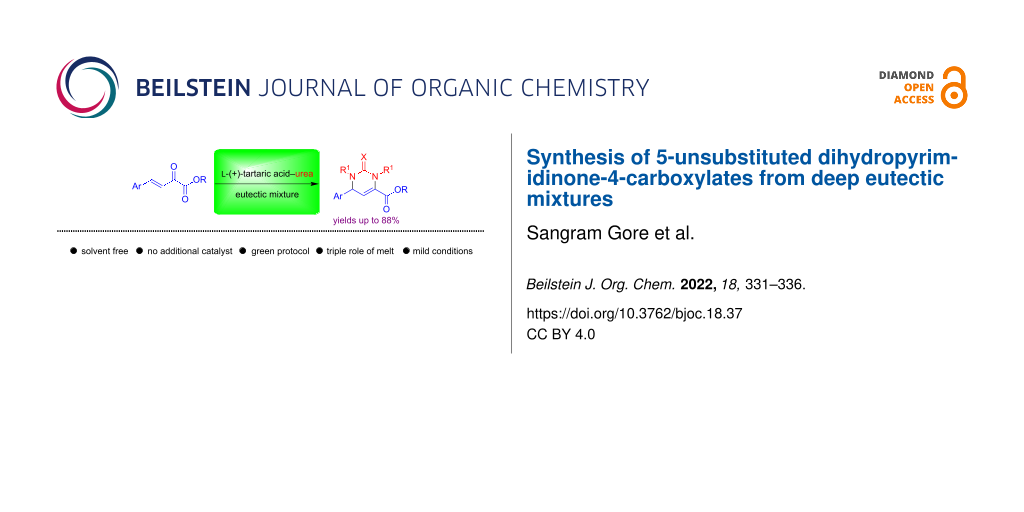
Abstract
A facile one-pot synthesis of 5-unsubstituted dihydropyrimidinones from β,γ-unsaturated ketoesters in low melting ʟ-(+)-tartaric acid–N,N-dimethylurea mixtures is reported. This solvent-free method is very general and provides easy access to 5-unsubstituted dihydropyrimidinone-4-carboxylate derivatives in good yields.
Graphical Abstract
Introduction
In recent years, dihydropyrimidinones (DHPMs) and their derivatives have attracted considerable attention due to the multifaceted pharmacological properties of this class of compounds [1-4]. The dihydropyrimidinone structure is found in calcium channel blockers [5-7], α1a adrenoceptor-selective antagonists [8,9], antihypertensive [10-13] and anti-inflammatory agents [14,15]. An interesting example is a rather simple DHPM derivative, monastrol, which specifically inhibits the motor activity of mitotic kinesin Eg5 and is therefore considered as a lead for the development of anticancer drugs [16].
Of particular interest are 5-unsubstituted DHPMs [17], such as compounds 1 and 2, which possess neuronal sodium channel blockade activities (Figure 1). Other examples are raltegravir, the first HIV-integrase inhibitor approved by the FDA for the treatment of HIV infection, derived from 5,6-dihydroxypyrimidine-4-carboxamide and N-methyl-4-hydroxypyrimidinone-carboxamide [18] and hydroxypyrimidinone carboxamide derivative P01, a potent inhibitor of Mycobacterium tuberculosis (Mtb) [19].
Figure 1: Biologically active functionalized DHPMs.
Figure 1: Biologically active functionalized DHPMs.
Owing to the biological significance of 5-unsubstituted dihydropyrimidinones, a variety of multistep protocols has been reported for the synthesis of 5-unsubstituted 3,4-dihydropyrimidin-2(1H)-ones [20,21]. Typically, dihydropyrimidinones are obtained via a Biginelli reaction leading to an ester group at C5 and an alkyl group at C6 position. A group of researchers from Merck reported the synthesis of 5-unsubstituted DHPMs via a Biginelli reaction followed by saponification of the ester and subsequent decarboxylation [22]. Later, Bussolari and McDonnell demonstrated the synthesis of 5-unsubstituted 3,4-dihydropyrimidinone-4-carboxylate derivatives by employing oxalacetic acid as a β-ketoester equivalent in the presence of TFA via a Biginelli reaction [23]. Lam and Fang reported the same synthesis under microwave conditions [24].
