture Techniques; Hernandez-Vergara, M. P.; Perez-Rostro, C. I., Eds.; IntechOpen, 2014. doi:10.5772/57375
Return to citation in text: [1] -
Shafi, J.; Tian, H.; Ji, M. Biotechnol. Biotechnol. Equip. 2017, 31, 446–459. doi:10.1080/13102818.2017.1286950
Return to citation in text: [1] -
Schallmey, M.; Singh, A.; Ward, O. P. Can. J. Microbiol. 2004, 50, 1–17. doi:10.1139/w03-076
Return to citation in text: [1] -
Elshaghabee, F. M. F.; Rokana, N.; Gulhane, R. D.; Sharma, C.; Panwar, H. Front. Microbiol. 2017, 8, No. 1490. doi:10.3389/fmicb.2017.01490
Return to citation in text: [1] -
Osei, E.; Kwain, S.; Mawuli, G. T.; Anang, A. K.; Owusu, K. B. A.; Camas, M.; Camas, A. S.; Ohashi, M.; Alexandru-Crivac, C. N.; Deng, H.; Jaspars, M.; Kyeremeh, K. Mar. Drugs 2019, 17, 9. doi:10.3390/md17010009
Return to citation in text: [1] -
Tetevi, G. M.; Kwain, S.; Mensah, T.; Camas, A. S.; Camas, M.; Dofuor, A. K.; Azerigyik, F. A.; Oluwabusola, E.; Deng, H.; Jaspars, M.; Kyeremeh, K. Molbank 2019, 4, M1094. doi:10.3390/m1094
Return to citation in text: [1] -
Kwain, S.; Tetevi, G. M.; Mensah, T.; Camas, A. S.; Camas, M.; Dofuor, A. K.; Azerigyik, F. A.; Oluwabusola, E.; Deng, H.; Jaspars, M.; Kyeremeh, K. Molbank 2019, 3, M1080. doi:10.3390/m1080
Return to citation in text: [1] -
Vijayasarathy, S.; Prasad, P.; Fremlin, L. J.; Ratnayake, R.; Salim, A. A.; Khalil, Z.; Capon, R. J. J. Nat. Prod. 2016, 79, 421–427. doi:10.1021/acs.jnatprod.5b01125
Return to citation in text: [1] -
Ayon, N. J.; Sharma, A. D.; Gutheil, W. G. J. Am. Soc. Mass Spectrom. 2019, 30, 448–458. doi:10.1007/s13361-018-2093-9
Return to citation in text: [1] -
Dewapriya, P.; Prasad, P.; Damodar, R.; Salim, A. A.; Capon, R. J. Org. Lett. 2017, 19, 2046–2049. doi:10.1021/acs.orglett.7b00638
Return to citation in text: [1] -
Wang, M.; Carver, J. J.; Phelan, V. V.; Sanchez, L. M.; Garg, N.; Peng, Y.; Nguyen, D. D.; Watrous, J.; Kapono, C. A.; Luzzatto-Knaan, T.; Porto, C.; Bouslimani, A.; Melnik, A. V.; Meehan, M. J.; Liu, W. T.; Crüsemann, M.; Boudreau, P. D.; Esquenazi, E.; Sandoval-Calderón, M.; Bandeira, N. Nat. Biotechnol. 2016, 34, 828–837. doi:10.1038/nbt.3597
Return to citation in text: [1] -
Vargas, F.; Weldon, K. C.; Sikora, N.; Wang, M.; Zhang, Z.; Gentry, E. C.; Panitchpakdi, M. W.; Caraballo-Rodriguez, A. M.; Dorreistein, P.; Jarmusch, A. K. Rapid Commun. Mass Spectrom. 2020, 34, e8725. doi:10.1002/rcm.8725
Return to citation in text: [1] -
