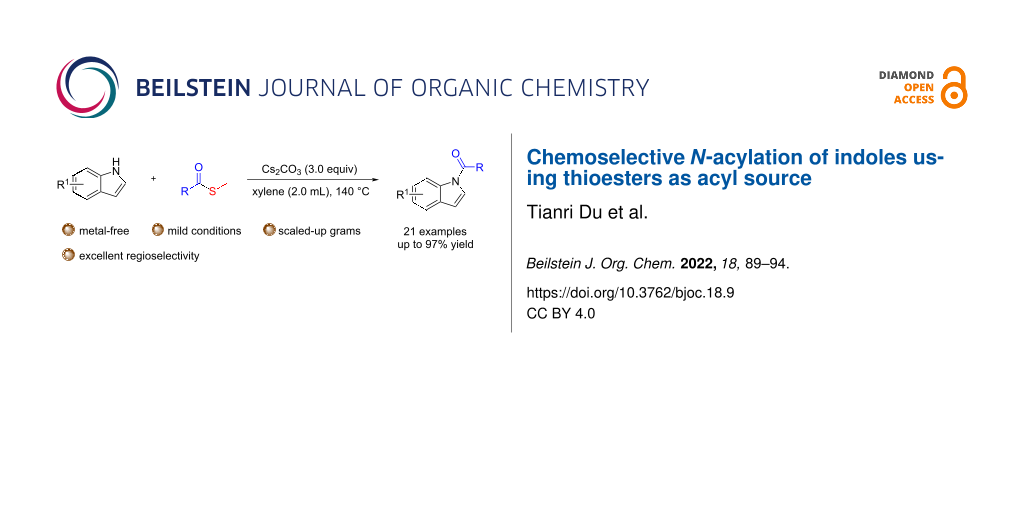
Abstract
The selective acylation of indoles often requires sensitive and reactive acyl chloride derivatives. Here, we report a mild, efficient, functional group tolerant, and highly chemoselective N-acylation of indoles using thioesters as a stable acyl source. A series of indoleamides have been obtained with moderate to good yields. In addition, heterocycles, such as carbazole, can also be used as nucleophiles in this reaction.
Graphical Abstract
Introduction
Molecules containing N-acylindoles have attracted wide attention in the synthetic polymers and pharmaceutical industry because of their unique structural, chemical, and biological properties [1]. For example, indomethacin is a nonselective inhibitor of COX1 and COX2, which is used for treating fever, pain and swelling [2]. Indole analog L-768242 exhibits nanomolar potencies (Ki) with superior selectivity for the hCB2 receptor over the human central cannabinoid (hCB1) receptor [3] (Figure 1).
Figure 1: Representative pharmaceuticals containing N-acylindole moieties.
Figure 1: Representative pharmaceuticals containing N-acylindole moieties.
Indole has multiple reactive sites, and chemoselective N- or C-functionalization of indoles is a challenging and important task [4,5]. Acylation of indoles frequently takes place at the C3 position because of the relatively strong electron cloud density. As N-acylated indoles are a widespread motif in many pharmaceuticals and natural products [6-8], selective N-acylation of indoles is very important. However, this process often requires unstable and reactive acyl chloride, which results in a poor functional group tolerance. Thus, developing a simple and efficient method for the synthesis of N-acylindoles becomes much attractive [9-12]. In 2009, Scheidt developed a dehydrogenative approach using indoles and alcohols catalyzed by tetrapropylammonium perruthenate [13] (Scheme 1, A1). In 2012, Sarpong successfully carried out chemoselective acylation of the N(sp2)–H bond by using a catalytic amount of DBU for the preparation of indoleamides [14] (Scheme 1, A2). Subsequently, Sundén reported an efficient chemoselective method for the synthesis of indoleamide by oxidative organocatalytic reaction of indole derivatives and conjugated aldehydes under NHC catalysis [15] (Scheme 1, A1).
Scheme 1: A) Strategies for the synthesis of N-acylindoles; B) thioester as dicarbonylation reagent; C) recent work of our group; D) this work.