Very recently, Kambappa and co-workers reported a one-pot synthesis of 5-unsubstituted dihydropyrimidinone-4-carboxylate using gem-dibromomethylarene, oxalacetic acid, and urea [25]. Here the gem-dibromomethylarene moiety serves as an aldehyde equivalent. In addition, utilizing aromatic ketones as a β-ketoester equivalent, the synthesis of 5-unsubstituted DHPM bearing two aryl groups at the C4 and C6 positions was also reported [26]. Although several synthetic routes to 5-unsubstituted DHPM have been reported, there is still need for improvements in terms of higher yields, shorter reaction times, less hazardous or corrosive reagents, and fewer synthetic steps. Here, we report the facile and economic access to 5-unsubstituted DHPMs using a melt procedure avoiding organic solvents. The method is based on our previous reports of synthesis of organic molecules in the melt [27].
Results and Discussion
Since 5-unsubstituted DHPMs bearing the carboxylic acid moiety at the C4 position allow versatile further functionalization and are biologically interesting DHPMs [17-21], we envisioned an environmentally benign cyclocondensation protocol using low melting mixtures as a green reaction medium. We have established low melting mixtures [28-31] based on carbohydrates, urea, and inorganic salts as an alternative to conventional solvents for carrying out a variety of organic transformations [32]. The stable melts are environmentally friendly as they are readily available from bulk renewable resources. Their simple production allows the replacement of organic solvents. The melts are stable against air and have very low vapor pressures resembling the properties of ionic liquids. In addition, the polarity of these melts is very high [33]. Recently, we have explored several organic transformations such as coupling reaction, cycloaddition reaction, synthesis of glycosylurea, dihydropyrimidinones, pyrimidopyrimidinediones, and functionalized indole derivatives in this novel and green reaction medium [34-38].
We have also developed an efficient method for the synthesis of trisubstituted hydantoin derivatives from β,γ-unsaturated ketoacids [39]. In the present study, in continuation of our interest in the synthesis of functionalized DHPMs [27], we utilized β,γ-unsaturated ketoesters and subjected them to the melt conditions to achieve the synthesis of 5-unsubstituted DHPMs.
We envisaged that the simple Michael addition reaction of urea derivatives with β,γ-unsaturated ketoesters and subsequent intramolecular condensation could lead to 5-unsubstituted DHPM derivatives. β,γ-Unsaturated ketoester 7, derived from benzaldehyde and pyruvic acid [40], on exposure to ʟ-(+)-tartaric acid–N,N-dimethylurea (DMU) melt underwent smooth reaction to furnish the corresponding 5-unsubstituted dihydropyrimidinone-4-carboxylate derivative 8 in good yield (entry 1, Table 1). Encouraged by this observation, we tested the generality of this methodology by employing various electron-donating as well as electron-withdrawing groups on the aryl ring and the results are summarized in Table 1. The electron-rich (E)-ethyl 4-(4-methoxyphenyl)-2-oxobut-3-enoate (11) furnished the corresponding 5-unsubsituted DHPM derivative 12 on treatment with the ʟ-(+)-tartaric acid–DMU melt in very good yield (entry 3, Table 1). Moreover, electron-deficient β,γ-unsaturated ketoesters, such as (E)-ethyl 4-(4-nitrophenyl)-2-oxobut-3-enoate (17), afforded the corresponding 5-unsubstituted DHPM derivative 18 in good yield (entry 6, Table 1). Similarly, heteroaromatic aldehyde derived ketoester 21, also underwent the tandem reaction to give the corresponding 5-unsubstituted DHPM derivative 22 in moderate yield (entry 9, Table 1). In addition to the aromatic part, the ester moiety of the β,γ-unsaturated ketoesters was also varied. (E)-Methyl 4-(2-azidophenyl)-2-oxobut-3-enoate (23), on exposure to the melt medium, yielded the corresponding 5-unsubstituted DHPM 24 (entry 10, Table 1) [41]. The scope of this method was further extended by employing this protocol to the synthesis of a thio derivative of 5-unsubstituted DHPM. Since thiourea does not form a clear melt with tartaric acid, the tartaric acid–choline chloride melt was employed for the reaction involving thiourea as one of the reactants. Hence, (E)-ethyl 4-(4-bromophenyl)-2-oxobut-3-enoate (19) on treatment with tartaric acid–choline chloride melt by employing thiourea as one of the reactants furnished the corresponding thio derivative of 5-unsubstituted DHPM derivative (entry 8, Table 1). The melt medium plays a triple role as solvent, catalyst and as reactant and furnishes the functionalized 5-unsubstituted dihydropyrimidinone-4-carboxylate derivatives.