Nothias, L.-F.; Petras, D.; Schmid, R.; Dührkop, K.; Rainer, J.; Sarvepalli, A.; Protsyuk, I.; Ernst, M.; Tsugawa, H.; Fleischauer, M.; Aicheler, F.; Aksenov, A. A.; Alka, O.; Allard, P.-M.; Barsch, A.; Cachet, X.; Caraballo-Rodriguez, A. M.; Da Silva, R. R.; Dang, T.; Garg, N.; Gauglitz, J. M.; Gurevich, A.; Isaac, G.; Jarmusch, A. K.; Kameník, Z.; Kang, K. B.; Kessler, N.; Koester, I.; Korf, A.; Le Gouellec, A.; Ludwig, M.; Martin H., C.; McCall, L.-I.; McSayles, J.; Meyer, S. W.; Mohimani, H.; Morsy, M.; Moyne, O.; Neumann, S.; Neuweger, H.; Nguyen, N. H.; Nothias-Esposito, M.; Paolini, J.; Phelan, V. V.; Pluskal, T.; Quinn, R. A.; Rogers, S.; Shrestha, B.; Tripathi, A.; van der Hooft, J. J. J.; Vargas, F.; Weldon, K. C.; Witting, M.; Yang, H.; Zhang, Z.; Zubeil, F.; Kohlbacher, O.; Böcker, S.; Alexandrov, T.; Bandeira, N.; Wang, M.; Dorrestein, P. C. Nat. Methods 2020, 17, 905–908. doi:10.1038/s41592-020-0933-6
Return to citation in text: [1] -
de Oliveira, J. A. M.; Williams, D. E.; Andersen, R. J.; Sarragiotto, M. H.; Baldoqui, D. C. J. Braz. Chem. Soc. 2020, 31, 357–363. doi:10.21577/0103-5053.20190188
Return to citation in text: [1] -
Zhi, Y.; Wu, Q.; Xu, Y. Sci. Rep. 2017, 7, 40976. doi:10.1038/srep40976
Return to citation in text: [1] -
Carrillo, C.; Teruel, J. A.; Aranda, F. J.; Ortiz, A. Biochim. Biophys. Acta, Biomembr. 2003, 1611, 91–97. doi:10.1016/s0005-2736(03)00029-4
Return to citation in text: [1] [2] -
Buchoux, S.; Lai-Kee-Him, J.; Garnier, M.; Tsan, P.; Besson, F.; Brisson, A.; Dufourc, E. J. Biophys. J. 2008, 95, 3840–3849. doi:10.1529/biophysj.107.128322
Return to citation in text: [1] -
Deleu, M.; Bouffioux, O.; Razafindralambo, H.; Paquot, M.; Hbid, C.; Thonart, P.; Jacques, P.; Brasseur, R. Langmuir 2003, 19, 3377–3385. doi:10.1021/la026543z
Return to citation in text: [1]
| 1. | Cote, C. K.; Heffron, J. D.; Bozue, J. A.; Welkos, S. L.; Liu, D. Bacillus anthracis and other Bacillus species. In Molecular Medical Microbiology, 2nd ed.; Schwartzman, J.; Tang, Y.-W.; Sussman, M.; Poxton, I., Eds.; Academic Press: Boston, USA, 2015; pp 1789–1844. doi:10.1016/b978-0-12-397169-2.00102-5 |
| 7. | Raddadi, N.; Crotti, E.; Rolli, E.; Marasco, R.; Fava, F.; Daffonchio, D. The Most Important Bacillus Species in Biotechnology. In Bacillus thuringiensis Biotechnology; Sansinenea, E., Ed.; Springer: Dordrecht, 2012; pp 347–366. doi:10.1007/978-94-007-3021-2_17 |