Scheme 1: A) Strategies for the synthesis of N-acylindoles; B) thioester as dicarbonylation reagent; C) recen...
In 2019, Jiang and co-workers reported a dicarbonylation of amine and aryl borates using α-ketothioester as a stable 1,2-dicarbonyl reagent [16] (Scheme 1, B). Recently, we applied S-methyl butanethioate in a Pd-catalyzed transthioetherification or transthioesterification of aryl halides for the synthesis of thioethers and thioesters [17] (Scheme 1, C). In addition, we also used this reagent to trap alkylcopper(I) intermediates and to form C−S bonds [18]. To the best of our knowledge, thioesters have not been developed as indole N-amidation reagent. Based on our continuous interest on thioesters, herein, we report an efficient and chemoselective protocol for the synthesis of indoleamide derivatives with thioesters as acyl source.
Results and Discussion
At the beginning of our studies, we selected 3-methyl-1H-indole (1a) and S-methyl butanethioate (2a) as the model substrates to establish this procedure. As shown in Table 1, different bases were tried to improve the amidation reaction, and Cs2CO3 was found the most suitable choice (Table 1, entry 1). NaOt-Bu can also be used in this reaction and 82% yield could be obtained. In addition, NaOH and K2CO3 were not suitable in this procedure (Table 1, entries 3 and 4). Also the reaction did not work in the absence of Cs2CO3 (Table 1, entry 5). Solvent screening indicated that xylene was the best choice, and DMF, THF, and MeOH were not suitable for this reaction (Table 1, entries 7–9). When Cs2CO3 was reduced from 3.0 equiv to 2.0 equiv, 85% of the desired product could be obtained (Table 1, entry 10). Subsequently, we conducted a temperature optimization and 73% of the product was observed at 100 °C (Table 1, entry 11).
Table 1: Optimization of the reaction conditionsa.
|
|
||
| Entry | Variation from standard conditions | Yield (%)b |
| 1 | none | 97 |
| 2 | NaOt-Bu instead of Cs2CO3 | 82 |
| 3 | NaOH instead of Cs2CO3 | trace |
| 4 | K2CO3 instead of Cs2CO3 | trace |
| 5 | without Cs2CO3 | NRc |
| 6 | toluene instead of xylene | 89 |
| 7 | DMF instead of xylene | 0 |
| 8 | THF instead of xylene | 0 |
| 9 | MeOH instead of xylene | 0 |
| 10 | 2.0 equiv Cs2CO3 | 85 |
| 11 | 100 °C instead of 140 °C | 73 |
aReaction conditions: 1a (0.2 mmol, 1.0 equiv), 2a (0.6 mmol, 3.0 equiv), Cs2CO3 (0.6 mmol, 3.0 equiv), xylene (2.0 mL), 140 °C, 12 h. bYield was determined by GC using n-dodecane as the internal standard. cNR = no reaction.
Under the optimized reaction conditions, the scope of the reaction by variation of indoles and thioesters was tested. As shown in Scheme 2, a variety of functional groups, such as -OMe, -F and -I, are tolerated providing the desired products with moderate to excellent yields (Scheme 2, 3c–e). In addition, various methyl thioesters could also participate in this reaction smoothly (Scheme 2, 3g–r). Interestingly, S-methyl benzothioate and S-methyl 4-methylbenzothioate could also take part in this reaction and converted into the corresponding products 3s and 3t in 93% and 96% yield, respectively. Notably, carbazole could also be acylated with thioesters and 84% yield of the desired product was obtained (Scheme 2, 3u).
Scheme 2: Reactions of thioesters and indoles. Reaction conditions: 1 (0.2 mmol, 1.0 equiv), 2 (0.6 mmol, 3.0 equiv), Cs2CO3 (0.6 mmol, 3.0 equiv), xylene (2.0 mL), 140 °C, 12 h. Isolated yields are shown.