Table 1: Synthesis of 5-unsubstituted DHPMs.a
|
|
||||
| Entry | Substrate | Time (h) | Product | Yieldb (%) |
| 1 |
7 |
2 |
8 |
83 |
| 2 |
9 |
2.5 |
10 |
88c |
| 3 |
11 |
3 |
12 |
87 |
| 4 |
13 |
8 |
14 |
71 |
| 5 |
15 |
2.5 |
16 |
84 |
| 6 |
17 |
1.5 |
18 |
77 |
| 7 |
19 |
2 |
20 |
70 |
| 8 |
19 |
6 |
20A |
48d |
| 9 |
21 |
5 |
22 |
60 |
| 10 |
23 |
1.5 |
24 |
53 |
| 11 |
25 |
1.5 |
26 |
85 |
| 12 |
27 |
3.5 |
28 |
51 |
aβ,γ-unsaturated ketoester (1 mmol) in ʟ-(+)-tartaric acid–DMU melt at 70 °C; bisolated yield; cat 90 °C in ʟ-(+)-tartaric acid–urea (2:3) melt; dreaction carried out in the presence of thiourea in ʟ-(+)-tartaric acid–choline chloride melt (1:2) at 90 °C.
Conclusion
In conclusion, a novel one-pot approach has been developed for the synthesis of 5-unsubstituted dihydropyrimidinone-4-carboxylate derivatives in good yields under environmentally benign conditions. Electron-rich as well as electron-deficient, highly functionalized β,γ-unsaturated ketoesters proved to be excellent substrates in this cyclocondensation reaction. The carboxylic ester substitution at C4 position provides the option for further chemical transformations on the DHPM skeleton. We hope that this environmentally benign one-pot method will find application in the synthesis of 5-unsubstituted dihydropyrimidinones.
Supporting Information
| Supporting Information File 1: Experimental procedures, characterization of products, copies of NMR spectra. | ||
| Format: PDF | Size: 2.1 MB | Download |
References
-
Biginelli, P. Gazz. Chim. Ital. 1893, 23, 360–416.
Return to citation in text: [1] -
Matos, L. H. S.; Masson, F. T.; Simeoni, L. A.; Homem-de-Mello, M. Eur. J. Med. Chem. 2018, 143, 1779–1789. doi:10.1016/j.ejmech.2017.10.073
Return to citation in text: [1] -
Ismaili, L.; Monnin, J.; Etievant, A.; Arribas, R. L.; Viejo, L.; Refouvelet, B.; Soukup, O.; Janockova, J.; Hepnarova, V.; Korabecny, J.; Kucera, T.; Jun, D.; Andrys, R.; Musilek, K.; Baguet, A.; García-Frutos, E. M.; De Simone, A.; Andrisano, V.; Bartolini, M.; de los Ríos, C.; Marco-Contelles, J.; Haffen, E. ACS Chem. Neurosci. 2021, 12, 1328–1342. doi:10.1021/acschemneuro.0c00803
Return to citation in text: [1] -
Li, Y.; Tan, T.; Zhao, Y.; Wei, Y.; Wang, D.; Chen, R.; Tao, L. ACS Macro Lett. 2020, 9, 1249–1254. doi:10.1021/acsmacrolett.0c00496
Return to citation in text: [1] -
Atwal, K. S.; Rovnyak, G. C.; Schwartz, J.; Moreland, S.; Hedberg, A.; Gougoutas, J. Z.; Malley, M. F.; Floyd, D. M. J. Med. Chem. 1990, 33, 1510–1515. doi:10.1021/jm00167a035
Return to citation in text: [1] -
Atwal, K. S.; Rovnyak, G. C.; Kimball, S. D.; Floyd, D. M.; Moreland, S.; Swanson, B. N.; Gougoutas, J. Z.; Schwartz, J.; Smillie, K. M.; Malley, M. F. J. Med. Chem. 1990, 33, 2629–2635. doi:10.1021/jm00171a044
Return to citation in text: [1] -
Cho, H.; Ueda, M.; Shima, K.; Mizuno, A.; Hayashimatsu, M.; Ohnaka, Y.; Takeuchi, Y.; Hamaguchi, M.; Aisaka, K.; Hidaka, T.; Kawai, M.; Takeda, M.; Ishihara, T.; Funahashi, K.; Satoh, F.; Morita, M.; Noguchi, T. J. Med. Chem. 1989, 32, 2399–2406. doi:10.1021/jm00130a029