| 8. | Kandi, V. J. Med. Microb. Diagn. 2016, 5, No. 2. doi:10.4172/2161-0703.1000e130 |
| 9. | Nemutanzhela, M. E.; Roets, Y.; Gardiner, N.; Lalloo, R. The use and benefits of Bacillus based biological agents in aquaculture. In Sustainable Aquaculture Techniques; Hernandez-Vergara, M. P.; Perez-Rostro, C. I., Eds.; IntechOpen, 2014. doi:10.5772/57375 |
| 10. | Shafi, J.; Tian, H.; Ji, M. Biotechnol. Biotechnol. Equip. 2017, 31, 446–459. doi:10.1080/13102818.2017.1286950 |
| 11. | Schallmey, M.; Singh, A.; Ward, O. P. Can. J. Microbiol. 2004, 50, 1–17. doi:10.1139/w03-076 |
| 12. | Elshaghabee, F. M. F.; Rokana, N.; Gulhane, R. D.; Sharma, C.; Panwar, H. Front. Microbiol. 2017, 8, No. 1490. doi:10.3389/fmicb.2017.01490 |
| 4. | Sansinenea, E.; Ortiz, A. Biotechnol. Lett. 2011, 33, 1523–1538. doi:10.1007/s10529-011-0617-5 |
| 5. | Harwood, C. R.; Mouillon, J.-M.; Pohl, S.; Arnau, J. FEMS Microbiol. Rev. 2018, 42, 721–738. doi:10.1093/femsre/fuy028 |
| 6. | Boottanun, P.; Potisap, C.; Hurdle, J. G.; Sermswan, R. W. AMB Express 2017, 7, No. 16. doi:10.1186/s13568-016-0302-0 |
| 3. | Cihan, A. C.; Tekin, N.; Ozcan, B.; Cokmus, C. Braz. J. Microbiol. 2012, 43, 309–324. doi:10.1590/s1517-83822012000100037 |
| 2. | Checinska, A.; Paszczynski, A.; Burbank, M. Annu. Rev. Food Sci. Technol. 2015, 6, 351–369. doi:10.1146/annurev-food-030713-092332 |
| 22. | de Oliveira, J. A. M.; Williams, D. E.; Andersen, R. J.; Sarragiotto, M. H.; Baldoqui, D. C. J. Braz. Chem. Soc. 2020, 31, 357–363. doi:10.21577/0103-5053.20190188 |
| 24. | Carrillo, C.; Teruel, J. A.; Aranda, F. J.; Ortiz, A. Biochim. Biophys. Acta, Biomembr. 2003, 1611, 91–97. doi:10.1016/s0005-2736(03)00029-4 |
| 25. | Buchoux, S.; Lai-Kee-Him, J.; Garnier, M.; Tsan, P.; Besson, F.; Brisson, A.; Dufourc, E. J. Biophys. J. 2008, 95, 3840–3849. doi:10.1529/biophysj.107.128322 |
| 26. | Deleu, M.; Bouffioux, O.; Razafindralambo, H.; Paquot, M.; Hbid, C.; Thonart, P.; Jacques, P.; Brasseur, R. Langmuir 2003, 19, 3377–3385. doi:10.1021/la026543z |
| 19. | Wang, M.; Carver, J. J.; Phelan, V. V.; Sanchez, L. M.; Garg, N.; Peng, Y.; Nguyen, D. D.; Watrous, J.; Kapono, C. A.; Luzzatto-Knaan, T.; Porto, C.; Bouslimani, A.; Melnik, A. V.; Meehan, M. J.; Liu, W. T.; Crüsemann, M.; Boudreau, P. D.; Esquenazi, E.; Sandoval-Calderón, M.; Bandeira, N. Nat. Biotechnol. 2016, 34, 828–837. doi:10.1038/nbt.3597 |