Scheme 2: Reactions of thioesters and indoles. Reaction conditions: 1 (0.2 mmol, 1.0 equiv), 2 (0.6 mmol, 3.0...
With the established method for the N-acylation of indoles, a 2 mmol scale reaction was carried out. The reaction of 3-methyl-1H-indole (1a) and S-methyl butanethioate (2a) proceeded smoothly and 1-(3-methyl-1H-indol-1-yl)butan-1-one (3a) was obtained with 62% isolated yield (0.25 g, Scheme 3). The results indicate that this N-acylation reaction of indole has great potential in practical synthesis.
Some control experiments were conducted to explore the reaction mechanism of this transformation (Scheme 4). When S-methyl decanethioate (2i) was adopted without Cs2CO3, no decomposition product was observed (Scheme 4, reaction 1). When S-methyl decanethioate (2i) was treated under the standard reaction conditions, 56% of 2i was recovered (Scheme 4, reaction 2). Furthermore, without Cs2CO3, no desired product could be obtained (Scheme 4, reaction 3). These results indicate that Cs2CO3 plays an important role in the N-acylation process of indoles. The reaction of decanoic acid and 3-methyl-1H-indole (1a) was also conducted under the standard conditions, and no desired product was obtained, illustrating that 1-(3-methyl-1H-indol-1-yl)decan-1-one (3i) was not transformed from decanoic acid (4) (Scheme 4, reaction 4).
A plausible reaction mechanism has been proposed based on the results of the control experiments. As shown in Scheme 5, the reaction starts with a base-promoted deprotonation of indole forming intermediate A. In the next step nucleophilic substitution between intermediate A and 2a occurs to give the desired N-acylindole product and CsSCH3 as byproduct [19-21] (Scheme 5).
Conclusion
In conclusion, a chemoselective N-acylation of synthetically valuable indoles has been developed by using thioesters as a stable acyl source, a variety of N-acylated indoles could be obtained efficiently. Beside indole, carbazole can also take part in this reaction.
Supporting Information
| Supporting Information File 1: Experimental part and NMR spectra. | ||
| Format: PDF | Size: 3.7 MB | Download |
Funding
The authors are thankful for the financial support from the National Natural Science Foundation of China (22101005), the National Key Research and Development Program of China (2021YFD1700100), the Natural Science Foundation of Anhui Province (1908085MC71), Key Research and Development Program of Anhui Province (202104a06020008), Key Experimental Foundation for Quality and Safety of Agricultural Products in Anhui Province.
References
-
Lancianesi, S.; Palmieri, A.; Petrini, M. Chem. Rev. 2014, 114, 7108–7149. doi:10.1021/cr400676v
Return to citation in text: [1] -
Shen, T. Y.; Windholz, T. B.; Rosegay, A.; Witzel, B. E.; Wilson, A. N.; Willett, J. D.; Holtz, W. J.; Ellis, R. L.; Matzuk, A. R.; Lucas, S.; Stammer, C. H.; Holly, F. W.; Sarett, L. H.; Risley, E. A.; Nuss, G. W.; Winter, C. A. J. Am. Chem. Soc. 1963, 85, 488–489. doi:10.1021/ja00887a038