Return to citation in text: [1] -
Barrow, J. C.; Nantermet, P. G.; Selnick, H. G.; Glass, K. L.; Rittle, K. E.; Gilbert, K. F.; Steele, T. G.; Homnick, C. F.; Freidinger, R. M.; Ransom, R. W.; Kling, P.; Reiss, D.; Broten, T. P.; Schorn, T. W.; Chang, R. S. L.; O'Malley, S. S.; Olah, T. V.; Ellis, J. D.; Barrish, A.; Kassahun, K.; Leppert, P.; Nagarathnam, D.; Forray, C. J. Med. Chem. 2000, 43, 2703–2718. doi:10.1021/jm990612y
Return to citation in text: [1] -
Jain, K. S.; Bariwal, J. B.; Kathiravan, M. K.; Phoujdar, M. S.; Sahne, R. S.; Chauhan, B. S.; Shah, A. K.; Yadav, M. R. Bioorg. Med. Chem. 2008, 16, 4759–4800. doi:10.1016/j.bmc.2008.02.091
Return to citation in text: [1] -
Atwal, K. S.; Swanson, B. N.; Unger, S. E.; Floyd, D. M.; Moreland, S.; Hedberg, A.; O'Reilly, B. C. J. Med. Chem. 1991, 34, 806–811. doi:10.1021/jm00106a048
Return to citation in text: [1] -
Rovnyak, G. C.; Atwal, K. S.; Hedberg, A.; Kimball, S. D.; Moreland, S.; Gougoutas, J. Z.; O'Reilly, B. C.; Schwartz, J.; Malley, M. F. J. Med. Chem. 1992, 35, 3254–3263. doi:10.1021/jm00095a023
Return to citation in text: [1] -
Grover, G. J.; Dzwonczyk, S.; McMullen, D. M.; Normandin, D. E.; Parham, C. S.; Sleph, P. G.; Moreland, S. J. Cardiovasc. Pharmacol. 1995, 26, 289–294. doi:10.1097/00005344-199508000-00015
Return to citation in text: [1] -
Farghaly, A. M.; AboulWafa, O. M.; Elshaier, Y. A. M.; Badawi, W. A.; Haridy, H. H.; Mubarak, H. A. E. Med. Chem. Res. 2019, 28, 360–379. doi:10.1007/s00044-019-02289-6
Return to citation in text: [1] -
Kappe, C. O. Eur. J. Med. Chem. 2000, 35, 1043–1052. doi:10.1016/s0223-5234(00)01189-2
Return to citation in text: [1] -
Zhou, M.; Wang, Y.; Lin, X.; Wan, J.; Wen, C. Front. Pharmacol. 2021, 11, 624059. doi:10.3389/fphar.2020.624059
Return to citation in text: [1] -
Mayer, T. U.; Kapoor, T. M.; Haggarty, S. J.; King, R. W.; Schreiber, S. L.; Mitchison, T. J. Science 1999, 286, 971–974. doi:10.1126/science.286.5441.971
Return to citation in text: [1] -
Kong, K.-H.; Chen, Y.; Ma, X.; Chui, W. K.; Lam, Y. J. Comb. Chem. 2004, 6, 928–933. doi:10.1021/cc049910t
Return to citation in text: [1] [2] -
Summa, V.; Petrocchi, A.; Bonelli, F.; Crescenzi, B.; Donghi, M.; Ferrara, M.; Fiore, F.; Gardelli, C.; Gonzalez Paz, O.; Hazuda, D. J.; Jones, P.; Kinzel, O.; Laufer, R.; Monteagudo, E.; Muraglia, E.; Nizi, E.; Orvieto, F.; Pace, P.; Pescatore, G.; Scarpelli, R.; Stillmock, K.; Witmer, M. V.; Rowley, M. J. Med. Chem. 2008, 51, 5843–5855. doi:10.1021/jm800245z
Return to citation in text: [1] [2] -
Oh, S.; Park, Y.; Engelhart, C. A.; Wallach, J. B.; Schnappinger, D.; Arora, K.; Manikkam, M.; Gac, B.; Wang, H.; Murgolo, N.; Olsen, D. B.; Goodwin, M.; Sutphin, M.; Weiner, D. M.; Via, L. E.; Boshoff, H. I. M.; Barry, C. E., III. J. Med. Chem. 2018, 61, 9952–9965. doi:10.1021/acs.jmedchem.8b00883
Return to citation in text: [1] [2] -