| 20. | Vargas, F.; Weldon, K. C.; Sikora, N.; Wang, M.; Zhang, Z.; Gentry, E. C.; Panitchpakdi, M. W.; Caraballo-Rodriguez, A. M.; Dorreistein, P.; Jarmusch, A. K. Rapid Commun. Mass Spectrom. 2020, 34, e8725. doi:10.1002/rcm.8725 |
| 21. | Nothias, L.-F.; Petras, D.; Schmid, R.; Dührkop, K.; Rainer, J.; Sarvepalli, A.; Protsyuk, I.; Ernst, M.; Tsugawa, H.; Fleischauer, M.; Aicheler, F.; Aksenov, A. A.; Alka, O.; Allard, P.-M.; Barsch, A.; Cachet, X.; Caraballo-Rodriguez, A. M.; Da Silva, R. R.; Dang, T.; Garg, N.; Gauglitz, J. M.; Gurevich, A.; Isaac, G.; Jarmusch, A. K.; Kameník, Z.; Kang, K. B.; Kessler, N.; Koester, I.; Korf, A.; Le Gouellec, A.; Ludwig, M.; Martin H., C.; McCall, L.-I.; McSayles, J.; Meyer, S. W.; Mohimani, H.; Morsy, M.; Moyne, O.; Neumann, S.; Neuweger, H.; Nguyen, N. H.; Nothias-Esposito, M.; Paolini, J.; Phelan, V. V.; Pluskal, T.; Quinn, R. A.; Rogers, S.; Shrestha, B.; Tripathi, A.; van der Hooft, J. J. J.; Vargas, F.; Weldon, K. C.; Witting, M.; Yang, H.; Zhang, Z.; Zubeil, F.; Kohlbacher, O.; Böcker, S.; Alexandrov, T.; Bandeira, N.; Wang, M.; Dorrestein, P. C. Nat. Methods 2020, 17, 905–908. doi:10.1038/s41592-020-0933-6 |
| 16. | Vijayasarathy, S.; Prasad, P.; Fremlin, L. J.; Ratnayake, R.; Salim, A. A.; Khalil, Z.; Capon, R. J. J. Nat. Prod. 2016, 79, 421–427. doi:10.1021/acs.jnatprod.5b01125 |
| 17. | Ayon, N. J.; Sharma, A. D.; Gutheil, W. G. J. Am. Soc. Mass Spectrom. 2019, 30, 448–458. doi:10.1007/s13361-018-2093-9 |
| 18. | Dewapriya, P.; Prasad, P.; Damodar, R.; Salim, A. A.; Capon, R. J. Org. Lett. 2017, 19, 2046–2049. doi:10.1021/acs.orglett.7b00638 |
| 13. | Osei, E.; Kwain, S.; Mawuli, G. T.; Anang, A. K.; Owusu, K. B. A.; Camas, M.; Camas, A. S.; Ohashi, M.; Alexandru-Crivac, C. N.; Deng, H.; Jaspars, M.; Kyeremeh, K. Mar. Drugs 2019, 17, 9. doi:10.3390/md17010009 |
| 14. | Tetevi, G. M.; Kwain, S.; Mensah, T.; Camas, A. S.; Camas, M.; Dofuor, A. K.; Azerigyik, F. A.; Oluwabusola, E.; Deng, H.; Jaspars, M.; Kyeremeh, K. Molbank 2019, 4, M1094. doi:10.3390/m1094 |
| 15. | Kwain, S.; Tetevi, G. M.; Mensah, T.; Camas, A. S.; Camas, M.; Dofuor, A. K.; Azerigyik, F. A.; Oluwabusola, E.; Deng, H.; Jaspars, M.; Kyeremeh, K. Molbank 2019, 3, M1080. doi:10.3390/m1080 |
| 23. | Zhi, Y.; Wu, Q.; Xu, Y. Sci. Rep. 2017, 7, 40976. doi:10.1038/srep40976 |
| 24. | Carrillo, C.; Teruel, J. A.; Aranda, F. J.; Ortiz, A. Biochim. Biophys. Acta, Biomembr. 2003, 1611, 91–97. doi:10.1016/s0005-2736(03)00029-4 |
© 2022 Nartey et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.