Return to citation in text: [1] -
Yang, Y.; Duan, X.-H.; Deng, J.-Y.; Jin, B.; Jia, H.-M.; Liu, B.-L. Bioorg. Med. Chem. Lett. 2011, 21, 5594–5597. doi:10.1016/j.bmcl.2011.06.077
Return to citation in text: [1] -
Fang, W.; Deng, Q.; Xu, M.; Tu, T. Org. Lett. 2013, 15, 3678–3681. doi:10.1021/ol401550h
Return to citation in text: [1] -
Ottoni, O.; Cruz, R.; Alves, R. Tetrahedron 1998, 54, 13915–13928. doi:10.1016/s0040-4020(98)00865-5
Return to citation in text: [1] -
Dhanoa, D. S.; Bagley, S. W.; Chang, R. S. L.; Lotti, V. J.; Chen, T. B.; Kivlighn, S. D.; Zingaro, G. J.; Siegl, P. K. S.; Patchett, A. A.; Greenlee, W. J. J. Med. Chem. 1993, 36, 4230–4238. doi:10.1021/jm00078a013
Return to citation in text: [1] -
Welstead, W. J., Jr.; Stauffer, H. F., Jr.; Sancilio, L. F. J. Med. Chem. 1974, 17, 544–547. doi:10.1021/jm00251a019
Return to citation in text: [1] -
Zeng, J.; Zhang, Z.; Zhu, Q.; Jiang, Z.; Zhong, G. Molecules 2020, 25, 1189. doi:10.3390/molecules25051189
Return to citation in text: [1] -
Umehara, A.; Ueda, H.; Tokuyama, H. J. Org. Chem. 2016, 81, 11444–11453. doi:10.1021/acs.joc.6b02097
Return to citation in text: [1] -
Zhou, X.-Y.; Chen, X. Synthesis 2019, 51, 516–521. doi:10.1055/s-0037-1609937
Return to citation in text: [1] -
Kikugawa, Y. Synthesis 1981, 460–461. doi:10.1055/s-1981-29483
Return to citation in text: [1] -
Sundberg, R. J. Ketones, aldehydes, and carboxylic acids derived from indole. In The chemistry of indoles; Blomquist, A. T., Ed.; Organic chemsitry: a series of monographs, Vol. 18; Academic Press: New York and London, 1970; pp 401–430. doi:10.1016/b978-0-12-676950-0.50014-6
Return to citation in text: [1] -
Maki, B. E.; Scheidt, K. A. Org. Lett. 2009, 11, 1651–1654. doi:10.1021/ol900306v
Return to citation in text: [1] -
Heller, S. T.; Schultz, E. E.; Sarpong, R. Angew. Chem., Int. Ed. 2012, 51, 8304–8308. doi:10.1002/anie.201203976
Return to citation in text: [1] -
Ta, L.; Sundén, H. Chem. Commun. 2018, 54, 531–534. doi:10.1039/c7cc08672e
Return to citation in text: [1] -
Wang, M.; Dai, Z.; Jiang, X. Nat. Commun. 2019, 10, 2661. doi:10.1038/s41467-019-10651-w
Return to citation in text: [1] -
Li, Y.; Bao, G.; Wu, X.-F. Chem. Sci. 2020, 11, 2187–2192. doi:10.1039/c9sc05532k
Return to citation in text: [1] -
Tian, Q.; Xu, S.; Zhang, C.; Liu, X.; Wu, X.; Li, Y. J. Org. Chem. 2021, 86, 8797–8804. doi:10.1021/acs.joc.1c00665
Return to citation in text: [1] -
Hardee, D. J.; Kovalchuke, L.; Lambert, T. H. J. Am. Chem. Soc. 2010, 132, 5002–5003. doi:10.1021/ja101292a
Return to citation in text: [1] -
Laha, J. K.; Kaur Hunjan, M.; Hegde, S.; Gupta, A. Org. Lett. 2020, 22, 1442–1447. doi:10.1021/acs.orglett.0c00041
Return to citation in text: [1] -