Wang, Z.-T.; Xu, L.-W.; Xia, C.-G.; Wang, H.-Q. Tetrahedron Lett. 2004, 45, 7951–7953. doi:10.1016/j.tetlet.2004.08.107
Return to citation in text: [1] [2] -
Ren, Y.-M.; Cai, C. Monatsh. Chem. 2009, 140, 49–52. doi:10.1007/s00706-008-0011-8
Return to citation in text: [1] [2] -
Steele, T. G.; Coburn, C. A.; Patane, M. A.; Bock, M. G. Tetrahedron Lett. 1998, 39, 9315–9318. doi:10.1016/s0040-4039(98)02155-8
Return to citation in text: [1] -
Bussolari, J. C.; McDonnell, P. A. J. Org. Chem. 2000, 65, 6777–6779. doi:10.1021/jo005512a
Return to citation in text: [1] -
Fang, Z.; Lam, Y. Tetrahedron 2011, 67, 1294–1297. doi:10.1016/j.tet.2010.11.075
Return to citation in text: [1] -
Kambappa, V.; Kotturappa, C. G.; Manjunath, R. S.; Shivaramu, P. D. J. Heterocycl. Chem. 2020, 57, 3475–3482. doi:10.1002/jhet.4066
Return to citation in text: [1] -
Sabitha, G.; Reddy, K. B.; Srinivas, R.; Yadav, J. S. Helv. Chim. Acta 2005, 88, 2996–2999. doi:10.1002/hlca.200590242
Return to citation in text: [1] -
Gore, S.; Baskaran, S.; Koenig, B. Green Chem. 2011, 13, 1009–1013. doi:10.1039/c1gc00009h
Return to citation in text: [1] [2] -
Abbott, A. P.; Boothby, D.; Capper, G.; Davies, D. L.; Rasheed, R. K. J. Am. Chem. Soc. 2004, 126, 9142–9147. doi:10.1021/ja048266j
Return to citation in text: [1] -
Carriazo, D.; Serrano, M. C.; Gutiérrez, M. C.; Ferrer, M. L.; del Monte, F. Chem. Soc. Rev. 2012, 41, 4996–5014. doi:10.1039/c2cs15353j
Return to citation in text: [1] -
Zhang, Q.; De Oliveira Vigier, K.; Royer, S.; Jérôme, F. Chem. Soc. Rev. 2012, 41, 7108–7146. doi:10.1039/c2cs35178a
Return to citation in text: [1] -
Ruß, C.; König, B. Green Chem. 2012, 14, 2969–2982. doi:10.1039/c2gc36005e
Return to citation in text: [1] -
Imperato, G.; Eibler, E.; Niedermaier, J.; König, B. Chem. Commun. 2005, 1170–1172. doi:10.1039/b414515a
Return to citation in text: [1] -
Imperato, G.; Höger, S.; Lenoir, D.; König, B. Green Chem. 2006, 8, 1051–1055. doi:10.1039/b603660k
Return to citation in text: [1] -
Ilgen, F.; König, B. Green Chem. 2009, 11, 848–854. doi:10.1039/b816551c
Return to citation in text: [1] -
Imperato, G.; Vasold, R.; König, B. Adv. Synth. Catal. 2006, 348, 2243–2247. doi:10.1002/adsc.200600248
Return to citation in text: [1] -
Ruß, C.; Ilgen, F.; Reil, C.; Luff, C.; Haji Begli, A.; König, B. Green Chem. 2011, 13, 156–161. doi:10.1039/c0gc00468e
Return to citation in text: [1] -
Gore, S.; Baskaran, S.; Koenig, B. Adv. Synth. Catal. 2012, 354, 2368–2372. doi:10.1002/adsc.201200257
Return to citation in text: [1] -
Gore, S.; Baskaran, S.; König, B. Org. Lett. 2012, 14, 4568–4571. doi:10.1021/ol302034r
Return to citation in text: [1] -
Gore, S.; Chinthapally, K.; Baskaran, S.; König, B. Chem. Commun. 2013, 49, 5052–5054. doi:10.1039/c3cc41254g
Return to citation in text: [1] -
Stecher, E. D.; Incorvia, M. J.; Kerben, B.; Lavine, D.; Oen, M.; Suhl, E. J. Org. Chem. 1973, 38, 4453–4457. doi:10.1021/jo00965a024
Return to citation in text: [1] -
The lower yield might be attributed to the acid-mediated reaction of the azide.