Jiang, Y.-Y.; Liu, T.-T.; Zhang, R.-X.; Xu, Z.-Y.; Sun, X.; Bi, S. J. Org. Chem. 2018, 83, 2676–2685. doi:10.1021/acs.joc.7b03107
Return to citation in text: [1]
| 1. | Lancianesi, S.; Palmieri, A.; Petrini, M. Chem. Rev. 2014, 114, 7108–7149. doi:10.1021/cr400676v |
| 6. | Dhanoa, D. S.; Bagley, S. W.; Chang, R. S. L.; Lotti, V. J.; Chen, T. B.; Kivlighn, S. D.; Zingaro, G. J.; Siegl, P. K. S.; Patchett, A. A.; Greenlee, W. J. J. Med. Chem. 1993, 36, 4230–4238. doi:10.1021/jm00078a013 |
| 7. | Welstead, W. J., Jr.; Stauffer, H. F., Jr.; Sancilio, L. F. J. Med. Chem. 1974, 17, 544–547. doi:10.1021/jm00251a019 |
| 8. | Zeng, J.; Zhang, Z.; Zhu, Q.; Jiang, Z.; Zhong, G. Molecules 2020, 25, 1189. doi:10.3390/molecules25051189 |
| 4. | Fang, W.; Deng, Q.; Xu, M.; Tu, T. Org. Lett. 2013, 15, 3678–3681. doi:10.1021/ol401550h |
| 5. | Ottoni, O.; Cruz, R.; Alves, R. Tetrahedron 1998, 54, 13915–13928. doi:10.1016/s0040-4020(98)00865-5 |
| 3. | Yang, Y.; Duan, X.-H.; Deng, J.-Y.; Jin, B.; Jia, H.-M.; Liu, B.-L. Bioorg. Med. Chem. Lett. 2011, 21, 5594–5597. doi:10.1016/j.bmcl.2011.06.077 |
| 19. | Hardee, D. J.; Kovalchuke, L.; Lambert, T. H. J. Am. Chem. Soc. 2010, 132, 5002–5003. doi:10.1021/ja101292a |
| 20. | Laha, J. K.; Kaur Hunjan, M.; Hegde, S.; Gupta, A. Org. Lett. 2020, 22, 1442–1447. doi:10.1021/acs.orglett.0c00041 |
| 21. | Jiang, Y.-Y.; Liu, T.-T.; Zhang, R.-X.; Xu, Z.-Y.; Sun, X.; Bi, S. J. Org. Chem. 2018, 83, 2676–2685. doi:10.1021/acs.joc.7b03107 |
| 2. | Shen, T. Y.; Windholz, T. B.; Rosegay, A.; Witzel, B. E.; Wilson, A. N.; Willett, J. D.; Holtz, W. J.; Ellis, R. L.; Matzuk, A. R.; Lucas, S.; Stammer, C. H.; Holly, F. W.; Sarett, L. H.; Risley, E. A.; Nuss, G. W.; Winter, C. A. J. Am. Chem. Soc. 1963, 85, 488–489. doi:10.1021/ja00887a038 |
| 17. | Li, Y.; Bao, G.; Wu, X.-F. Chem. Sci. 2020, 11, 2187–2192. doi:10.1039/c9sc05532k |
| 14. | Heller, S. T.; Schultz, E. E.; Sarpong, R. Angew. Chem., Int. Ed. 2012, 51, 8304–8308. doi:10.1002/anie.201203976 |
| 18. | Tian, Q.; Xu, S.; Zhang, C.; Liu, X.; Wu, X.; Li, Y. J. Org. Chem. 2021, 86, 8797–8804. doi:10.1021/acs.joc.1c00665 |
| 13. | Maki, B. E.; Scheidt, K. A. Org. Lett. 2009, 11, 1651–1654. doi:10.1021/ol900306v |
| 9. | Umehara, A.; Ueda, H.; Tokuyama, H. J. Org. Chem. 2016, 81, 11444–11453. doi:10.1021/acs.joc.6b02097 |
| 10. | Zhou, X.-Y.; Chen, X. Synthesis 2019, 51, 516–521. doi:10.1055/s-0037-1609937 |
| 11. | Kikugawa, Y. Synthesis 1981, 460–461. doi:10.1055/s-1981-29483 |
| 12. | Sundberg, R. J. Ketones, aldehydes, and carboxylic acids derived from indole. In The chemistry of indoles; Blomquist, A. T., Ed.; Organic chemsitry: a series of monographs, Vol. 18; Academic Press: New York and London, 1970; pp 401–430. doi:10.1016/b978-0-12-676950-0.50014-6 |
| 16. | Wang, M.; Dai, Z.; Jiang, X. Nat. Commun. 2019, 10, 2661. doi:10.1038/s41467-019-10651-w |
© 2022 Du et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.