Return to citation in text: [1]
| 1. | Biginelli, P. Gazz. Chim. Ital. 1893, 23, 360–416. |
| 2. | Matos, L. H. S.; Masson, F. T.; Simeoni, L. A.; Homem-de-Mello, M. Eur. J. Med. Chem. 2018, 143, 1779–1789. doi:10.1016/j.ejmech.2017.10.073 |
| 3. | Ismaili, L.; Monnin, J.; Etievant, A.; Arribas, R. L.; Viejo, L.; Refouvelet, B.; Soukup, O.; Janockova, J.; Hepnarova, V.; Korabecny, J.; Kucera, T.; Jun, D.; Andrys, R.; Musilek, K.; Baguet, A.; García-Frutos, E. M.; De Simone, A.; Andrisano, V.; Bartolini, M.; de los Ríos, C.; Marco-Contelles, J.; Haffen, E. ACS Chem. Neurosci. 2021, 12, 1328–1342. doi:10.1021/acschemneuro.0c00803 |
| 4. | Li, Y.; Tan, T.; Zhao, Y.; Wei, Y.; Wang, D.; Chen, R.; Tao, L. ACS Macro Lett. 2020, 9, 1249–1254. doi:10.1021/acsmacrolett.0c00496 |
| 14. | Kappe, C. O. Eur. J. Med. Chem. 2000, 35, 1043–1052. doi:10.1016/s0223-5234(00)01189-2 |
| 15. | Zhou, M.; Wang, Y.; Lin, X.; Wan, J.; Wen, C. Front. Pharmacol. 2021, 11, 624059. doi:10.3389/fphar.2020.624059 |
| 26. | Sabitha, G.; Reddy, K. B.; Srinivas, R.; Yadav, J. S. Helv. Chim. Acta 2005, 88, 2996–2999. doi:10.1002/hlca.200590242 |
| 10. | Atwal, K. S.; Swanson, B. N.; Unger, S. E.; Floyd, D. M.; Moreland, S.; Hedberg, A.; O'Reilly, B. C. J. Med. Chem. 1991, 34, 806–811. doi:10.1021/jm00106a048 |
| 11. | Rovnyak, G. C.; Atwal, K. S.; Hedberg, A.; Kimball, S. D.; Moreland, S.; Gougoutas, J. Z.; O'Reilly, B. C.; Schwartz, J.; Malley, M. F. J. Med. Chem. 1992, 35, 3254–3263. doi:10.1021/jm00095a023 |
| 12. | Grover, G. J.; Dzwonczyk, S.; McMullen, D. M.; Normandin, D. E.; Parham, C. S.; Sleph, P. G.; Moreland, S. J. Cardiovasc. Pharmacol. 1995, 26, 289–294. doi:10.1097/00005344-199508000-00015 |
| 13. | Farghaly, A. M.; AboulWafa, O. M.; Elshaier, Y. A. M.; Badawi, W. A.; Haridy, H. H.; Mubarak, H. A. E. Med. Chem. Res. 2019, 28, 360–379. doi:10.1007/s00044-019-02289-6 |
| 27. | Gore, S.; Baskaran, S.; Koenig, B. Green Chem. 2011, 13, 1009–1013. doi:10.1039/c1gc00009h |
| 8. | Barrow, J. C.; Nantermet, P. G.; Selnick, H. G.; Glass, K. L.; Rittle, K. E.; Gilbert, K. F.; Steele, T. G.; Homnick, C. F.; Freidinger, R. M.; Ransom, R. W.; Kling, P.; Reiss, D.; Broten, T. P.; Schorn, T. W.; Chang, R. S. L.; O'Malley, S. S.; Olah, T. V.; Ellis, J. D.; Barrish, A.; Kassahun, K.; Leppert, P.; Nagarathnam, D.; Forray, C. J. Med. Chem. 2000, 43, 2703–2718. doi:10.1021/jm990612y |
| 9. | Jain, K. S.; Bariwal, J. B.; Kathiravan, M. K.; Phoujdar, M. S.; Sahne, R. S.; Chauhan, B. S.; Shah, A. K.; Yadav, M. R. Bioorg. Med. Chem. 2008, 16, 4759–4800. doi:10.1016/j.bmc.2008.02.091 |
| 24. | Fang, Z.; Lam, Y. Tetrahedron 2011, 67, 1294–1297. doi:10.1016/j.tet.2010.11.075 |
| 5. | Atwal, K. S.; Rovnyak, G. C.; Schwartz, J.; Moreland, S.; Hedberg, A.; Gougoutas, J. Z.; Malley, M. F.; Floyd, D. M. J. Med. Chem. 1990, 33, 1510–1515. doi:10.1021/jm00167a035 |
| 6. | Atwal, K. S.; Rovnyak, G. C.; Kimball, S. D.; Floyd, D. M.; Moreland, S.; Swanson, B. N.; Gougoutas, J. Z.; Schwartz, J.; Smillie, K. M.; Malley, M. F. J. Med. Chem. 1990, 33, 2629–2635. doi:10.1021/jm00171a044 |
| 7. | Cho, H.; Ueda, M.; Shima, K.; Mizuno, A.; Hayashimatsu, M.; Ohnaka, Y.; Takeuchi, Y.; Hamaguchi, M.; Aisaka, K.; Hidaka, T.; Kawai, M.; Takeda, M.; Ishihara, T.; Funahashi, K.; Satoh, F.; Morita, M.; Noguchi, T. J. Med. Chem. 1989, 32, 2399–2406. doi:10.1021/jm00130a029 |
| 25. | Kambappa, V.; Kotturappa, C. G.; Manjunath, R. S.; Shivaramu, P. D. J. Heterocycl. Chem. 2020, 57, 3475–3482. doi:10.1002/jhet.4066 |
| 19. | Oh, S.; Park, Y.; Engelhart, C. A.; Wallach, J. B.; Schnappinger, D.; Arora, K.; Manikkam, M.; Gac, B.; Wang, H.; Murgolo, N.; Olsen, D. B.; Goodwin, M.; Sutphin, M.; Weiner, D. M.; Via, L. E.; Boshoff, H. I. M.; Barry, C. E., III. J. Med. Chem. 2018, 61, 9952–9965. doi:10.1021/acs.jmedchem.8b00883 |
| 22. | Steele, T. G.; Coburn, C. A.; Patane, M. A.; Bock, M. G. Tetrahedron Lett. 1998, 39, 9315–9318. doi:10.1016/s0040-4039(98)02155-8 |
| 18. | Summa, V.; Petrocchi, A.; Bonelli, F.; Crescenzi, B.; Donghi, M.; Ferrara, M.; Fiore, F.; Gardelli, C.; Gonzalez Paz, O.; Hazuda, D. J.; Jones, P.; Kinzel, O.; Laufer, R.; Monteagudo, E.; Muraglia, E.; Nizi, E.; Orvieto, F.; Pace, P.; Pescatore, G.; Scarpelli, R.; Stillmock, K.; Witmer, M. V.; Rowley, M. J. Med. Chem. 2008, 51, 5843–5855. doi:10.1021/jm800245z |
| 23. | Bussolari, J. C.; McDonnell, P. A. J. Org. Chem. 2000, 65, 6777–6779. doi:10.1021/jo005512a |
| 17. | Kong, K.-H.; Chen, Y.; Ma, X.; Chui, W. K.; Lam, Y. J. Comb. Chem. 2004, 6, 928–933. doi:10.1021/cc049910t |
| 16. | Mayer, T. U.; Kapoor, T. M.; Haggarty, S. J.; King, R. W.; Schreiber, S. L.; Mitchison, T. J. Science 1999, 286, 971–974. doi:10.1126/science.286.5441.971 |
| 20. | Wang, Z.-T.; Xu, L.-W.; Xia, C.-G.; Wang, H.-Q. Tetrahedron Lett. 2004, 45, 7951–7953. doi:10.1016/j.tetlet.2004.08.107 |
| 21. | Ren, Y.-M.; Cai, C. Monatsh. Chem. 2009, 140, 49–52. doi:10.1007/s00706-008-0011-8 |
| 32. | Imperato, G.; Eibler, E.; Niedermaier, J.; König, B. Chem. Commun. 2005, 1170–1172. doi:10.1039/b414515a |
| 17. | Kong, K.-H.; Chen, Y.; Ma, X.; Chui, W. K.; Lam, Y. J. Comb. Chem. 2004, 6, 928–933. doi:10.1021/cc049910t |
| 18. | Summa, V.; Petrocchi, A.; Bonelli, F.; Crescenzi, B.; Donghi, M.; Ferrara, M.; Fiore, F.; Gardelli, C.; Gonzalez Paz, O.; Hazuda, D. J.; Jones, P.; Kinzel, O.; Laufer, R.; Monteagudo, E.; Muraglia, E.; Nizi, E.; Orvieto, F.; Pace, P.; Pescatore, G.; Scarpelli, R.; Stillmock, K.; Witmer, M. V.; Rowley, M. J. Med. Chem. 2008, 51, 5843–5855. doi:10.1021/jm800245z |
| 19. | Oh, S.; Park, Y.; Engelhart, C. A.; Wallach, J. B.; Schnappinger, D.; Arora, K.; Manikkam, M.; Gac, B.; Wang, H.; Murgolo, N.; Olsen, D. B.; Goodwin, M.; Sutphin, M.; Weiner, D. M.; Via, L. E.; Boshoff, H. I. M.; Barry, C. E., III. J. Med. Chem. 2018, 61, 9952–9965. doi:10.1021/acs.jmedchem.8b00883 |
| 20. | Wang, Z.-T.; Xu, L.-W.; Xia, C.-G.; Wang, H.-Q. Tetrahedron Lett. 2004, 45, 7951–7953. doi:10.1016/j.tetlet.2004.08.107 |
| 21. | Ren, Y.-M.; Cai, C. Monatsh. Chem. 2009, 140, 49–52. doi:10.1007/s00706-008-0011-8 |
| 28. | Abbott, A. P.; Boothby, D.; Capper, G.; Davies, D. L.; Rasheed, R. K. J. Am. Chem. Soc. 2004, 126, 9142–9147. doi:10.1021/ja048266j |
| 29. | Carriazo, D.; Serrano, M. C.; Gutiérrez, M. C.; Ferrer, M. L.; del Monte, F. Chem. Soc. Rev. 2012, 41, 4996–5014. doi:10.1039/c2cs15353j |
| 30. | Zhang, Q.; De Oliveira Vigier, K.; Royer, S.; Jérôme, F. Chem. Soc. Rev. 2012, 41, 7108–7146. doi:10.1039/c2cs35178a |
| 31. | Ruß, C.; König, B. Green Chem. 2012, 14, 2969–2982. doi:10.1039/c2gc36005e |
| 40. | Stecher, E. D.; Incorvia, M. J.; Kerben, B.; Lavine, D.; Oen, M.; Suhl, E. J. Org. Chem. 1973, 38, 4453–4457. doi:10.1021/jo00965a024 |
| 41. | The lower yield might be attributed to the acid-mediated reaction of the azide. |
| 39. | Gore, S.; Chinthapally, K.; Baskaran, S.; König, B. Chem. Commun. 2013, 49, 5052–5054. doi:10.1039/c3cc41254g |
| 27. | Gore, S.; Baskaran, S.; Koenig, B. Green Chem. 2011, 13, 1009–1013. doi:10.1039/c1gc00009h |
| 33. | Imperato, G.; Höger, S.; Lenoir, D.; König, B. Green Chem. 2006, 8, 1051–1055. doi:10.1039/b603660k |
| 34. | Ilgen, F.; König, B. Green Chem. 2009, 11, 848–854. doi:10.1039/b816551c |
| 35. | Imperato, G.; Vasold, R.; König, B. Adv. Synth. Catal. 2006, 348, 2243–2247. doi:10.1002/adsc.200600248 |
| 36. | Ruß, C.; Ilgen, F.; Reil, C.; Luff, C.; Haji Begli, A.; König, B. Green Chem. 2011, 13, 156–161. doi:10.1039/c0gc00468e |
| 37. | Gore, S.; Baskaran, S.; Koenig, B. Adv. Synth. Catal. 2012, 354, 2368–2372. doi:10.1002/adsc.201200257 |
| 38. | Gore, S.; Baskaran, S.; König, B. Org. Lett. 2012, 14, 4568–4571. doi:10.1021/ol302034r |
© 2022 Gore et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.